Introduction
Breast cancer is the most common malignant tumor in
women and the second leading cause of cancer-associated mortality
amongst women worldwide (2009–2010) (1,2). In 2008,
the World Health Organization estimated that >1.2 million people
were diagnosed with breast cancer globally (3). Breast cancer may be classified into
different subgroups depending on the expression of estrogen
receptor (ER), progesterone receptor or human epidermal growth
factor receptor 2. These subgroups present with distinct molecular
backgrounds and exhibit diverse clinical behavior and treatment
responses (4,5). Among all types of breast cancer, tumors
with negative expression of ER, which accounts for 25–30% of all
types of breast cancer (6), are known
for their aggressive nature and high metastatic potential (7).
At present, therapeutic strategies for breast cancer
include surgery, radiation and chemotherapy (8). However, despite advances in multimodal
treatments, no effective systemic therapy has been established.
Drug resistance is considered to be one of the most important
factors influencing the clinical outcomes of patients (9). Therefore, the discovery of novel
therapeutic approaches is required to advance the treatment
outcomes of patients with ER-negative breast cancer. Numerous
studies have illustrated that the acridone nucleus, in addition to
its derivatives, possess the property of a potent anticancer effect
(10,11). However, the efficacy of acridone
against human breast cancer is yet to be reported.
In the present study, the cytotoxic effects of
acridone on the MDA-MB-231 human ER-negative breast cancer cell
line was investigated, in addition to its underlying mechanisms
in vitro. To the best of our knowledge, the present study
was the first to demonstrate that acridone inhibited the
proliferation of MDA-MB-231 cells. It was also revealed that
acridone significantly inhibited the mRNA and protein expression
level of ATP binding cassette subfamily G member 2 (ABCG2). These
findings provide evidence that acridone may possess the potential
to be used for the clinical treatment of human breast cancer.
Materials and methods
Cell lines and regents
The MDA-MB-231 cell line was purchased from the
American Type Culture Collection (ATCC; Manassas, VA, USA).
Dulbecco's modified Eagle's medium (DMEM), fetal bovine serum (FBS)
and penicillin-streptomycin were purchased from Gibco (Thermo
Fisher Scientific, Inc., Waltham, MA, USA). MTT was supplied by
Sigma-Aldrich (Merck KGaA, Darmstadt, Germany). Protease inhibitor
cocktail tablets and phosphatase inhibitor cocktail tablets were
purchased from Roche Applied Science (Mannheim, Germany). Trypsin
and TRIzol were purchased from Invitrogen (Thermo Fisher
Scientific, Inc.). Mouse anti-ABCG2 antibody was purchased from
Abcam (Cambridge, MA, USA). Mouse anti-β-actin antibody was
purchased from Cell Signaling Technology, Inc. (Danvers, MA,
USA).
Cell culture
MDA-MB-231 cells were cultured in DMEM supplemented
with 10% FBS and 100 µg/ml penicillin-100 U/ml streptomycin at 37°C
in a humidified incubator containing 5% CO2.
Cell viability assays
MDA-MB-231 cells were seeded in 96-well plates at a
density of 2.5×103 cells/ml in 200 µl DMEM medium and
cultured at 37°C overnight. Subsequently, cells were treated at
37°C with multiple concentrations of acridone (0.1, 0.5 and 1.0 µM)
for 48 h. Cells treated with medium with 0.1% DMSO were regarded as
the negative control. Next, 20 µl MTT (5 mg/ml in PBS) was added to
each well and incubated at 37°C for a further 4 h. Subsequently,
the medium was discarded and 200 µl DMSO was added to each well to
dissolve the purple formazan crystals. The optical density (OD) at
570 nm was detected using a microplate reader (BioTek Instruments,
Inc., Winooski, VT, USA).
RNA isolation and reverse
transcription-quantitative polymerase chain reaction (RT-qPCR)
MDA-MB-231 cells were treated with multiple
concentrations of acridone (0.1, 0.5 and 1.0 µM) at 37°C for 48 h.
In brief, total cellular RNA was isolated using TRIzol reagent
(Sigma-Aldrich; Merck KGaA) according to the manufacturer's
instructions. First strand cDNA was prepared using the SYBR Green
master kit (Applied Biosystems; Thermo Fisher Scientific, Inc.)
according to the manufacturer's instructions. The following primers
were used: 5′-TATAGCTCAGATCATTGTCACAGTC-3′ (sense) and
5′-GTTGGTCGTCAGGAAGAAGAG-3′ (antisense) for ABCG2. The PCR assay
was performed using the SYBR Premix Ex Taq system (Takara
Biotechnology Co., Ltd., Dalian, China), and the thermocycling
conditions were as follows: 95°C for 15 min, 40 cycles of 95°C for
30 sec, 55°C for 30 min and 72°C for 30 sec. β-actin was used as a
loading control. Relative gene expression was obtained following
normalization with endogenous β-actin and determination of the
difference in threshold cycle (Cq) between treated and untreated
cells using the 2−∆∆Cq method (12).
Western blotting
MDA-MB-231 cells (2×106 cells/dish) were
seeded in 100 mm culture dishes and cultured in DMEM at 37°C
overnight, followed by treatment with multiple concentrations of
acridone (0.1, 0.5 and 1 µM) at 37°C for 48 h. Following
trypsinization, cells were transferred to centrifuge tubes and
centrifuged at 4°C, 800 × g for 3 min. The supernatant was
discarded and the cell pellets were washed using ice-cold PBS 2–3
times. RIPA buffer [250 mmol/l NaCl, 50 mmol/l HEPES, 5 mmol/l
egtazic acid, 20 mmol/l ethylenediaminetetraacetic acid (pH 8.0),
0.1% Triton X-100, 2 mg/ml leupeptin, 2 mg/ml aprotinin and 1 mM
phenylmethylsulfonyl fluoride] containing protease inhibitor
cocktail tablets and phosphatase inhibitor (Roche Applied Science)
was used to dissolve the pellets for 30 min at 4°C. Once the cell
lysate was centrifuged at 12,000 × g at 4°C for 15 min, supernatant
was collected and stored at −80°C. Using the BCA protein assay kit
(Beyotime Institute of Biotechnology, Haimen, China) according to
the manufacturer's instructions, protein concentration was
determined. Cell lysates (30 µg/lane) were separated using 10%
SDS-PAGE, transferred to polyvinylidene fluoride membranes, blocked
with 5% fat-free dried milk for 2 h at room temperature and probed
with ABCG2 antibody (1:500; cat. no. ab207732; Abcam, Cambridge,
UK) and horseradish peroxidase-conjugated goat anti-rabbit
immunoglobulin G secondary antibody (1:1,000; cat. no. ab6702;
Abcam). Immunoreactive bands were visualized using enhanced
chemiluminescence system (ImageQuant LAS 4000; General Electric
Company, Fairfield, CT, USA) with LabImage version 2.7.1 (Kapelan
Bio-Imaging, Leipzig, Germany).
Statistical analysis
Each experiment was performed at least three times
and analyzed using GraphPad Prism 5.0 software (GraphPad Software,
Inc., La Jolla, CA, USA). The data are presented as the mean ±
standard deviation and analyzed using one-way analysis of variance
followed by Tukey's multiple comparison test. P<0.05 was
considered to indicate a statistically significant difference.
Results
Anti-proliferation effect of acridone
on MDA-MB-231 cells in vitro
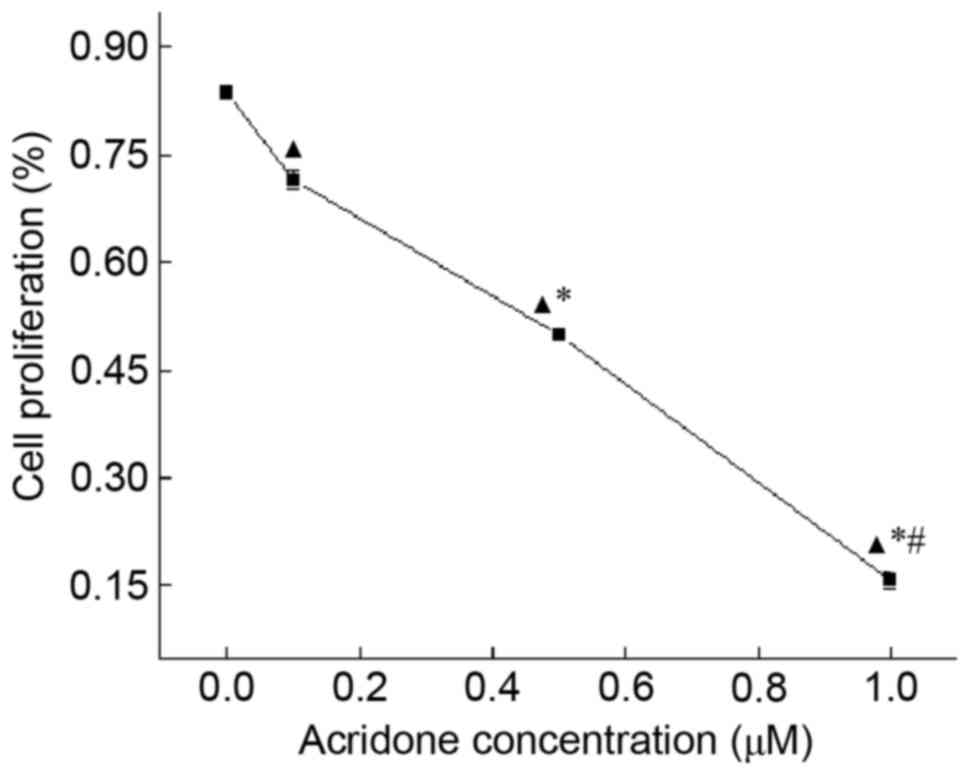
Acridone demonstrated an anti-proliferation effect
on MDA-MB-231 cells, which was determined using an MTT assay
(Table I; Fig. 1). Compared with control group,
acridone significantly decreased the proliferation of MDA-MB-231
cells in a dose-dependent manner (P<0.05). At 48 h, the OD
values of 0.0, 0.1, 0.5 and 1.0 µM acridone were 0.836±0.009,
0.714±0.013, 0.498±0.005 and 0.156±0.011, respectively. The results
of the present study indicated that acridone may inhibit the
proliferation of MDA-MB-231 cells.
 | Table I.Effects of acridone on the
proliferation of MDA-MB-231 cells. |
Table I.
Effects of acridone on the
proliferation of MDA-MB-231 cells.
| Acridone
concentration (µM) | Optical density
value |
|---|
| 0.0 | 0.836±0.009 |
| 0.1 | 0.714±0.013 |
| 0.5 |
0.498±0.005a |
| 1.0 |
0.156±0.011b |
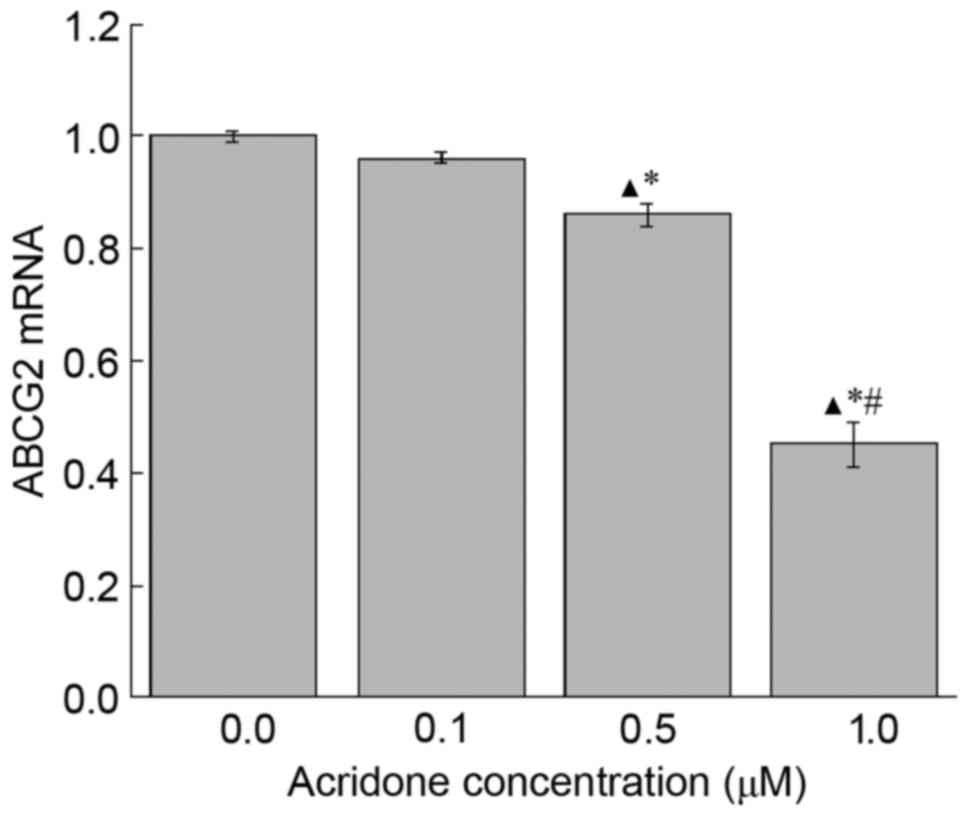
Effect of acridone on ABCG2 mRNA
expression levels in MDA-MB-231 cells
To further study the mechanism underlying the
anti-proliferation function of acridone, RT-qPCR was performed to
examine the mRNA expression levels of ABCG2. As presented in
Fig. 2, 0.5 and 1.0 µM acridone
treatment significantly decreased ABCG2 mRNA expression levels
compared with the control group (P<0.05). However, there was no
significant difference (P>0.05) identified between the 0.1 µM
acridone treatment group and the control group in the mRNA
expression levels of ABCG2. The data revealed that, compared with
control group, acridone may decrease ABCG2 mRNA expression at 0.5
and 1.0 µM doses.
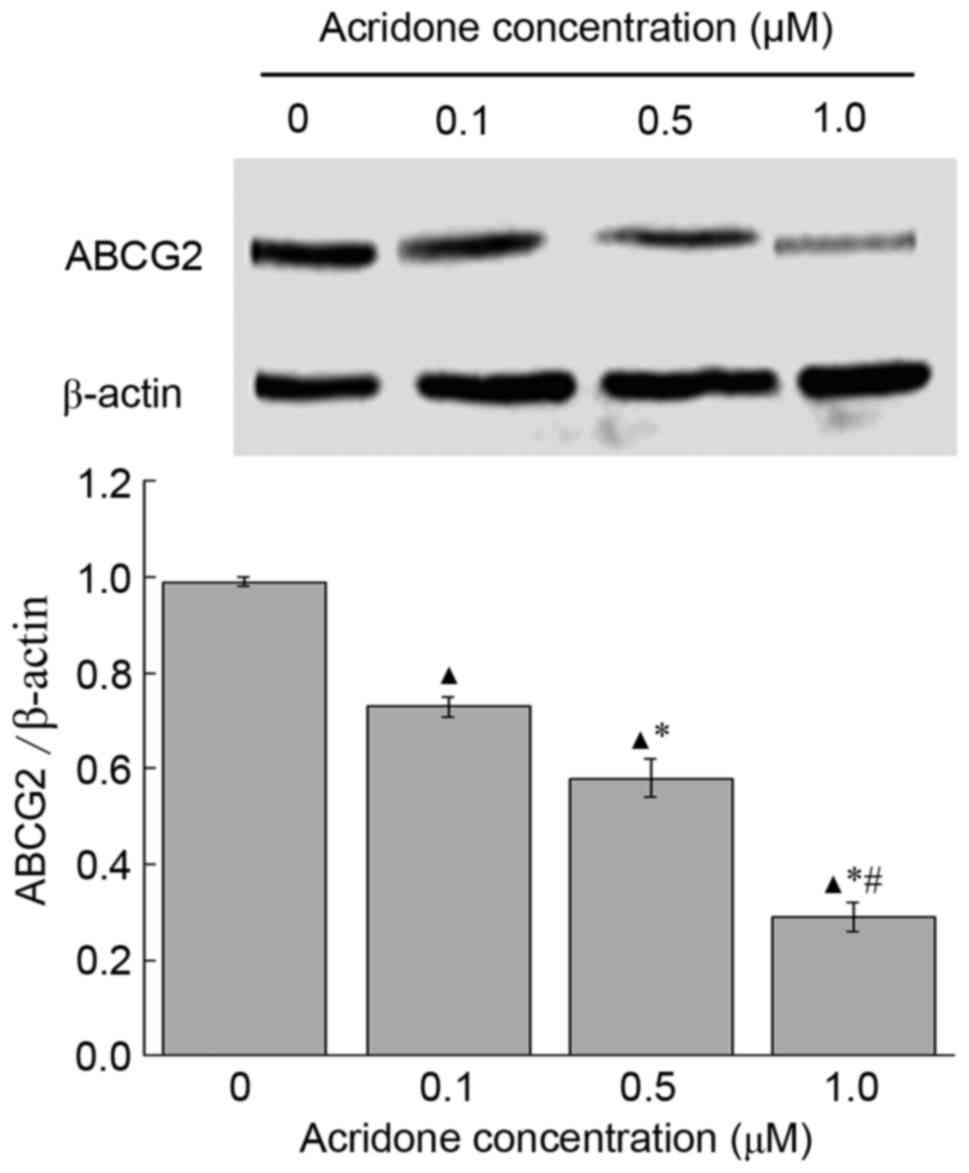
Effects of acridone on the expression
levels of ABCG2 protein in MDA-MB-231 cells
Expression levels of the ABCG2 protein in MDA-MB-231
cells treated with acridone were detected using western blotting.
Compared with control group, the expression level of ABCG2 was
significantly downregulated in a dose-dependent manner following
acridone treatment (P<0.05; Fig.
3). The protein levels of ABCG2 in the control group were
significantly increased compared with those in the 0.1, 0.5 and 1.0
µM acridone treatment groups (P<0.05; Fig. 3). The results of the present study
revealed that, compared with control group, acridone may decrease
ABCG2 protein expression at 0.1, 0.5 and 1.0 µM doses.
Discussion
There are numerous functions of the acridone nucleus
in addition to its derivatives that have been reported in previous
studies, including anticancer (13–16),
anti-herpes (17), anti-malarial
(18,19), antivirus (20), anti-allergy (21) and anti-leishmanial (22) activities. These features are
attributed to the semi planar heterocyclic structure, which
interacts with different biomolecular targets.
However, the antitumor function of acridone against
human breast cancer and its mechanism of action remain to be
elucidated. Therefore, the purpose of the present study was to
examine the effect of acridone on human breast cancer and to
elucidate the underlying molecular mechanisms. The present study
revealed, to the best of our knowledge for the first time, that
acridone inhibited the proliferation of MDA-MB-231 cells in
vitro. In addition, the molecular mechanisms by which acridone
affects MDA-MB-231 cells were revealed.
Membrane transporters serve a critical function in
regulating drug resistance. In the last decade, the ATP-binding
cassette protein superfamily has received notable attention,
particularly the protein ABCG2 (23).
This ATP-binding cassette transporter serves a crucial function in
mediating drug efflux and is associated with the primary or
acquired drug resistance presented in clinical settings. A number
of studies have attempted to develop specific inhibitors targeting
ABCG2 (24,25). In consideration of the potent
inhibitory effect of acridone, the present study further explored
whether ABCG2 is associated with the underlying mechanism of the
inhibitory effect of acridone. Compared with control group, the
mRNA and protein expression levels of ABCG2 were decreased in
acridone-treated MDA-MB-231 cells, indicating that acridone
treatment may be associated with ABCG2-dependent cell proliferation
inhibition.
To conclude, the present study demonstrated that
acridone may inhibit the proliferation of MDA-MB-231 cells, in
addition to downregulating the mRNA and protein expression levels
of ABCG2. These results revealed details regarding the mechanisms
behind the effect of acridone on MDA-MB-231 and suggest that
acridone may be a potential candidate for the development of novel
treatment strategies for human breast cancer. However, the specific
molecular targets of acridone and the signaling pathways affected
in vitro require further study.
References
|
1
|
Coughlin SS and Ekwueme DU: Breast cancer
as a global health concern. Cancer Epidemiol. 33:315–318. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Jemal A, Center MM, DeSantis C and Ward
EM: Global patterns of cancer incidence and mortality rates and
trends. Cancer Epidemiol Biomarkers Prev. 19:1893–1907. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Kasami M, Uematsu T, Honda M, Yabuzaki T,
Sanuki J, Uchida Y and Sugimura H: Comparison of estrogen receptor,
progesterone receptor and Her-2 status in breast cancer pre- and
post-neoadjuvant chemotherapy. Breast. 17:523–527. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Rouzier R, Perou CM, Symmans WF, Ibrahim
N, Cristofanilli M, Anderson K, Hess KR, Stec J, Ayers M, Wagner P,
et al: Breast cancer molecular subtypes respond differently to
preoperative chemotherapy. Clin Cancer Res. 11:5678–5685. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Nguyen PL, Taghian AG, Katz MS, Niemierko
A, Abi Raad RF, Boon WL, Bellon JR, Wong JS, Smith BL and Harris
JR: Breast cancer subtype approximated by estrogen receptor,
progesterone receptor, and HER-2 is associated with local and
distant recurrence after breast-conserving therapy. J Clin Oncol.
26:2373–2378. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Huang CS, Lin CH, Lu YS and Shen CY:
Unique features of breast cancer in Asian women-breast cancer in
Taiwan as an example. J Steroid Biochem Mol Biol. 118:300–303.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kennecke H, Yerushalmi R, Woods R, Cheang
MC, Voduc D, Speers CH, Nielsen TO and Gelmon K: Metastatic
behavior of breast cancer subtypes. J Clin Oncol. 28:3271–3277.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Morgan G, Ward R and Barton M: The
contribution of cytotoxic chemotherapy to 5-year survival in adult
malignancies. Clin Oncol (R Coll Radiol). 16:549–560. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kuczynski EA, Sargent DJ, Grothey A and
Kerbel RS: Drug rechallenge and treatment beyond
progression-implications for drug resistance. Nat Rev Clin Oncol.
10:571–587. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zambre VP, Murumkar PR, Giridhar R and
Yadav MR: Development of highly predictive 3D-QSAR CoMSIA models
for anthraquinone and acridone derivatives as telomerase inhibitors
targeting G-quadruplex DNA telomere. J Mol Graph Model. 29:229–239.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Cuenca F, Moore MJ, Johnson K, Guyen B, De
Cian A and Neidle S: Design, synthesis and evaluation of
4,5-di-substituted acridone ligands with high G-quadruplex affinity
and selectivity, together with low toxicity to normal cells. Bioorg
Med Chem Lett. 19:5109–5113. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Su TL, Kohler B, Chou TC, Chun MW and
Watanabe KA: Synthesis of the acridone alkaloids glyfoline and
congeners. Structure-activity relationship studies of cytotoxic
acridones. J Med Chem. 35:2703–2710. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Cholewiński G, Dzierzbicka K and
Kolodziejczyk AM: Natural and synthetic acridines/acridones as
antitumor agents: Their biological activities and methods of
synthesis. Pharmacol Rep. 63:305–336. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Koba M and Baczek T: Physicochemical
interaction of antitumor acridinone derivatives with DNA in view of
QSAR studies. Med Chem Res. 20:1385–1393. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Prasad VV Rajendra, Peters GJ, Lemos C,
Kathmann I and Mayur YC: Cytotoxicity studies of some novel fluoro
acridone derivatives against sensitive and resistant cancer cell
lines and their mechanistic studies. Eur J Pharm Sci. 43:217–224.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Yamamoto N, Furukawa H, Ito Y, Yoshida S,
Maeno K and Nishiyama Y: Anti-herpesvirus activity of
citrusinine-I, a new acridone alkaloid, and related compounds.
Antiviral Res. 12:21–36. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Basco LK, Mitaku S, Skaltsounis AL,
Ravelomanantsoa N, Tillequin F, Koch M and Le Bras J: In vitro
activities of furoquinoline and acridone alkaloids against
Plasmodium falciparum. Antimicrob Agents Chemother. 38:1169–1171.
1994. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Valdés AF: Acridine and acridinones: Old
and new structures with antimalarial activity. Open Med Chem J.
5:11–20. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Stankiewicz-Drogoń A, Dörner B, Erker T
and Boguszewska-Chachulska AM: Synthesis of new acridone
derivatives, inhibitors of NS3 helicase, which efficiently and
specifically inhibit subgenomic HCV replication. J Med Chem.
53:3117–3126. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Chukaew A, Ponglimanont C, Karalai C and
Tewtrakul S: Potential anti-allergic acridone alkaloids from the
roots of Atalantia monophylla. Phytochemistry. 69:2616–2620. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Delmas F, Avellaneda A, Di Giorgio C,
Robin M, De Clercq E, Timon-David P and Galy JP: Synthesis and
antileishmanial activity of (1,3-benzothiazol-2-yl)
amino-9-(10H)-acridinone derivatives. Eur J Med Chem. 39:685–690.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Holohan C, Van Schaeybroeck S, Longley DB
and Johnston PG: Cancer drug resistance: An evolving paradigm. Nat
Rev Cancer. 13:714–726. 2013. View
Article : Google Scholar : PubMed/NCBI
|
|
24
|
Pérez-Tomás R: Multidrug resistance:
Retrospect and prospects in anti-cancer drug treatment. Curr Med
Chem. 13:1859–1876. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Wu CP, Ohnuma S and Ambudkar SV:
Discovering natural product modulators to overcome multidrug
resistance in cancer chemotherapy. Curr Pharm Biotechnol.
12:609–620. 2011. View Article : Google Scholar : PubMed/NCBI
|