Introduction
Gastric cancer is the fourth most common cancer
globally and was the second-highest cause of cancer-associated
mortality in 2014 (1–4). Although various factors are implicated
in gastric cancer development, the underlying mechanisms remain
unclear (5). Cancer stem cells serve
a key function in gastric tumor initiation, metastasis and
recurrence (6). Treatment procedures
that eliminate gastric cancer stem cells may improve longevity as
well as increase the cure rate (7–9).
Identifying gastric cancer in the early stages may help prevent
gastric cancer progression. Understanding how cancer stem cells are
involved in the mechanisms underlying cancer invasion and
recurrence is vital in overcoming treatment failure.
The molecular markers that identify gastric cancer
stem cells are useful in understanding the pathogenic nature of
gastric cancer. Gastric cancer stem cells are derived from stem
cells of gastric origin (10–12). Such cells have tumor-propagating
properties but are difficult to identify in different pathological
stages of cancer due to their phenotypic similarities in comparison
with non-cancerous stem cells, yet certain cell surface marker
including CD44 are used to identify them (13). Raf kinase inhibitor protein (RKIP)
acts as a metastasis suppressor by inhibiting multiple cell
survival mechanisms and thereby inducing apoptosis (14,15) in
multiple types of cancer, including colon, prostate and breast
cancers and melanoma (16–19). However, the function of RKIP in the
development of gastric cancer stem cells and cancer progression
remains to be fully understood. Peroxiredoxins are a group of
enzymes that act as antioxidants and exert control over tumor
development (20). As cancer
develops, cancer cells develop resistance against
apoptosis-inducing oxidative damage (21). However, the function of peroxiredoxins
in carcinogenesis, in particular the activity of peroxiredoxins in
cancer stem cells, remains to be fully understood. Using a murine
gastric cancer model, the present study examined the expression of
RKIP and peroxiredoxin 2 in gastric cancer stem cells.
Materials and methods
Experimental animals
Male BALB/c (The Jackson Laboratory, Wuhan, China;
age, 3 months; weight, 25–28 g) mice were used in the present
study. Each group comprised 6 mice, maintained in controlled
conditions (temperature, 26–28°C; humidity, 50–60%) and fed ad
libitum. The present study and protocols were approved by the
Animal Care and Ethical Committee of Jining First People's Hospital
(Jinning, China). To induce gastric cancer, the chemical carcinogen
N-methyl-N-nitrosourea (MNU) was provided with drinking water at a
concentration of 200 ppm. Following daily intake of MNU, the mice
developed initial tumors by week 4 and developed aggressive tumors
by week 8, as determined by histological analysis. MNU is a
standard protocol for developing murine gastric cancer (22,23).
Following tumor development, initial and aggressive tumor samples
were collected from mice sacrificed at weeks 4 and 8, respectively,
and samples (4–12 mm size) from control mice, initial and
aggressive tumor tissues were collected. A total of 6 mice were
sacrificed at week 4, and the remaining mice at week 8. Humane
end-points were set at a loss of 3–5 g body weight in mice with
initial tumors, and a loss of up to 8 in mice with aggressive
tumors. The tumors ranged in size, with initial and aggressive
tumors measuring between 4–6 and 8–12 mm, respectively.
Histological analysis and
immunohistochemistry
To perform histological and immunohistochemical
analysis, tissues were initially fixed in a 10% formaldehyde
fixative solution at room temperature for 48 h. Tissues were then
embedded in paraffin for sectioning. Using a microtome, the
paraffin-embedded tissues were sliced into 6 µm sections. The
sections were then placed on glass slides and deparaffinized by
heating in a slide warming table at 70°C for 5 min. The slides were
then immersed in xylene to remove wax and subsequently immersed in
alcohol solution and then rehydrated with distilled water. To
observe histological alterations, the slides were stained with
hematoxylin (catalog no., HHS16; Sigma-Aldrich; Merck KGaA,
Darmstadt, Germany) for 7 min and eosin (catalog no., HT110132;
Sigma-Aldrich; Merck KGaA) for 30 sec at room temperature and
visualized under the light microscope at ×20 magnification (Eclipse
Ti2, Nikon, Tokyo, Japan). For immunohistochemistry, the processed
slides were immersed in 0.3% H2O2 in 1X PBS
for 15 min at room temperature to block endogenous peroxidase
activity. The sections were then incubated with 4% bovine serum
albumin (BSA; catalog no., A6003; Sigma-Aldrich; Merck KGaA)
blocking buffer in PBS for 1 h at room temperature and further
incubated with anti-RKIP antibody (cat. no., epr2875Y; GeneTex,
Inc., Irvine, CA, USA; 1:400 dilution), anti-peroxiredoxin 2
antibody (cat. no., ab71533; Abcam, Cambridge, UK; 1:250 dilution)
or anti-cluster of differentiation 44 (CD44) antibody (cat. no.,
ab51037; Abcam; 1:300 dilution) overnight at 4°C. Following washing
with 1X TBST, the slides were incubated with the horseradish
peroxidase-conjugated anti-rabbit IgG (cat. no., ab6721; Abcam;
1:2,000 dilution) for 30 min at room temperature.
3,3′-diaminobenzidine solution (5 mg in 10 ml) was subsequently
applied to stain the antibody binding site. Following staining, the
slides were visualized under the light microscope (Eclipse Ti2,
Nikon, Tokyo, Japan) and the images were captured under
magnification, ×20.
Western blot analysis
Protein samples were prepared by homogenizing the
tissue samples in 2X protein sample buffer (2 ml Tris (1 M, pH
6.8), 4.6 ml glycerol (50%), 1.6 ml SDS (10%), 0.4 ml bromophenol
blue (0.5%) and 0.4 ml β-mercaptoethanol) on ice to release the
cell lysates from control, initial tumor and aggressive gastric
cancer tissues. The prepared samples were immediately heated in a
boiling water bath for 5 min and allowed to cool. The concentration
of protein present in each sample were estimated by Lowry method
(24). The present study used the
same method for performing western blot analysis as a previous
study (25). The cell lysate with 70
µg protein was separated using 12% SDS-PAGE. Once the gel was run
to the bottom, the separated proteins were transferred to a
polyvinylidene fluoride membrane. The membrane was subsequently
blocked at room temperature for 2 h using 4% BSA in 1X TBST to
prevent non-specific binding and additionally incubated with
anti-RKIP antibody (cat. no., epr2875Y; GeneTex, Inc; 1:1,000
dilution), anti-peroxiredoxin 2 antibody (cat. no., ab71533; Abcam;
1:2,000 dilution) or anti-CD44 antibody (cat. no., ab51037; Abcam;
1:4,000 dilution) overnight at 4°C. Tubulin antibody (cat. no.,
Ab6046; Abcam; 1:500 dilution) was used for the control. Following
washing with 1X TBST, the membrane was incubated with alkaline
phosphatase-conjugated anti-Rabbit IgG; cat. no., ab97048; Abcam;
1:3,000) for 1 h at room temperature to identify the binding of the
primary antibodies. Following two additional washes with 1X TBST
solution, the membrane was developed with
5-Bromo-4-chloro-3-indolyl phosphate/Nitro blue tetrazolium
(catalog no., B1911; Sigma Aldrich; Merck KGaA) to obtain the
signal.
Results
In vivo mice model for gastric
cancer
In vitro models using gastric cancer cell
lines have certain disadvantages as the responses of the cells and
the associated carcinogenic mechanisms differ among different cell
lines and compared with in vivo models. Therefore, an animal
model that mimics human microenvironments is essential for
understanding the different stages of gastric cancer (26,27). A
murine gastric cancer model was established using the carcinogen
MNU. MNU induced the first gastric tumors in week 4 and resulted in
an aggressive form of gastric cancer in week 8, which are observed
through histological complication of tissue sections (6 µm) that
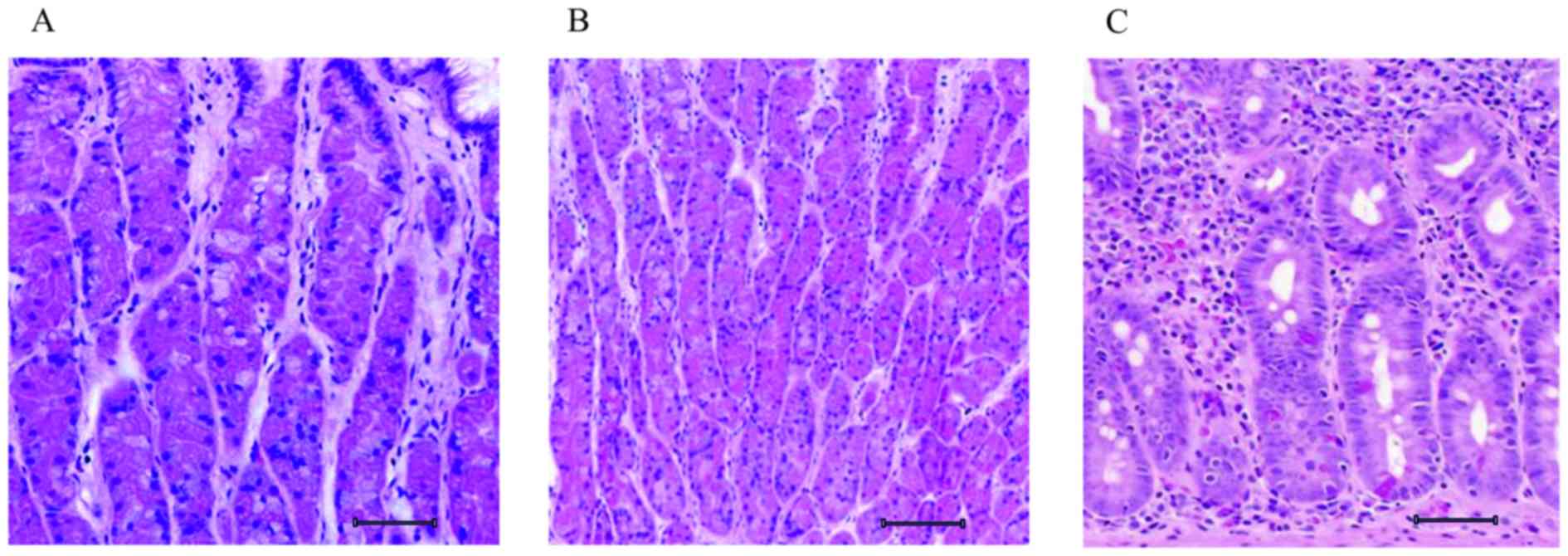
are observed with hematoxylin and eosin staining (Fig. 1). The control gastric tissue was
uniform in pattern, with loosely packed cells (Fig. 1A). Following MNU intake, mice
developed initial tumors in week 4; the histological section
revealed a proliferative mass of cells with increased cell density
(Fig. 1B) when compared with control.
As the tumor developed to an aggressive form in week 8, the
histological section revealed a clustering of cellular patterns
with large, elongated nuclei (Fig.
1C).
CD44 expression and its association
with gastric cancer stem cells
CD44 expression is associated with the extracellular
matrix. CD44 acts as an adhesion molecule that determines cell
proliferation and cell survival (28,29). CD44
expression is associated with gastric cancer stem cell development
(30). In the present study, CD44 was
used as a standard marker for gastric cancer stem cells to
determine the key function of RKIP and peroxiredoxin 2 in gastric
cancer stem cells. Immunohistochemical analysis was used to examine
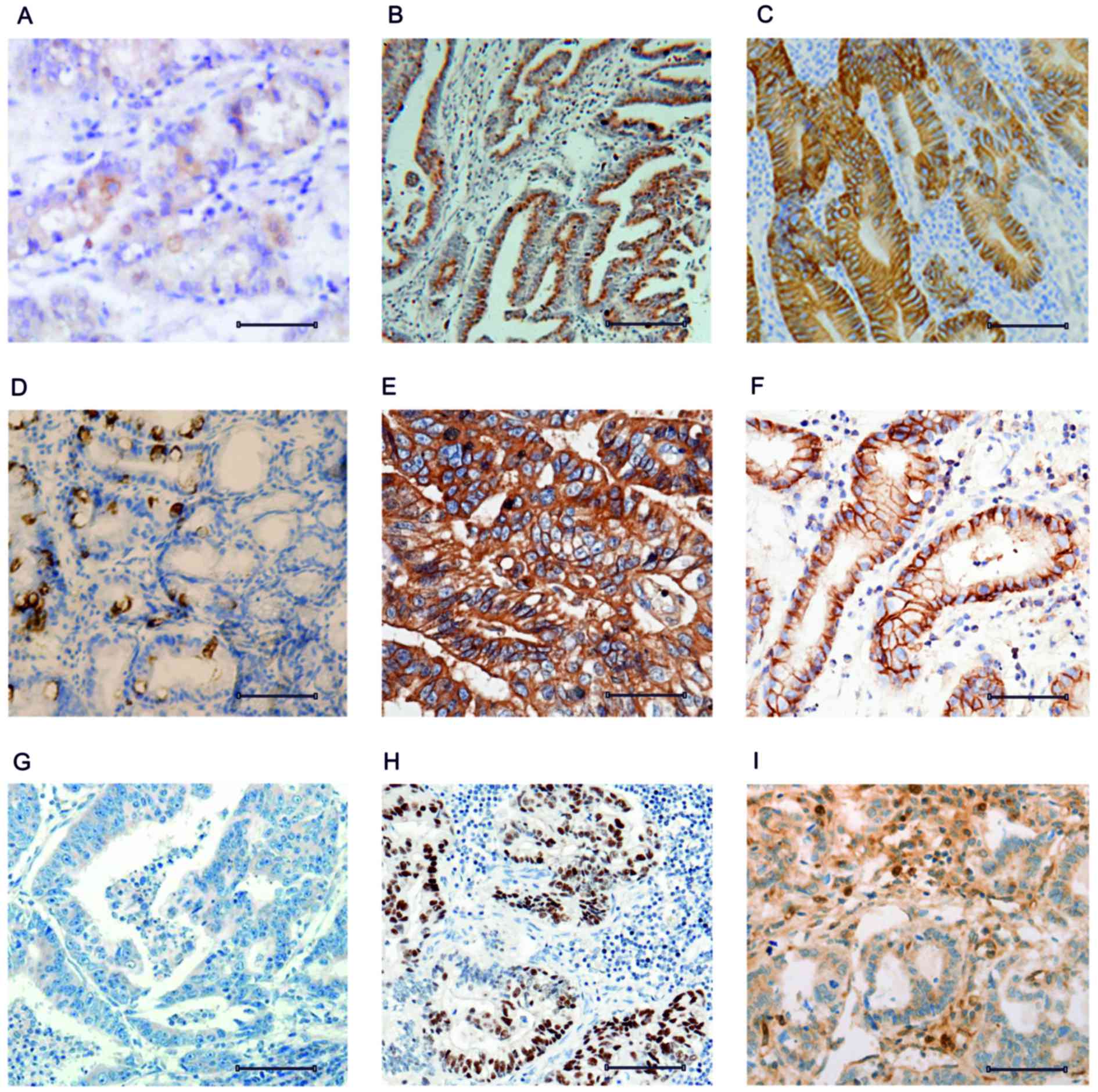
the expression pattern of CD44 in control, initial and aggressive
gastric cancer tissue samples. The control tissue revealed diluted
expression of the CD44+ cells that were spread over the tissue
layer (Fig. 2A). The transition of
normal tissue to initial gastric cancer tissue in week 4 was
associated with increased expression of CD44 (Fig. 2B). The transition of initial gastric
cancer tissue to aggressive gastric cancer tissue in week 8 was
associated with an additional increase in CD44 expression (Fig. 2C).
Comparative analysis of CD44, RKIP and
peroxiredoxin 2 expressions
RKIP acts as a metastasis suppressor and regulates
tumor progression (31). The present
study comparatively analyzed CD44, RKIP and peroxiredoxin 2
expression to elucidate their functions in gastric tumor
regulation. The expression pattern of RKIP was analyzed in
non-cancerous tissue and revealed similar expression to CD44
(Fig. 2D). However, the increase of
RKIP expression in initial cancer tissue compared with control
tissue was greater compared with the increase in CD44 (Fig. 2E). The results suggested that RKIP
initially functions as a tumor suppressor, and is able to exert
control over tumor progression. However, RKIP expression was
decreased in the aggressive stage of tumor development to levels
comparable to the control tissue (Fig.
2F). Similarly, peroxiredoxin 2expression was analyzed and was
revealed to be sequentially upregulated as the tumor progressed
from normal tissue to initial cancer tissue to aggressive cancer
tissue (Fig. 2G-I). The control
tissue revealed low expression of peroxiredoxin 2 (Fig. 2G), while the initial cancer tissue
revealed increased expression compared with the control (Fig. 2H). The aggressive cancer tissue
exhibited an additional increase in peroxiredoxin 2 expression.
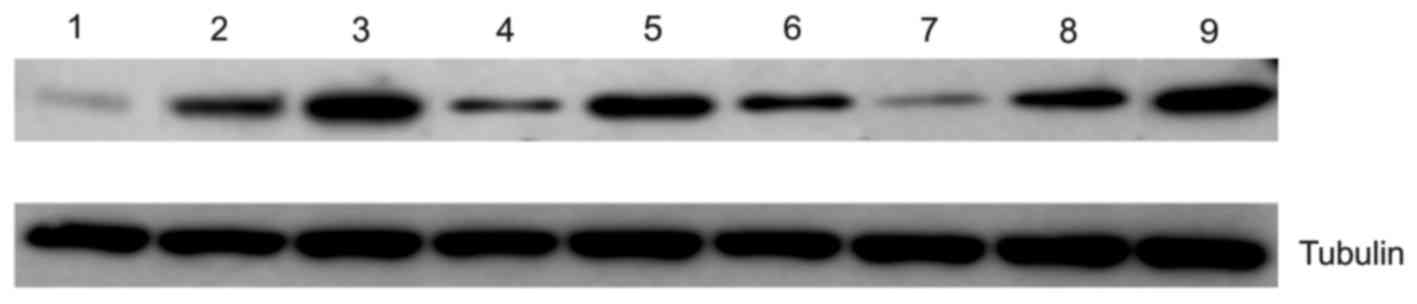
Western blotting analysis
The data obtained using immunohistochemical analysis
were further validated using western blotting analysis. The results
of western blotting determined that expression of CD44 (Fig. 3; lane 1–3) and peroxiredoxin 2
increased with tumor progression (Fig.
3; lane 7–9). Immunohistochemical analysis revealed that RKIP
was overexpressed in initial gastric cancer tissue compared with
control tissue, but expression was decreased in aggressive gastric
cancer tissue compared with initial cancer tissue (Fig. 3; lane 4–6).
Discussion
The study of stem cell markers at different
pathological stages remains challenging due to changes in marker
expression profiles. Identifying novel stem cell markers may assist
in improving the understanding of how tumors progress and the
mechanisms by which tumors are initiated (32). The present study used CD44, a
well-studied tumor marker associated with gastric cancer stem cells
(13). CD44 was used to assess the
expression patterns of RKIP and peroxiredoxin 2 under different
pathological conditions.
Previous studies have suggested that peroxiredoxin 2
expression decreases in certain types of cancer tissue and
increases in others. For example, certain studies have demonstrated
that peroxiredoxin 2 expression decreases in lung cancer tissue
when compared with control tissues (31), while others reported that
peroxiredoxin 2 expression increases in other types of human
cancers (34). Therefore, the results
concerning peroxiredoxin 2 expression in the present study require
further examination. In addition, the gastric cancer model was
successfully established in the BALB/c mice used in the present
study, and demonstrated pathological conditions similar to human
gastric cancer models (35).
The histological results also assisted in
understanding how the tumors progressed as the mice continued to be
treated with MNU (Fig. 1A-C).
Similarly, the tumor progression assisted in understanding the
expression patterns of different protein markers. Comparative
immunohistochemical analysis suggested CD44 and RKIP expressions
influenced gastric tumor development. The present study suggested
that RKIP negatively regulated initial tumor development and
exerted less control over tumor development in the aggressive
stage, during which CD44 expression increased (Fig 2A-F). While the present study supported
the previously reported mechanism of tumor metastasis suppression
by RKIP (34), this mechanism was
observed only in the initial tumor stage. The present study
demonstrated that peroxiredoxin 2 expression was upregulated as
gastric tumors progressed (Fig. 2G-I)
and exhibits a similar linear expression pattern to CD44.
In summary, the present study successfully
established a murine gastric cancer model using the chemical
carcinogen MNU, and comparatively analyzed the expression patterns
of CD44, RKIP and peroxiredoxin 2 during gastric cancer stem cell
expansion, potentially assisting to inform the design of novel
therapeutic interventions in gastric cancer.
References
|
1
|
Parkin DM, Bray F, Ferlay J and Pisani P:
Global cancer statistics, 2002. CA Cancer J Clin. 55:74–108. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Ferlay J, Shin HR, Bray F, Forman D,
Mathers C and Parkin DM: Estimates of worldwide burden of cancer in
2008: GLOBOCAN 2008. Int J Cancer. 127:2893–2917. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Siegel R, Ward E, Brawley O and Jemal A:
Cancer statistic, 2011: The impact of eliminating socioeconomic and
racial disparities on premature cancer deaths. Ca Cancer J Clin.
61:212–236. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
DeSantis CE, Lin CC, Mariotto AB, Siegel
RL, Stein KD, Kramer JL, Alteri R, Robbins AS and Jemal A: Cancer
treatment and survivorship statistics, 2014. CA Cancer J Clin.
64:252–271. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Singh SR: Gastric cancer stem cells: A
novel therapeutic target. Cancer Lett. 338:110–119. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Zhao Y, Feng F and Zhou YN: Stem cells in
gastric cancer. World J Gastroenterol. 21:112–123. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Roboz GJ and Guzman M: Acute myeloid
leukemia stem cells: Seek and destroy. Expert Rev Hematol.
2:663–672. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Valent P: Targeting of leukemia-initiating
cells to develop curative drug therapies: Straightforward but
nontrivial concept. Current Cancer Drug Targets. 11:56–71. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Korkaya H and Wicha MS: Selective
targeting of cancer stem cells. BioDrugs. 21:299–310. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zhu L, Gibson P, Currle DS, Tong Y,
Richardson RJ, Bayazitov IT, Poppleton H, Zakharenko S, Ellison DW
and Gilbertson RJ: Prominin 1 marks intestinal stem cells that are
susceptible to neoplastic transformation. Nature. 457:603–607.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Barker N, Ridgway RA, Van Es JH, van de
Wetering M, Begthel H, van den Born M, Danenberg E, Clarke AR,
Sansom OJ and Clevers H: Crypt stem cells as the cells-of-origin of
intestinal cancer. Nature. 457:608–611. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Cozzio A, Passegué E, Ayton PM, Karsunky
H, Cleary ML and Weissman IL: Similar MLL-associated leukemias
arising from self-renewing stem cells and short-lived myeloid
progenitors. Genes Dev. 17:3029–3035. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Yeung K, Seitz T, Li S, Janosch P,
McFerran B, Kaiser C, Fee F, Katsanakis KD, Rose DW, Mischak H, et
al: Suppression of Raf-1 kinase activity and MAP kinase signalling
by RKIP. nature. 401:173–177. 1999. View
Article : Google Scholar : PubMed/NCBI
|
|
14
|
Chatterjee D, Bai Y, Wang Z, Beach S, Mott
S, Roy R, Braastad C, Sun Y, Mukhopadhyay A and Aggarwal BB: RKIP
sensitizes prostate and breast cancer cells to drug-induced
apoptosis. J Biol Chem. 279:17515–17523. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Al-Mulla F, Hagan S, Behbehani AI, Bitar
MS, George SS, Going JJ, García JJ, Scott L, Fyfe N, Murray GI and
Kolch W: Raf kinase inhibitor protein expression in a survival
analysis of colorectal cancer patients. J Clin Oncol. 24:5672–5679.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Keller ET, Fu Z, Yeung K and Brennan M:
Raf kinase inhibitor protein: A prostate cancer metastasis
suppressor gene. Cancer Lett. 207:131–137. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Hagan S, Al-Mulla F, Mallon E, Oien K,
Ferrier R, Gusterson B, García JJ and Kolch W: Reduction of Raf-1
kinase inhibitor protein expression correlates with breast cancer
metastasis. Clin Cancer Res. 11:7392–7397. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Schuierer MM, Bataille F, Hagan S, Kolch W
and Bosserhoff A-K: Reduction in Raf kinase inhibitor protein
expression is associated with increased Ras-extracellular
signal-regulated kinase signaling in melanoma cell lines. Cancer
Res. 64:5186–5192. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Nyström T, Yang J and Molin M:
Peroxiredoxins, gerontogenes linking aging to genome instability
and cancer. Genes Dev. 26:2001–2008. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Szatrowski TP and Nathan CF: Production of
large amounts of hydrogen peroxide by human tumor cells. Cancer
Res. 51:794–798. 1991.PubMed/NCBI
|
|
21
|
Yamamoto M, Furihata C, Ogiu T, Tsukamoto
T, Inada Ki, Hirano K and Tatematsu M: Independent variation in
susceptibilities of six different mouse strains to induction of
pepsinogen-altered pyloric glands and gastric tumor
intestinalization by N-methyl-N-nitrosourea. Cancer Lett.
179:121–132. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Hayakawa Y, Fox JG, Gonda T, Worthley DL,
Muthupalani S and Wang TC: Mouse models of gastric cancer. Cancers
(Basel). 5:92–130. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Hamidouche Z, Haÿ E, Vaudin P, Charbord P,
Schüle R, Marie PJ and Fromigué O: FHL2 mediates
dexamethasone-induced mesenchymal cell differentiation into
osteoblasts by activating Wnt/beta-catenin signaling-dependent
Runx2 expression. FASEB J. 22:3813–3822. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Waterborg JH: The Lowry method for protein
quantitation. protein Protoc Handb. 1–9. 2002.
|
|
25
|
Abate-Shen C: Deregulated homeobox gene
expression in cancer: Cause or consequence? Nat Re Cancer.
2:777–785. 2002. View
Article : Google Scholar
|
|
26
|
Yu S, Yang M and Nam KT: Mouse models of
gastric carcinogenesis. J Gastric Cancer. 14:67–86. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Jang BI, Li Y, Graham DY and Cen P: The
role of CD44 in the pathogenesis, diagnosis and therapy of gastric
cancer. Gut Liver. 5:397–405. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Ghaffarzadehgan K, Jafarzadeh M, Raziee
HR, Sima HR, Esmaili-Shandiz E, Hosseinnezhad H, Taghizadeh-Kermani
A, Moaven O and Bahrani M: Expression of cell adhesion molecule
CD44 in gastric adenocarcinoma and its prognostic importance. World
J Gastroenterol. 14:6376–6381. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Nosrati A, Naghshvar F and Khanari S:
Cancer stem cell markers CD44, CD133 in primary gastric
adenocarcinoma. Int J Mol Cell Med. 3:279–286. 2014.PubMed/NCBI
|
|
30
|
Li X, Wang J, Xu Z, Ahmad A, Li E, Wang Y,
Qin S and Wang Q: Expression of sox2 and oct4 and their clinical
significance in human non-small-cell lung cancer. Int J Mol Sci.
13:7663–7675. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Park YH, Kim SU, Lee BK, Kim HS, Song IS,
Shin HJ, Han YH, Chang KT, Kim JM, Lee DS, et al: Prx I suppresses
K-ras-driven lung tumorigenesis by opposing redox-sensitive
ERK/cyclin D1 pathway. Antioxid Redox Signal. 19:482–496. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Diao S, Zhang Jf, Wang H, He ML, Lin MC,
Chen Y and Kung HF: Proteomic identification of microRNA-122a
target proteins in hepatocellular carcinoma. Proteomics.
10:3723–3731. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Takaishi S, Okumura T, Tu S, Wang SS,
Shibata W, Vigneshwaran R, Gordon SA, Shimada Y and Wang TC:
Identification of gastric cancer stem cells using the cell surface
marker CD44. Stem Cells. 27:1006–1020. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Beshir AB, Ren G, Magpusao AN, Barone LM,
Yeung KC and Fenteany G: Raf kinase inhibitor protein suppresses
nuclear factor-κB-dependent cancer cell invasion through negative
regulation of matrix metalloproteinase expression. Cancer Lett.
299:137–149. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Jin DZ, Jung HC, Kim JM, Kim JS, Song IS
and Kim CY: Establishment of BALB/c mice model infected with
Helicobacter pylori. Korean J Intern Med. 14:55–63. 1999.
View Article : Google Scholar : PubMed/NCBI
|