Introduction
Helicobacter pylori (H. pylori)
infection is now accepted as a crucial event in the development of
atrophic gastritis, and is implicated in the development of gastric
carcinoma (1–3). Gastric cancer develops incrementally,
beginning with chronic inflammation, and progressing to atrophic
inflammation, intestinal metaplasia, dysplasia and finally, frank
malignancy (4). Whereas the majority
of infected individuals are asymptomatic, chronic H. pylori
infection in susceptible individuals is associated with variable
degrees of mucosal damage (4). As a
result, only a small percentage of infected individuals develop
gastric cancer. The clinical outcome appears to be determined by
the interplay of bacterial virulence factors, host gastric mucosal
components and the environment. More specifically, a previous study
revealed that genetic variability affecting elements of the mucosal
immune system pivotally influences the clinical course of H.
pylori infection (5).
Nucleotide-binding oligomerization domain-containing
protein 2/caspase recruitment domain-containing protein 15
(NOD2/CARD15) is a member of the NOD-like receptor gene family. It
functions as an intracellular receptor for bacterial
lipopolysaccharide, and is involved in signal transduction leading
to the activation of the nuclear transcription factor-κB (NF-κB)
(6). Dysregulation of NOD2 signaling
is associated with the pathogenesis of numerous inflammatory
disorders (7). Indeed, NOD2
mutations are related to the occurrence of chronic inflammation of
the gastric mucosa associated with H. pylori infection,
development of intestinal metaplasia and dysplasia and ultimately
gastric cancer (6). Previously, it
was reported that the H. pylori bacterial Cag pathogenicity
island and a cooperative interaction between the Toll-like receptor
2 (TLR2)/NOD2 and NOD-like receptor pyrin domain containing 3
protein, regulate interleukin-1β production in H. pylori
infected dendritic cells (8).
Initiation of NOD2 signaling is mediated by receptor-interacting
protein 2 (RIP2), and the RIP2 interaction with NOD2 enhances NF-κB
activity, making it an important player in cellular immune response
(9). One study suggested that the
innate immune system, including the NOD2-RIP2 signaling pathway, is
involved in the pathogenesis of gastric inflammation and the
development of gastric cancer (10).
RIP2 is an intracellular serine/threonine kinase
that contains a caspase recruitment domain at its carboxy terminus.
Single nucleotide polymorphisms (SNPs) of the receptor interacting
serine/threonine kinase 2 (RIPK2) gene, encoding RIP2, are
associated with systemic lupus erythematosus (SLE) (11), and with the severity of childhood
atopic asthma (12). However,
although the association of NOD2 and TLR
polymorphisms with gastric cancer susceptibility have been
described, RIPK2 polymorphisms have not been studied in this
context (13,14).
The present study investigated the association
between RIPK2 gene polymorphisms and gastric cancer
susceptibility in a Japanese population. The present study also
investigated the association between RIPK2 polymorphisms and
the severity of chronic gastritis in subjects without gastric
cancer.
Materials and methods
Study subjects
All patients with gastric cancer attended the
Endoscopy Center of Kanazawa Medical University (Uchinada-machi,
Japan) or Fujita Health University (Kutsukake-cho, Japan) from
April 2005 to March 2015, and were diagnosed by endoscopy and
pathological examination of biopsy samples. Gastric cancer was
classified according to Lauren's classification (15). Non-cancer patients complaining of
abdominal discomfort underwent endoscopic examination, and were
diagnosed as having a gastric ulcer, duodenal ulcer, gastritis or
no apparent gastric disease. Patients who had severe systemic
disease and received non-steroidal anti-inflammatory drugs were
excluded from the present study. Finally, the study population
comprised 1,221 subjects whose polymorphisms could be clearly
analyzed, including 524 patients with gastric cancer (GC group,
mean age, 65.4±11.4; age range, 23–94; male/female, 371/133) and
697 subjects without gastric cancer (non-GC group; mean age,
60.9±13.6; age range, 22–93; male/female, 400/297). In 428/697
patients in the non-GC group (mean age, 60.0±13.3; age range,
26–93; male/female, 254/174), the severity of chronic gastritis was
assessed using antral biopsy specimens and classified according to
the updated Sydney system (16). All
pathology assessment was performed by a pathologist at Fujita
Health University Hospital who was blinded to any clinical
information. According to the estimated histological gastritis
scores, the atrophic gastritis group (AT group) was defined as
atrophy score ≥2 or metaplasia score ≥1, and the others were
classified into the non-atrophy group. In addition, serum
pepsinogen (PG) I/II levels were evaluated in 134/428 subjects
without gastric cancer. The Ethics Committees of Fujita Health
University and Kanazawa Medical University approved the protocol.
Written informed consent was obtained from all of the participating
patients prior to enrollment in the present study.
SNP selection and detection
A significant association between the rs16900627 SNP
and SLE susceptibility has been previously reported (11). The polymorphism is located in the 3′
untranslated region of RIPK2, a region rich in microRNA
binding sites, and is associated with other SNPs, including
rs7844627, 10504881. In addition, rs2230801, encoding a
non-synonymous substitution (Ile259Thr), was identified in
RIPK2. The distribution of the two SNP genotypes were
confirmed using the HapMap database (https://snpinfo.niehs.nih.gov/snpinfo/snptag.html) and
National Centre for Biotechnology Information (NCBI) SNP database
(http://www.ncbi.nlm.nih.gov/snp/)
(17). The present study selected the
rs16900627 and rs2230801 SNPs for further investigation on the
basis that they may affect innate immune signaling. For genotype
determination, the present study used polymerase chain
reaction-single strand conformation polymorphism (PCR-SSCP) methods
using DNA samples prepared from peripheral blood as reported
previously (18). All PCRs were
performed in a volume of 20 µl containing 0.1 µg of genomic DNA.
Primer pairs used to detect rs16900627 were as follows: Forward,
5′-CTGATGGAAGCCATTTTCACATTCAT-3′ and reverse,
5′-TCTGTCTCTGGTGGGTAAAGGGTAT-3. The DNA was denatured at 95°C for 3
min, followed by 35 cycles at 96°C for 15 sec, 50°C for 40 sec and
72°C for 30 sec, with a final extension at 72°C for 5 min. Primer
pairs used to detect rs2230801 were as follows: Forward,
5′-TCCTTTGCAGATAATGTATAGTGTGTCA-3′ and reverse,
5′-AGAGATCATACGTGCTCGGTGAGGT-3′. The DNA was denatured at 95°C for
3 min, followed by 35 cycles at 96°C for 15 sec, 58°C for 40 sec
and 72°C for 30 sec, with final extension at 72°C for 5 min.
Subsequently, 2 µl of both PCR products was denatured with 10 µl
formamide (Sigma-Aldrich; Merck KGaA, Darmstadt, Germany) at 90°C
for 5 min. SSCP was performed at 18°C using a GenePhor DNA
separation system with GeneGel Excel 12.5/24 (GE Healthcare,
Chicago, IL, USA), after which the denatured single strand DNA was
stained using a DNA Silver Staining kit (GE Healthcare) according
to the manufacturer's protocol.
Statistical analysis
Hardy-Weinberg equilibrium was assessed by
χ2 statistics. The age data are presented as the mean ±
standard deviation. Mean ages between two groups was compared by
Student's t-test. The ratios of H. pylori infection status
and gender were compared by Fisher's exact test. Differences of
genotype frequencies were determined by two-sided Fisher's exact
test. The odds ratios (OR) and 95% confidence intervals (CI) were
also determined by logistic regression with adjustment for age, sex
and H. pylori infection status. The Sydney system scores and
PG I/II ratio between two groups were compared by Mann Whitney
U-test. P<0.05 was considered to indicate a statistically
significant difference. Analyses were performed using STATA Version
13 (StataCorp LP, College Station, TX, USA).
Results
Characteristics of the study subjects
and the frequencies of genotypes
The characteristics of subjects in the present study
are summarized in Table I. The mean
age, male:female ratio and H. pylori positivity of the GC
group were significantly higher compared with those of the non-GC
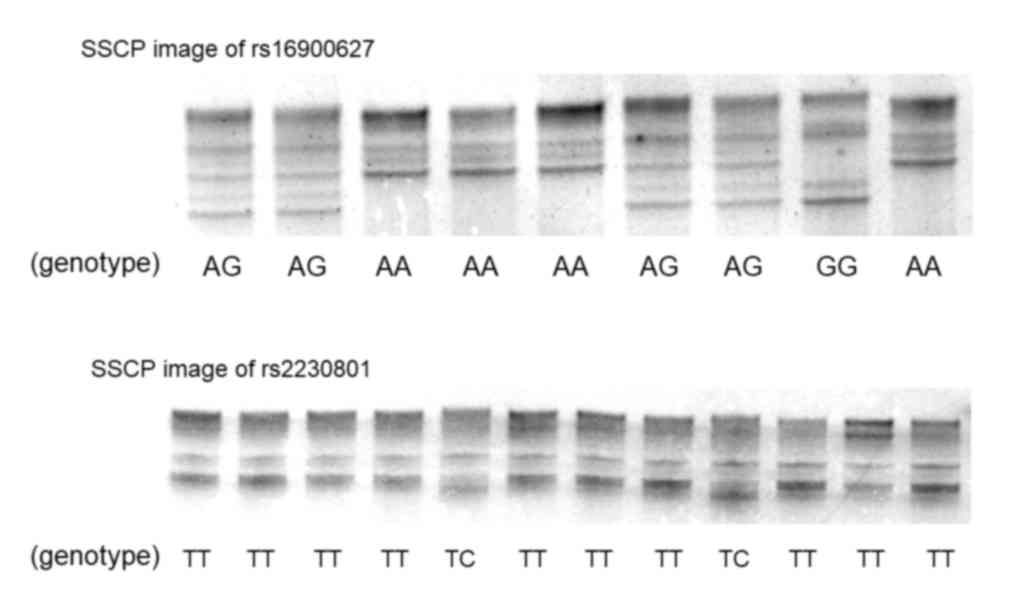
group. Single strand DNAs of rs16900627 and rs2230801 were clearly
separated by SSCP (Fig. 1). The
distribution of the rs16900627 genotype in 697 subjects without
gastric cancer was 554AA, 130AG and 13GG (Table I), and that of rs2230801 was 681TT and
16TC. The CC genotype of rs2230801 was not identified. The
distribution of genotypes was in Hardy-Weinberg equilibrium
(rs16900627 and rs2230801; P=0.12 and P=1.0, respectively). The
wild-type rs16900627 homozygote frequency was significantly lower
in the GC group compared with in the non-GC group (P=0.029),
whereas the frequencies of other genotypes were not significantly
different (Table I).
 | Table I.Characteristics of the subjects and
frequencies of genotypes. |
Table I.
Characteristics of the subjects and
frequencies of genotypes.
| Characteristic | Non-GC group | GC group | P-value |
|---|
| No. of subjects | 697 | 524 |
|
| Mean age ± SD | 60.9±13.6 | 65.4±11.4 | <0.001 |
| Male:female | 400:297 | 371:133 | <0.001 |
| H. pylori
positivity | 430/697 | 457/524 | <0.001 |
| rs16900627
(*351A>G) |
|
|
|
| AA | 554 | 395 | 0.029 |
| AG | 130 | 115 |
|
| GG | 13 | 14 | 0.432 |
| G allele
frequency | 11.2% | 13.6% | 0.071 |
| rs2230801 (776T>C,
Ile259Thr) |
|
|
|
| TT | 681 | 517 | 0.289 |
| TC | 16 | 7 |
|
| CC | 0 | 0 |
|
| C
allele frequency | 1.15% | 0.67% | 0.291 |
Association between gene polymorphisms
and gastric cancer susceptibility
By logistic regression analysis following adjustment
for age, sex and H. pylori infection status, the rs16900627
AG+GG genotypes were revealed to be significantly associated with
susceptibility to gastric cancer (OR, 1.38; 95% CI, 1.03–1.84;
P=0.032; Table II), and more
strongly associated with the intestinal type of gastric cancer (OR,
1.56; 95% CI, 1.11–2.20; P=0.011). In addition, if the number of
minor alleles was considered to be a co-variable (GG=2, AG=1 and
AA=0), it was also significantly associated with gastric cancer
susceptibility (OR, 1.37; 95% CI, 1.06–1.77; P=0.016), particularly
with the intestinal type (OR, 1.53; 95% CI, 1.13–2.07; P=0.0062;
Table II). Conversely, the rs2230801
genotype was not significantly associated with gastric cancer
susceptibility (Table III).
 | Table II.Association between rs16900627
polymorphisms and gastric cancer. |
Table II.
Association between rs16900627
polymorphisms and gastric cancer.
| A, G allele
carriers vs. the others |
|---|
|
|---|
|
rs16900627a | AA | AG | GG | AG+GG vs. AA, OR
(95% CI) | P-value |
|---|
| Non-GC (697) | 554 | 130 | 13 | Reference |
|
| Overall GC
(524) | 395 | 115 | 14 | 1.38
(1.03–1.84) | 0.032 |
| Intestinal
(309) | 227 | 74 | 8 | 1.56
(1.11–2.20) | 0.011 |
| Diffuse (212) | 166 | 40 | 6 | 1.15
(0.778–1.69) | 0.491 |
| Unknown | 2 | 1 | 0 | – | – |
|
| B, Co-variable:
The number of G allele |
|
|
rs16900627a | AA | AG | GG | No. of G allele,
OR (95% CI) | P-value |
|
| Non-GC (697) | 554 | 130 | 13 | Reference |
|
| Overall GC
(524) | 395 | 115 | 14 | 1.37
(1.06–1.77) | 0.016 |
| Intestinal
(309) | 227 | 74 | 8 | 1.53
(1.13–2.07) | 0.006 |
| Diffuse (212) | 166 | 40 | 6 | 1.20
(0.857–1.68) | 0.288 |
| Unknown | 2 | 1 | 0 | – | – |
 | Table III.Association between rs2230801
polymorphisms and gastric cancer. |
Table III.
Association between rs2230801
polymorphisms and gastric cancer.
|
rs2230801a | TT | TC | CC | TC vs. TT, OR (95%
CI) | P-value |
|---|
| Non-GC (697) | 681 | 16 | 0 | Reference |
|
| Overall GC
(524) | 517 | 7 | 0 | 0.531
(0.210–1.34) | 0.18 |
| Intestinal
(309) | 304 | 5 | 0 | 0.664
(0.229–1.92) | 0.45 |
| Diffuse (212) | 210 | 2 | 0 | 0.344
(0.077–1.53) | 0.16 |
| Unknown | 3 | 0 | 0 | – | – |
Association between gene polymorphisms
and severity of chronic gastritis
The characteristics of 428 subjects without gastric
cancer whose histological severity was assessed are presented in
Table IV. The genotype distribution
in this sample was not significantly different from that of the
non-GC group (rs16900627 and rs2230801; P=0.11 and P=1.0,
respectively). As the mean values of atrophy and metaplasia scores
were 1.13 and 0.652, respectively, the AT group was defined as
atrophy score ≥2 or metaplasia score ≥1. On this basis, mean age,
male:female ratio and H. pylori positivity in the AT group
were significantly higher compared with those in the non-AT group.
Furthermore, the frequency of the rs16900627 AA genotype was
significantly lower, and the minor allele frequency was also
significantly higher in the AT group (P=0.011 and P=0.0060,
respectively). By logistic regression analysis, the rs16900627
minor allele carriers had a significantly increased risk for
gastric mucosal atrophy (OR, 1.72; 95% CI, 1.01–2.96; P=0.048) and
the significance was increased when the number of minor alleles was
set as a co-variable (OR, 1.83; 95% CI, 1.14–2.93; P=0.011,
Table V). Conversely, there was no
significant association between rs2230801 and severity of gastric
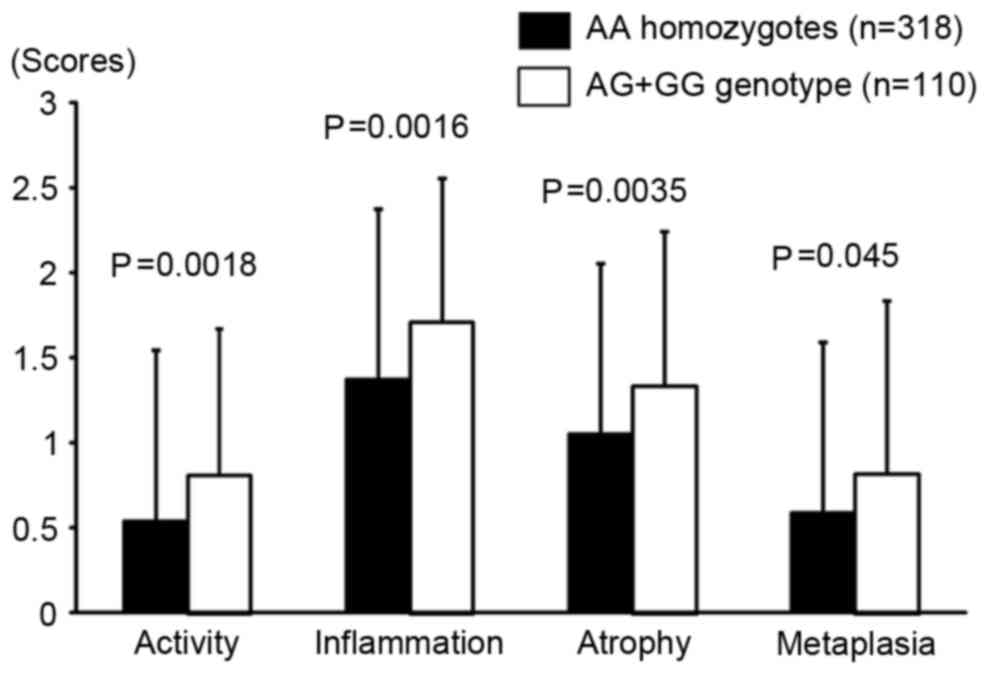
mucosal atrophy. In addition, all Sydney system scores were
significantly higher in rs16900627 minor allele carriers compared
with in AA homozygotes (Fig. 2). The
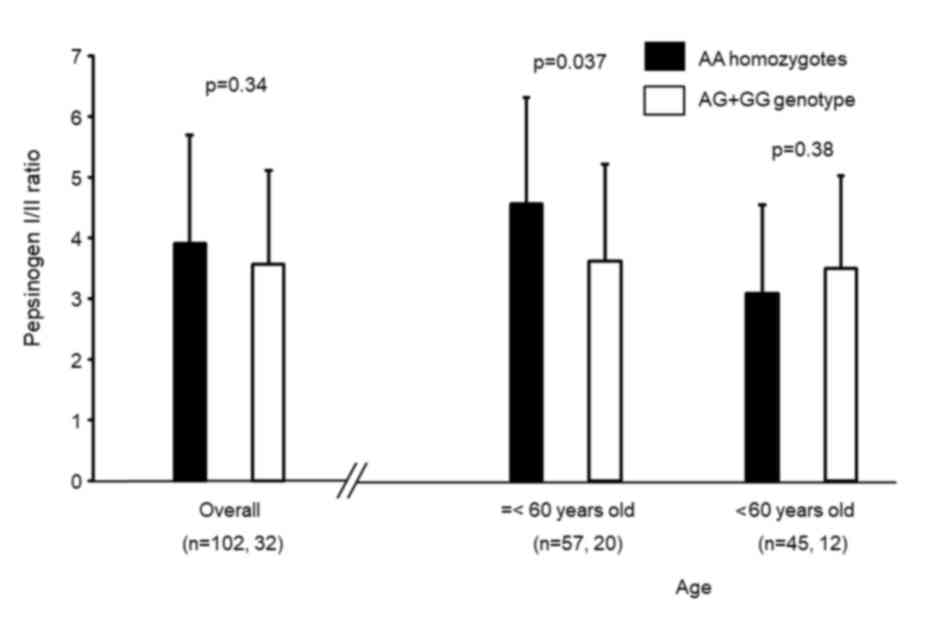
rs16900627 genotype distribution in 134 subjects whose serum
pepsinogens were determined was 102AA, 28AG and 4GG, which was not
significantly different from that in the non-GC group (P=0.56).
Overall, there was no significant difference in PG I/II ratio
between rs16900627 AA homozygotes and minor allele carriers
(Fig. 3). However, in subjects
younger than 60 years, the ratio was significantly lower in minor
allele carriers compared with in AA homozygotes (P=0.037).
 | Table IV.Characteristics and genotype
frequencies in the subjects that underwent severity of gastritis
evaluation. |
Table IV.
Characteristics and genotype
frequencies in the subjects that underwent severity of gastritis
evaluation.
| Characteristic | Total | Non-AT group | AT group | P-value |
|---|
| No. of
subjects | 428 | 232 | 196 |
|
| Mean age ± SD | 60.0±13.3 | 57.8±14.2 | 62.5±11.6 | <0.001 |
| Male: Female | 254:174 | 123:109 | 131:65 | 0.004 |
| H. pylori
positive rate | 278/428 | 94/232 | 184/196 | <0.001 |
| rs16900627
A>G |
|
|
|
|
| AA | 318 | 184 | 134 | 0.011 |
| AG | 98 | 44 | 54 |
|
| GG | 12 | 4 | 8 |
|
| G
allele frequency | 14.1% | 11.2% | 17.9% | 0.006 |
| rs2230801
T>C |
|
|
|
|
| TT | 416 | 228 | 188 |
|
| TC | 12 | 4 | 8 |
|
| CC | 0 | 0 | 0 |
|
| C
allele frequency | 1.40% | 0.86% | 2.04% |
|
 | Table V.Association between RIPK2
polymorphisms and gastric mucosal atrophy. |
Table V.
Association between RIPK2
polymorphisms and gastric mucosal atrophy.
| A, rs16900627, G
allele carriers vs. the others |
|---|
|
|---|
|
rs16900627a | AA | AG | GG | AG+GG vs. AA, OR
(95% CI) | P-value |
|---|
| Non-AT group
(232) | 184 | 44 | 4 | Reference |
|
| AT group (196) | 134 | 54 | 8 | 1.72
(1.01–2.96) | 0.048 |
|
| rs16900627,
co-variable: the number of G allele |
|
| B,
rs16900627a | AA | AG | GG | No. of G allele,
OR (95% CI) | P-value |
|
| Non-AT group
(232) | 184 | 44 | 4 | Reference |
|
| AT group (196) | 134 | 54 | 8 | 1.83
(1.14–2.93) | 0.011 |
|
| C, rs2230801, TC
vs. TT |
|
|
rs2230801a | TT | TC | CC | TC vs. TT, OR
(95% CI) | P-value |
|
| Non-AT group
(232) | 228 | 4 | 0 | Reference |
|
| AT group (196) | 188 | 8 | 0 | 1.70
(0.441–6.99) | 0.472 |
Discussion
The immune system is composed of innate and adaptive
parts, and H. pylori infection induces both parts of the
host immune response (19). Recently,
the innate immune response to H. pylori was revealed to be
an important factor affecting gastric mucosal inflammation
(8,20). The present study previously reported a
significant association between the TLR2-196 to −174
deletion polymorphism and gastric cancer susceptibility in Japanese
patients (21), which was consistent
with other Japanese studies associating TLR gene polymorphisms to
gastric cancer (14). NOD2
polymorphisms are also associated with changes in gastric mucosa,
which lead to gastric cancer susceptibility (13,22,23). This
suggested that genetic variations affecting the innate immune
response may impact gastric cancer susceptibility. RIP2 is involved
in both innate and adaptive parts of the immune response (24). Therefore, RIPK2 genetic
variation may influence the severity of gastric mucosal
inflammation, the progression of gastric atrophy and the
development of gastric cancer.
The results of the present study provide the first
evidence, to the best of our knowledge, that RIPK2 genetic
polymorphisms are significantly associated with susceptibility to
gastric cancer in the Japanese population. The rs16900627 A>G
minor allele was associated with an increased risk for the
development of gastric cancer, particularly the intestinal type.
The frequency of rs16900627 in the Japanese population is reported
in the HapMap database. However, in the present study the
distribution of the genotype was different from the controls
without gastric cancer (P=0.045), even though the genotype
distribution in the control subjects was in Hardy-Weinberg
equilibrium. Conversely, the genotype distribution of 232 subjects
without histological gastric mucosal atrophy was the same as that
in the HapMap database (P=0.13). This discrepancy may be explained
by the fact that all of the control subjects included were patients
who had an endoscopic examination due to various symptoms,
including abdominal symptoms and abnormal findings revealed by a
health check. The frequency of rs2230801 is not reported in HapMap,
but the genotype distribution was in the Hardy-Weinberg equilibrium
in the control subjects.
To date, there have been few studies associating
RIPK2 genetic variations and clinical disease susceptibility
(11,12,25). Li
et al (11) reported that the
rs16900627 minor allele was significantly associated with SLE
susceptibility. This suggested that the mutant rs16900627 genotype
may be associated with an increased risk of chronic inflammation
via an alteration of innate immune responses, although the detailed
mechanisms remain unclear. Similarly, in the present study,
activity and inflammation scores based on the Sydney system were
higher in AG+GG genotypes compared with in AA homozygotes, although
H. pylori positivity was not significantly different (67.3
and 64.1%, respectively). Atrophy and metaplasia scores were also
significantly higher in AG+GG genotypes compared with in AA
homozygotes, and logistic regression analysis indicated that the
rs160900627 minor allele genotype was associated with an increased
risk with the progression of gastric mucosal atrophy.
However, by serological examination, significant
differences of PG I/II ratio between AG+GG genotypes and AA
homozygotes were not observed in older patients (age >60 years
old), although it was significantly different in younger age groups
(age ≤60 years old). Sydney system scores express the severity of
gastritis at a biopsy point, whereas PG I/II ratio express the
spread of mucosal atrophy (16,26). It
has been reported that the significant association between serum
and histological atrophy is more strongly observed in the corpus
compared with in the antrum (27).
The present study assessed the histological gastritis only in the
antrum, as the antrum is affected by H. pylori infection for
the longest period (4). This may be
the reason for the difference of results between histological and
serum atrophy. The present study suggested that the results of PG
I/II ratio indicated that the mucosal atrophy progressed more
extensively in AG+GG genotypes compared with in AA homozygotes at
an earlier stage of H. pylori infection, and that the
difference of the value of PG I/II ratio between the two groups may
be obscured by the mucosal atrophy spreading following a long
period. In addition, when the comparatively large deviation of the
PG I/II ration was considered, the sample size of the present study
may be too small.
The results of the present study demonstrated a
significant association between the rs16900627 minor genotype and
the intestinal type of gastric cancer. The intestinal type consists
of gland-like structures that mimic the intestinal glands, and a
series of precancerous intestinal type lesions are recognized,
beginning with chronic inflammation of the stomach and passing
through the intermediate stages of atrophic gastritis or intestinal
metaplasia (28). The results of the
present study suggested that the rs16900627 minor allele genotype
may be associated with the severity of gastric mucosal atrophy, and
may increase the risk for developing gastric mucosal atrophy
associated disorders, including intestinal type gastric cancer.
There was no significant association between
rs2230801, and gastric mucosal atrophy and gastric cancer
susceptibility. This polymorphism causes an Ile-to-Thr substitution
at amino acid 259, but is a minor variant in the NCBI SNP database
(http://www.ncbi.nlm.nih.gov/snp/).
Furthermore, there was no minor allele homozygote in the present
study. Overall, these findings suggested that this polymorphism had
little, if any, association with gastric disorders.
There were certain clinical limitations to the
present study. The present study included patients who visited the
Fujita Health University Hospital to undergo endoscopic examination
due to specific symptoms or further checks after a general health
check. The subjects who had no symptoms were included in the
control group. In addition, endoscopy is limited in its ability to
detect small histological neoplasia. The present study was not able
to confirm whether very small histological neoplasia were present
in the control group. Another limitation was that the present study
assessed histological gastritis using biopsy samples only from the
antrum. Further results may be provided if the severity of
gastritis in the corpus was assessed at the same time. The main
disadvantage was the relatively small sample size used in the
present study, particularly with respect to the number of subjects
that had their serum pepsinogen levels assessed. Due to the
relatively large deviation of pepsinogen levels, a greater sample
size will be required to assess the PG I/II ratio. The final
limitation of the study design was that only using samples stored
in a single center were analyzed retrospectively.
In conclusion, the results of the present study
suggest that the rs16900627 minor allele is associated with the
severity of gastric mucosal inflammation and the development of
gastric mucosal atrophy, and carriers of this allele may have an
increased risk for the development of gastric cancer, particularly
of the intestinal type. These results will be useful in predicting
which H. pylori infected patients will be at a higher risk
of developing gastric cancer.
References
|
1
|
Blaser MJ and Parsonnet J: Parasitism by
the ‘slow’ bacterium Helicobacter pylori leads to altered gastric
homeostasis and neoplasia. J Clin Invest. 94:4–8. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Huang JQ, Sridhar S, Chen Y and Hunt RH:
Meta-analysis of the relationship between Helicobacter pylori
seropositivity and gastric cancer. Gastroenterology. 114:1169–1179.
1998. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Uemura N, Okamoto S, Yamamoto S, Matsumura
N, Yamaguchi S, Yamakido M, Taniyama K, Sasaki N and Schlemper RJ:
Helicobacter pylori infection and the development of gastric
cancer. N Engl J Med. 345:784–789. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Correa P: Human gastric carcinogenesis: A
multistep and multifactorial process-First American Cancer Society
Award Lecture on Cancer Epidemiology and Prevention. Cancer Res.
52:6735–6740. 1992.PubMed/NCBI
|
|
5
|
Crabtree JE: Gastric mucosal inflammatory
responses to Helicobacter pylori. Aliment Pharmacol Ther. 10 Suppl
1:S29–S37. 1996. View Article : Google Scholar
|
|
6
|
Rosenstiel P, Hellmig S, Hampe J, Ott S,
Till A, Fischbach W, Sahly H, Lucius R, Fölsch UR, Philpott D and
Schreiber S: Influence of polymorphisms in the NOD1/CARD4 and
NOD2/CARD15 genes on the clinical outcome of Helicobacter pylori
infection. Cell Microbiol. 8:1188–1198. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Tigno-Aranjuez JT and Abbott DW:
Ubiquitination and phosphorylation in the regulation of NOD2
signaling and NOD2-mediated disease. Biochim Biophys Acta.
1823:2022–2028. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kim DJ, Park JH, Franchi L, Backert S and
Núñez G: The Cag pathogenicity island and cooperative interaction
between TLR2/NOD2 and NLRP3 regulate IL-1β production in
Helicobacter pylori-infected dendritic cells. Eur J Immunol.
43:2650–2658. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ogura Y, Inohara N, Benito A, Chen FF,
Yamaoka S and Nunez G: Nod2, a Nod1/Apaf-1 family member that is
restricted to monocytes and activates NF-kappaB. J Biol Chem.
276:4812–4818. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Li ZX, Wang YM, Tang FB, Zhang L, Zhang Y,
Ma JL, Zhou T, You WC and Pan KF: NOD1 and NOD2 genetic variants in
association with risk of gastric cancer and its precursors in a
Chinese population. PLoS One. 10:e01249492015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Li J, Tian J, Ma Y, Cen H, Leng RX, Lu MM,
Chen GM, Feng CC, Tao JH, Pan HF and Ye DQ: Association of RIP2
gene polymorphisms and systemic lupus erythematosus in a Chinese
population. Mutagenesis. 27:319–322. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Nakashima K, Hirota T, Suzuki Y, Akahoshi
M, Shimizu M, Jodo A, Doi S, Fujita K, Ebisawa M, Yoshihara S, et
al: Association of the RIP2 gene with childhood atopic asthma.
Allergol Int. 55:77–83. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Liu J, He C, Xu Q, Xing C and Yuan Y: NOD2
polymorphisms associated with cancer risk: A meta-analysis. PLoS
One. 9:e893402014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Castaño-Rodríguez N, Kaakoush NO, Goh KL,
Fock KM and Mitchell HM: The role of TLR2, TLR4 and CD14 genetic
polymorphisms in gastric carcinogenesis: A case-control study and
meta-analysis. PLoS One. 8:e603272013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Lauren P: The two histological main types
of gastric carcinoma: Diffuse and so-called intestinal-type
carcinoma. An attempt at a histo-clinical classification. Acta
Pathol Microbiol Scand. 64:31–49. 1965. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Dixon MF, Genta RM, Yardley JH and Correa
P: Classification and grading of gastritis. The updated sydney
system. International Workshop on the Histopathology of Gastritis,
Houston 1994. Am J Surg Pathol. 20:1161–1181. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Sherry ST, Ward MH, Kholodov M, Baker J,
Phan L, Smigielski EM and Sirotkin K: dbSNP: The NCBI database of
genetic variation. Nucleic Acids Res. 29:308–311. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Arisawa T, Tahara T, Ozaki K, Matsue Y,
Minato T, Yamada H, Nomura T, Hayashi R, Matsunaga K, Fukumura A,
et al: Association between common genetic variant of HRH2 and
gastric cancer risk. Int J Oncol. 41:497–503. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Algood HM and Cover TL: Helicobacter
pylori persistence: An overview of interactions between H. pylori
and host immune defenses. Clin Microbiol Rev. 19:597–613. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Rad R, Ballhorn W, Voland P, Eisenächer K,
Mages J, Rad L, Ferstl R, Lang R, Wagner H, Schmid RM, et al:
Extracellular and intracellular pattern recognition receptors
cooperate in the recognition of Helicobacter pylori.
Gastroenterology. 136:2247–2257. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Tahara T, Arisawa T, Wang F, Shibata T,
Nakamura M, Sakata M, Hirata I and Nakano H: Toll-like receptor 2
−196 to 174del polymorphism influences the susceptibility of
Japanese people to gastric cancer. Cancer Sci. 98:1790–1794. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Rigoli L, Di Bella C, Fedele F, Procopio
V, Amorini M, Lo Giudice G, Romeo P, Pugliatti F, Finocchiaro G,
Lucianò R and Caruso RA: TLR4 and NOD2/CARD15 genetic polymorphisms
and their possible role in gastric carcinogenesis. Anticancer Res.
30:513–517. 2010.PubMed/NCBI
|
|
23
|
Hnatyszyn A, Szalata M, Stanczyk J, Cichy
W and Slomski R: Association of c.802C>T polymorphism of
NOD2/CARD15 gene with the chronic gastritis and predisposition to
cancer in H. pylori infected patients. Exp Mol Pathol. 88:388–393.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Watanabe T, Asano N, Fichtner-Feigl S,
Gorelick PL, Tsuji Y, Matsumoto Y, Chiba T, Fuss IJ, Kitani A and
Strober W: NOD1 contributes to mouse host defense against
Helicobacter pylori via induction of type I IFN and activation of
the ISGF3 signaling pathway. J Clin Invest. 120:1645–1662. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Marcinek P, Jha AN, Shinde V,
Sundaramoorthy A, Rajkumar R, Suryadevara NC, Neela SK, van Tong H,
Balachander V, Valluri VL, et al: LRRK2 and RIPK2 variants in the
NOD 2-mediated signaling pathway are associated with susceptibility
to Mycobacterium leprae in Indian populations. PLoS One.
8:e731032013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Huang YK, Yu JC, Kang WM, Ma ZQ, Ye X,
Tian SB and Yan C: Significance of serum pepsinogens as a biomarker
for gastric cancer and atrophic gastritis screening: A systematic
review and meta-analysis. PLoS One. 10:e01420802015. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Lee JY, Kim N, Lee HS, Oh JC, Kwon YH,
Choi YJ, Yoon KC, Hwang JJ, Lee HJ, Lee A, et al: Correlations
among endoscopic, histologic and serologic diagnoses for the
assessment of atrophic gastritis. J Cancer Prev. 19:47–55. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Go MF: Review article: Natural history and
epidemiology of Helicobacter pylori infection. Aliment Pharmacol
Ther. 16 Suppl 1:S3–S15. 2002. View Article : Google Scholar
|