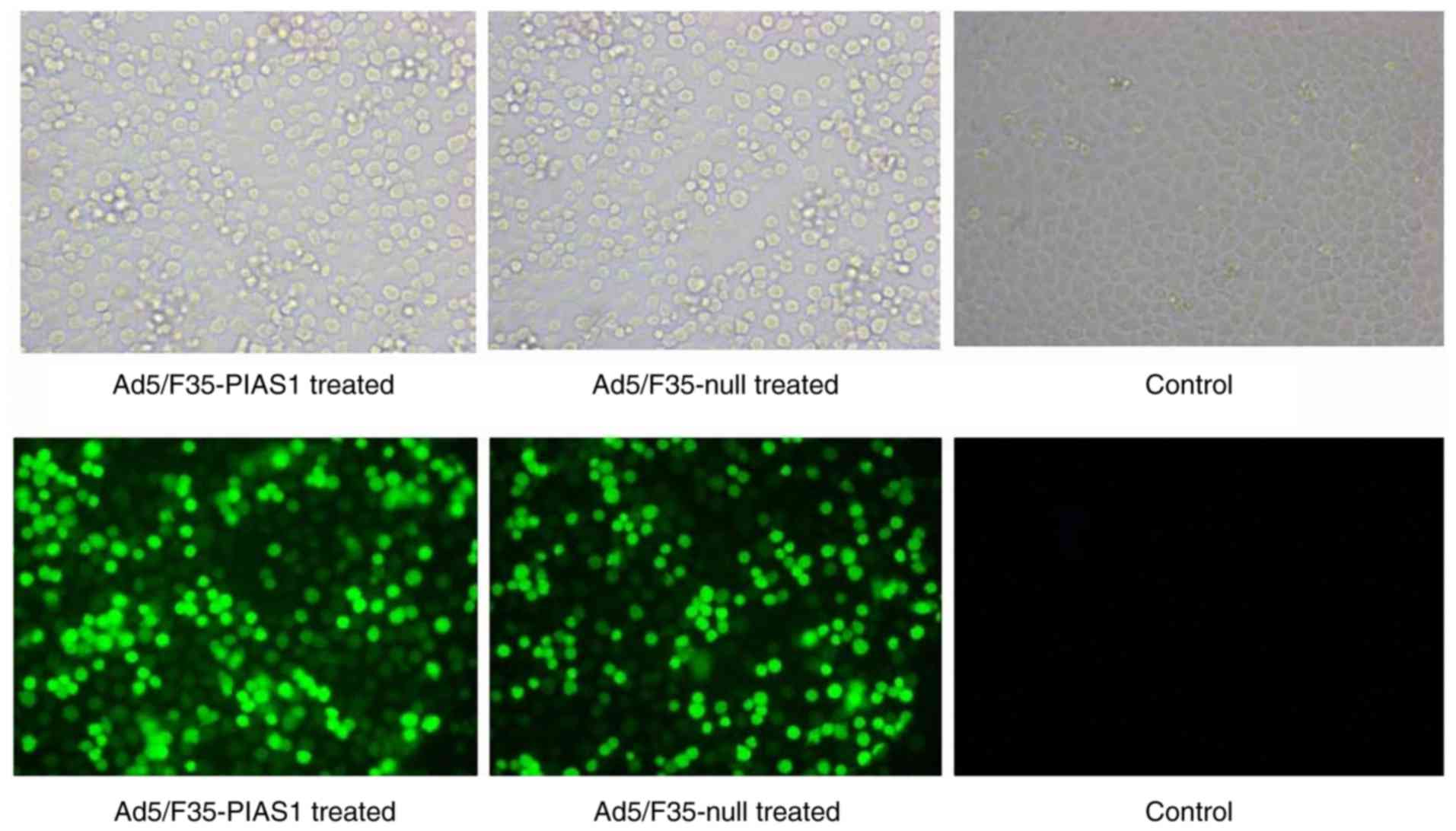
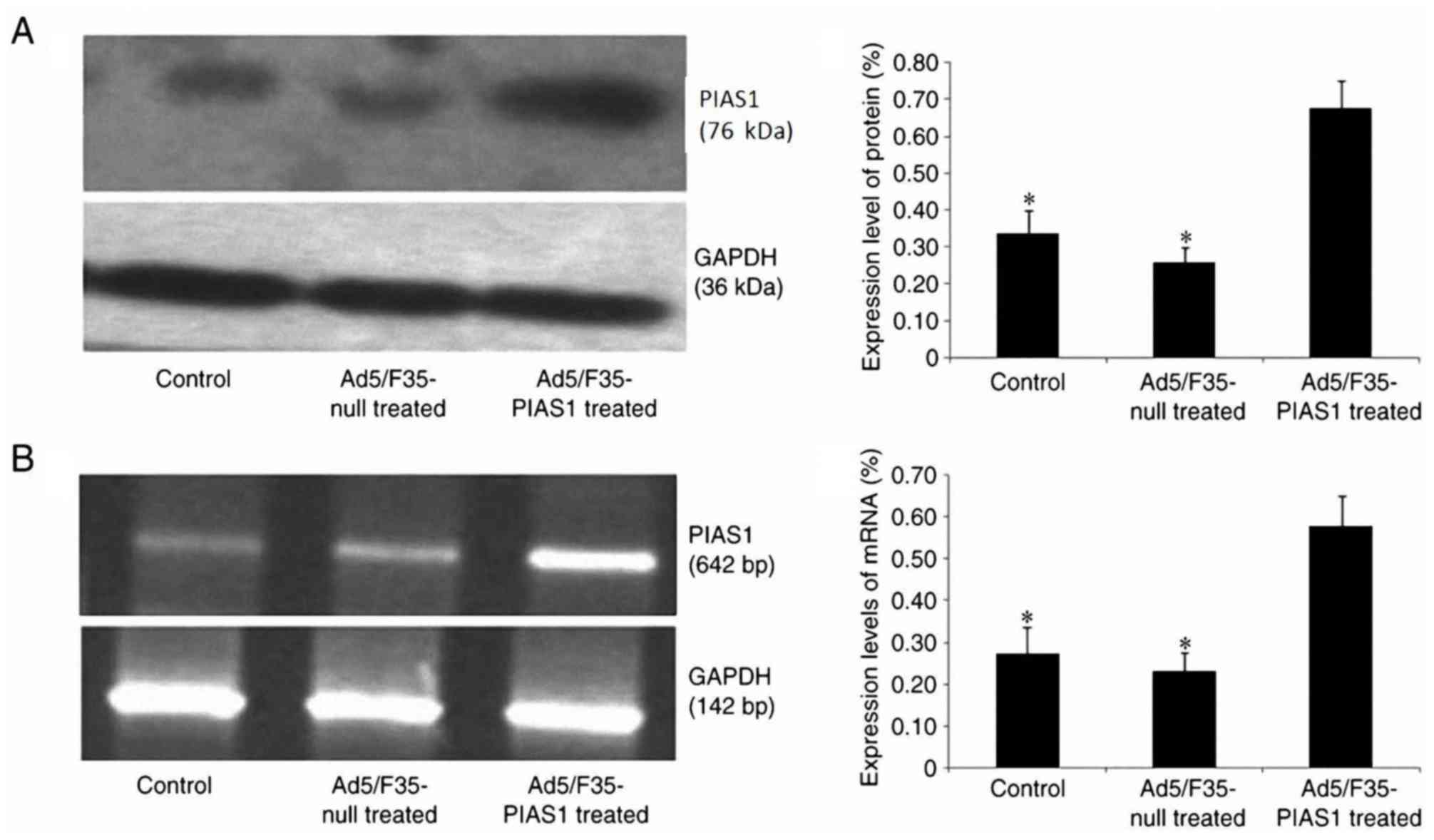
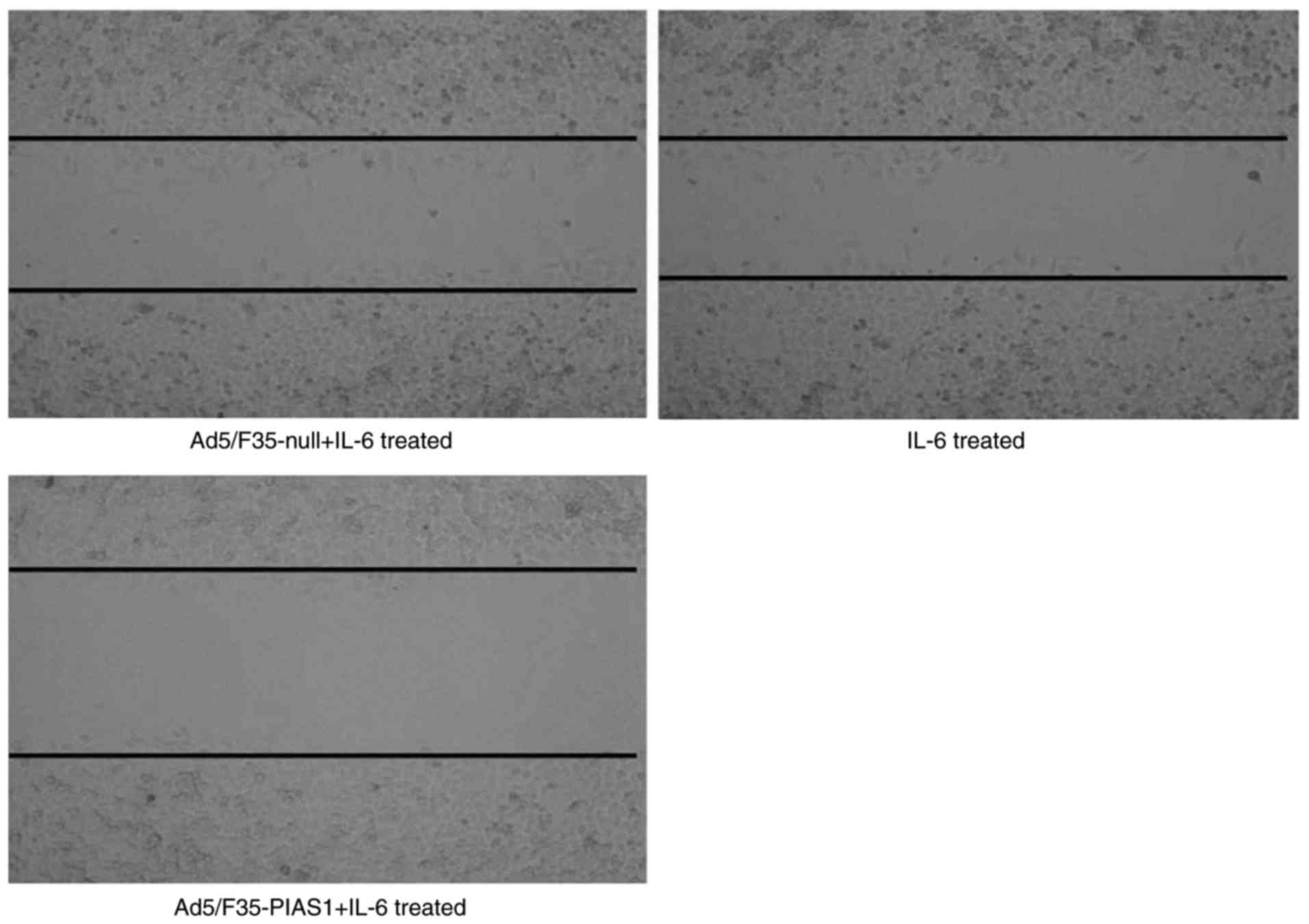
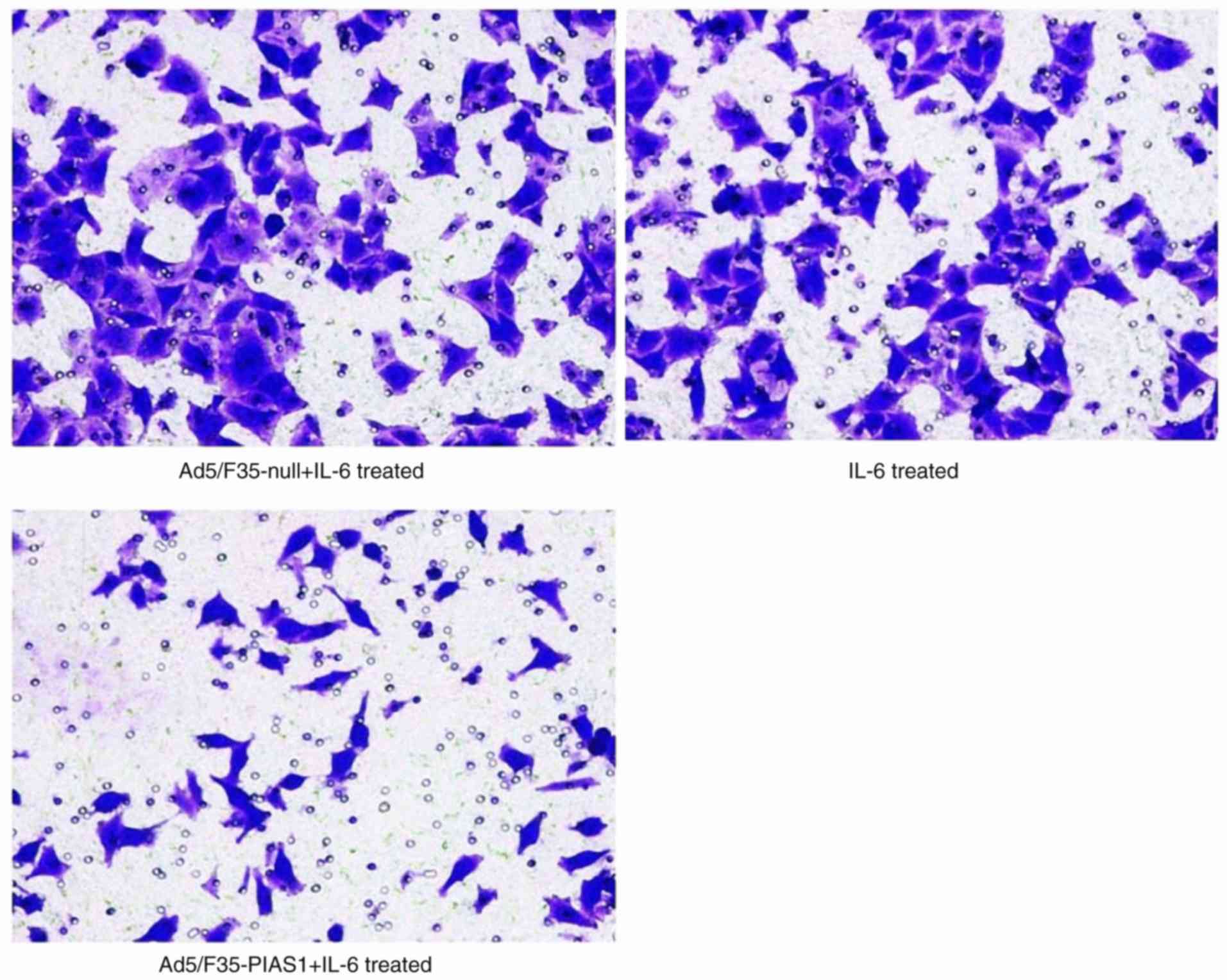
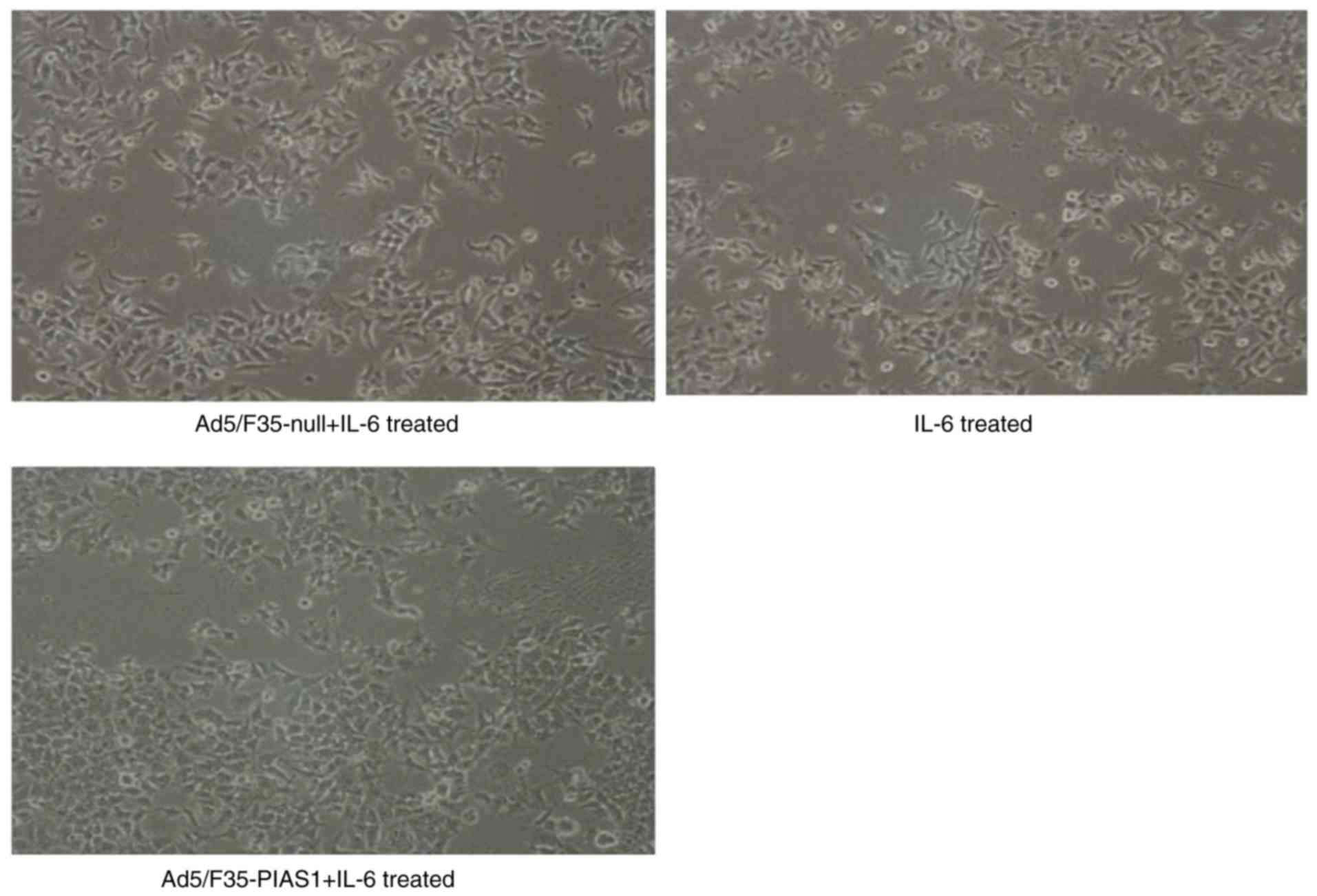
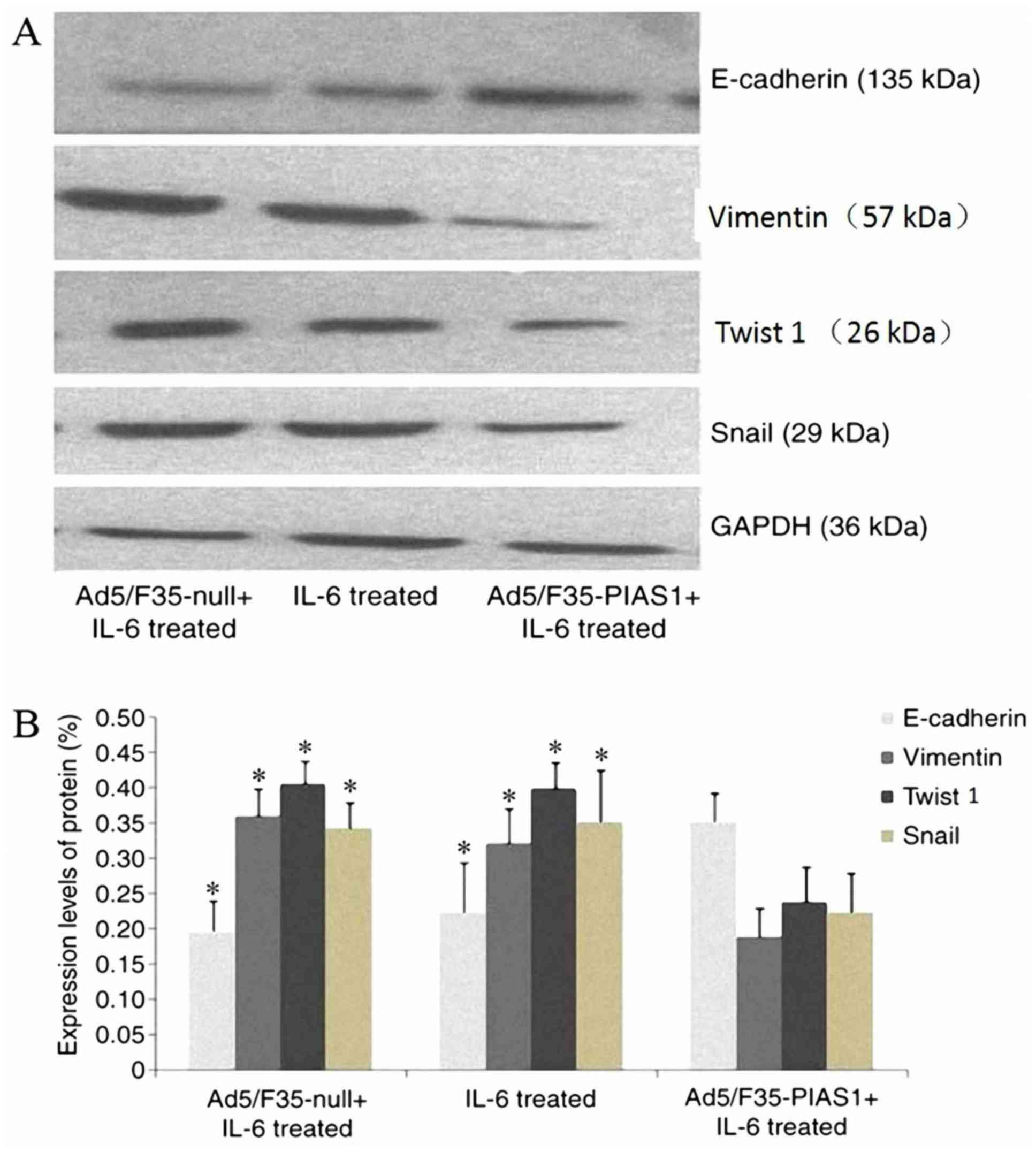
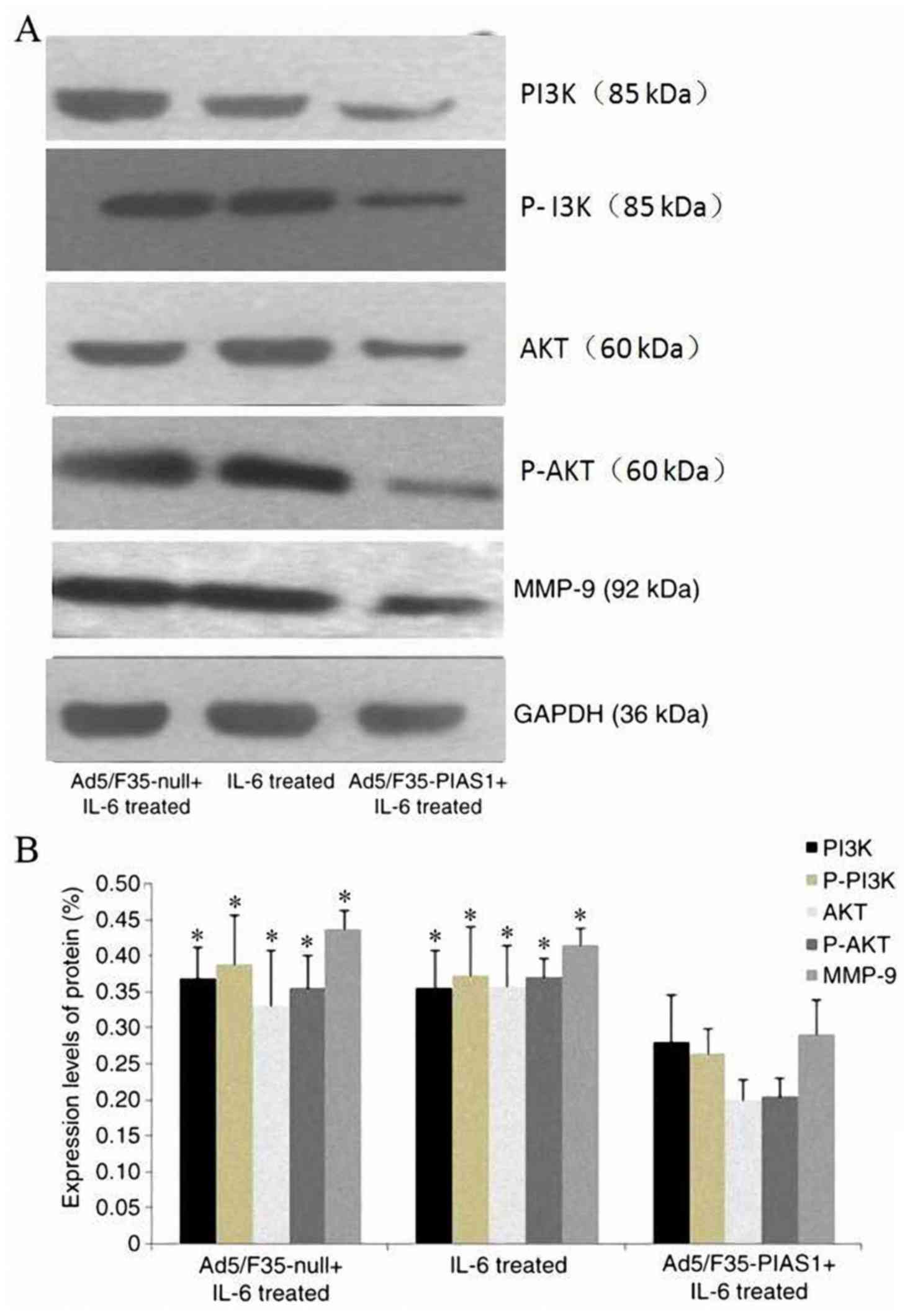
|
1
|
Röcken C: Ways to personalized medicine
for gastric cancer. Pathologe. 34:403–412. 2013.(In German).
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Kim HS, Kim JH, Kim JW and Kim BC:
Chemotherapy in elderly patients with gastric cancer. J Cancer.
7:88–94. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Wang SS, Jiang J, Liang XH and Tang YL:
Links between cancer stem cells and epithelial-mesenchymal
transition. Onco Targets Ther. 8:2973–2980. 2015.PubMed/NCBI
|
|
4
|
Huang L, Wu RL and Xu AM:
Epithelial-mesenchymal transition in gastric cancer. Am J Transl
Res. 7:2141–2158. 2015.PubMed/NCBI
|
|
5
|
Peng Z, Wang CX, Fang EH, Wang GB and Tong
Q: Role of epithelial-mesenchymal transition in gastric cancer
initiation and progression. World J Gastroenterol. 20:5403–5410.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Tanaka T and Kishimoto T: The biology and
medical implications of interleukin-6. Cancer Immunol Res.
2:288–294. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Huang C, Yang G, Jiang T, Zhu G, Li H and
Qiu Z: The effects and mechanisms of blockage of STAT3 signaling
pathway on IL-6 inducing EMT in human pancreatic cancer cells in
vitro. Neoplasma. 58:396–405. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Rodriguez JA, Huerta-Yepez S, Law IK,
Baay-Guzman GJ, Tirado-Rodriguez B, Hoffman JM, Iliopoulos D,
Hommes DW, Verspaget HW, Chang L, et al: Diminished expression of
CRHR2 in human colon cancer promotes tumor growth and EMT via
persistent IL-6/Stat3 signaling. Cell Mol Gastroenterol Hepatol.
1:610–630. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Dadakhujaev S, Salazar-Arcila C, Netherton
SJ, Chandhoke AS, Singla AK, Jirik FR and Bonni S: A novel role for
the SUMO E3 ligase PIAS1 in cancer metastasis. Oncoscience.
1:229–240. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Heo KS, Chang E, Takei Y, Le NT, Woo CH,
Sullivan MA, Morrell C, Fujiwara K and Abe J: Phosphorylation of
protein inhibitor of activated STAT1 (PIAS1) by MAPK-activated
protein kinase-2 inhibits endothelial inflammation via increasing
both PIAS1 transrepression and SUMO E3 ligase activity.
Arterioscler Thromb Vasc Biol. 33:321–329. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Chen P, Zhao D, Sun Y, Huang L, Zhang S
and Yuan Y: Protein inhibitor of activated STAT-1 is downregulated
in gastric cancer tissue and involved in cell metastasis. Oncol
Rep. 28:2149–2155. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Chen P, Huang LY and Yuan YZ: Construction
and identification of recombinant adenovirus with rat protein
inhibitor of activated STAT1 gene. Chin J Bilogicals. 24:431–434.
2011.
|
|
13
|
Chen P, Huang L, Sun Y and Yuan Y:
Upregulation of PIAS1 protects against sodium taurocholate-induced
severe acute pancreatitis associated with acute lung injury.
Cytokine. 54:305–314. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Ferlay J, Soerjomataram I, Dikshit R, Eser
S, Mathers C, Rebelo M, Parkin DM, Forman D and Bray F: Cancer
incidence and mortality worldwide: Sources, methods and major
patterns in GLOBOCAN 2012. Int J Cancer. 136:E359–E386. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Deng H, Huang X, Fan J, Wang L, Xia Q,
Yang X, Wang Z and Liu L: A variant of estrogen receptor-alpha,
ER-alpha36 is expressed in human gastric cancer and is highly
correlated with lymph node metastasis. Oncol Rep. 24:171–176.
2010.PubMed/NCBI
|
|
16
|
Mihmanli M, Ilhan E, Idiz UO, Alemdar A
and Demir U: Recent developments and innovations in gastric cancer.
World J Gastroenterol. 22:4307–4320. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Zhao J, Yang C, Guo S and Wu Y: GM130
regulates epithelial-to-mesenchymal transition and invasion of
gastric cancer cells via snail. Int J Clin Exp Pathol.
8:10784–10791. 2015.PubMed/NCBI
|
|
18
|
Choi YJ, Kim N, Chang H, Lee HS, Park SM,
Park JH, Shin CM, Kim JM, Kim JS, Lee DH and Jung HC: Helicobacter
pylori-induced epithelial-mesenchymal transition, a potential role
of gastric cancer initiation and an emergence of stem cells.
Carcinogenesis. 36:553–563. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Lander R, Nordin K and LaBonne C: The
F-box protein Ppa is a common regulator of core EMT factors Twist,
Snail, Slug, and Sip1. J Cell Biol. 194:17–25. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Tania M, Khan MA and Fu J: Epithelial to
mesenchymal transition inducing transcription factors and
metastatic cancer. Tumour Biol. 35:7335–7342. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Xu W, Yang Z and Lu N: A new role for the
PI3K/Akt signaling pathway in the epithelial-mesenchymal
transition. Cell Adh Migr. 9:317–324. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Yin K, Wang L, Zhang X, He Z, Xia Y, Xu J,
Wei S, Li B, Li Z, Sun G, et al: Netrin-1 promotes gastric cancer
cell proliferation and invasion via the receptor neogenin through
PI3K/AKT signaling pathway. Oncotarget. 8:51177–51189. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Willems L, Tamburini J, Chapuis N, Lacombe
C, Mayeux P and Bouscary D: PI3K and mTOR signaling pathways in
cancer: New data on targeted therapies. Curr Oncol Rep. 14:129–138.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Ho LJ, Luo SF and Lai JH: Biological
effects of interleukin-6: Clinical applications in autoimmune
diseases and cancers. Biochem Pharmacol. 97:16–26. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Fisher DT, Appenheimer MM and Evans SS:
The two faces of IL-6 in the tumor microenvironment. Semin Immunol.
26:38–47. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Nguyen DP, Li J and Tewari AK:
Inflammation and prostate cancer: The role of interleukin 6 (IL-6).
BJU Int. 113:986–992. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Lee SO, Yang X, Duan S, Tsai Y, Strojny
LR, Keng P and Chen Y: IL-6 promotes growth and
epithelial-mesenchymal transition of CD133+ cells of non-small cell
lung cancer. Oncotarget. 7:6626–6638. 2016.PubMed/NCBI
|
|
28
|
Hsu CP, Chen YL, Huang CC, Chou CC, Liu
CL, Hung CH, Kao TY and Chung YC: Anti-interleukin-6 receptor
antibody inhibits the progression in human colon carcinoma cells.
Eur J Clin Invest. 41:277–284. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Nowak DG, Cho H, Herzka T, Watrud K,
DeMarco DV, Wang VM, Senturk S, Fellmann C, Ding D, Beinortas T, et
al: MYC Drives Pten/Trp53-deficient proliferation and metastasis
due to IL6 Secretion and AKT suppression via PHLPP2. Cancer Discov.
5:636–651. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Li D, Sun X, Yan D, Huang J, Luo Q, Tang H
and Peng Z: Piwil2 modulates the proliferation and metastasis of
colon cancer via regulation of matrix metallopeptidase 9
transcriptional activity. Exp Biol Med (Maywood). 237:1231–1240.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Kim MA, Lee HS, Lee HE, Kim JH, Yang HK
and Kim WH: Prognostic importance of epithelial-mesenchymal
transition-related protein expression in gastric carcinoma.
Histopathology. 54:442–451. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Hsu WL, Ma YL, Hsieh DY, Liu YC and Lee
EH: STAT1 negatively regulates spatial memory formation and
mediates the memory-impairing effect of Aβ.
Neuropsychopharmacology. 39:746–758. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Kahyo T, Nishida T and Yasuda H:
Involvement of PIAS1 in the sumoylation of tumor suppressor p53.
Mol Cell. 8:713–718. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Wei J, Costa C, Ding Y, Zou Z, Yu L,
Sanchez JJ, Qian X, Chen H, Gimenez-Capitan A, Meng F, et al: mRNA
expression of BRCA1, PIAS1, and PIAS4 and survival after
second-line docetaxel in advanced gastric cancer. J Natl Cancer
Inst. 103:1552–1556. 2011. View Article : Google Scholar : PubMed/NCBI
|