Introduction
Primary liver cancer, which consists of 85–90%
hepatocellular carcinoma (HCC), is the fifth and seventh most
common cancer in men and women, respectively, worldwide, and ~85%
of cases occur in developing countries (1–3). Liver
cancer is the third most common cause of cancer-associated
mortality worldwide, and in China, it accounts for half of the
cancer cases and cancer-associated mortalities (1–3).
HCC is the leading cause of mortality from cancer in
rural China, and the primary challenges for improving the prognosis
of HCC are invasiveness, recurrence and metastasis (4–6). Surgical
resection and liver transplantation are the main curative
modalities for HCC (7,8). However, if the restrictive common
international criteria were applied, only 5–10% of patients would
be offered surgical resection (7).
The long-term prognosis for HCC remains poor, with a high 5-year
tumor recurrence rate and a low 5-year survival rate (7–9).
Transcatheter arterial chemoembolization has increased the chances
of conventional surgical treatments for HCC, and other clinical
treatments, including radiofrequency ablation and microwave
ablation, have been widely applied; however, improvements in the
prognosis and survival rate remain limited and poor (10). Rapid advances in cellular and
molecular techniques offer novel approaches for cancer treatments.
Numerous molecular markers associated with recurrence, metastasis
and invasiveness have been identified in tissue and serum, which
exhibit a prognostic significance and may be promising therapeutic
targets (8). However, the specificity
and reliability of these markers is poor (8). Therefore, it is necessary to identify a
novel reliable target molecule to improve the treatment of HCC.
In 1994, Ras homolog enriched in brain (RHEB) was
first identified and cloned while screening genes that regulate
neural activity (11). As a novel and
unique member of the Ras superfamily of G proteins, RHEB is a
conservative protein from yeast to humans, and plays a significant
role in regulating growth and cell cycle (12). With its GTPase activity, RHEB can
shift between the combination state with guanosine diphosphate and
guanosine-5′-triphosphate (GTP), which determines its molecular
biological function (11–13).
Previous studies demonstrated that RHEB is
overexpressed in various malignant tumors (14,15). In
breast cancer and in head and neck cancer, its overexpression
correlates directly with a worse prognosis (14–16). RHEB
is also overexpressed in skin cancer, and furthermore, depending on
transgenic overexpression technology, the hyperplasia and
canceration of the skin can be induced by increasing RHEB
expression in mouse keratin cells (14). Prostate cancer studies revealed that
RHEB is overexpressed in cancer tissues compared with adjacent
non-cancer tissues, and in androgen-independent prostate cancer
cell lines, RHEB overexpression is also observed (14–16). These
studies suggest that RHEB has oncogene functions; however, whether
RHEB plays an important function in the occurrence and progression
of HCC remains currently unknown.
Therefore, in the present study, the association
between RHEB expression and the clinicopathological features of HCC
was determined. The present study provides a basis for elucidating
the underlying molecular mechanisms of HCC.
Materials and methods
Cell cultures
Human liver cancer cell lines Hep3B, HepG2 and Huh-7
were obtained from the American Type Culture Collection (Manassas,
VA, USA), while SMMC-7721, MHCC-97-L and MHCC-97-H were a gift from
the Liver Cancer Institute, Zhongshan Hospital, Fudan University
(Shanghai, China). These cells were cultured in Dulbecco's modified
Eagle's medium (DMEM; Gibco; Thermo Fisher Scientific, Inc.,
Waltham, MA, USA) supplemented with 10% fetal bovine serum (Gibco;
Thermo Fisher Scientific, Inc.) and 1% penicillin-streptomycin
solution (Beyotime Institute of Biotechnology, Haimen, China) at
37°C in a 5% CO2 incubator.
Tumor formation assay
A total of 18 five-week-old male BALB/c nude mice,
(weight, ~18 g) were obtained from the Shanghai Experimental Animal
Center (Shanghai, China). The mice were kept under standard
laboratory conditions: 21–23°C controlled temperature, 50–65%
humidity and 12-h light-dark cycle lighting, with free access to
drinking water and chow. The condition of the mice was monitored
every other day. Tumor size was measured by a vernier caliper
weekly and calculated as (length × width2)/2. All
procedures were performed in accordance with the National
Institutes of Health Guide for the Care and Use of Laboratory
Animals (9).
HCC tissue collection and patient
follow-up
The surgically resected tissue samples, including 60
cases of diagnosed liver cancer with matching adjacent tissue
samples and 35 normal liver tissue samples from patients under
liver transplantation, were collected from the Eastern
Hepatobiliary Surgery Hospital, Second Military Medical University
(Shanghai, China) between May 2010 and May 2011. Of the 60 HCC
cases, 39 were males and 21 were females, with a median age of
52.0±8.9 years. Of these cases, 26 were clinical stage I, 15 were
stage II and 19 were stage III–IV. All tissue samples were stored
in liquid nitrogen within 30 min after surgical resection of the
biopsy for later use.
Ethics statement
Written informed consent from all patients and
approval from the Ethics Committee of the Eastern Hepatobiliary
Surgery Hospital were obtained. All animal experiments were
approved by the Institutional Animal Care and Use Committee of the
Second Military Medical University.
Gene microarray
Gene expression in cancer tissues and matching
adjacent tissues was assayed using a DNA microarray, and the
results were then analyzed using a gene map analysis software
(Human CHIP version 1; DNA Chip Research Inc., Tokyo, Japan).
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Total RNA was extracted from the cultured cells,
liver cancer and non-cancerous liver specimens using TRIzol regent
(Invitrogen; Thermo Fisher Scientific, Inc.), according to the
manufacturer's protocol. RT for complementary DNA was achieved by a
TaKaRa RNA PCR kit (Takara Biotechnology Co., Ltd., Dalian, China)
and detected using an RT-qPCR kit and 7900HT Fast Real-Time PCR
System (Applied Biosystems; Thermo Fisher Scientific, Inc.). The
primers for the RHEB gene are as follows: Forward
5′-ACTCCTACGATCCAACCATAGA-3′ and reverse
5′-TGGAGTATGTCTGAGGAAAGATAGA-3′, and the probe was
6-carboxyfluorescein (6-FAM)-5′-AGACACAGCCGGGCAAGATGAATA-3′-minor
groove binder (MGB). The GAPDH gene was used as an internal
control, and the primers were as follows: Forward
5′-CATGGGTGTGAACCATGAGA-3′ and reverse
5′-GAGTCCTTCCACGATACCAAAG-3′, and the probe was
FAM-5′-AGATCATCAGCAATGCCTCCTGCA-3′-MGB. The following cycling
conditions were used: 94°C for 5 min, followed by 94°C for 1 sec,
65°C for 15 sec and 72°C for 30 sec for 35 cycles. RHEB messenger
RNA (mRNA) from normal liver tissues and untreated cultured cells
was used as the control for RT-qPCR.
Immunohistochemical analysis
Sections (4-µm) were cut from paraffin blocks for
immunohistochemical staining. A rabbit anti-human RHEB monoclonal
antibody (ab92313) was purchased from Abcam (Cambridge, MA, USA),
and the PV-9000 immunohistochemical reagent kit was obtained from
Beijing Zhongshan Golden Bridge Biotechnology Co., Ltd. (Beijing,
China). RHEB protein expression in liver cancer was detected by
immunohistochemical staining with the PV-9000 kit according to the
manufacturer's protocol. After conventional dewaxing and hydration,
the sections were incubated in 3% H2O2 with
deionized water to block the endogenous peroxidase activity. A
microwave antigen retrieval procedure with
ethylenediaminetetraacetic acid (pH 8.0) was used, and then the
sections were cooled and incubated with rabbit anti-human RHEB
antibody (dilution 1:100) at 4°C overnight. Next, the sections were
incubated with a secondary anti-rabbit antibody (dilution 1:200;
8114; Cell Signaling Technology, Inc., Danvers, MA, USA) at 37°C
for 30 min, and then rinsed with 1X PBS. Finally,
3,3′-diaminobenzidine was used to develop the sections. The
sections were incubated at room temperature without light for 10
min, and the reaction was completed with distilled water. The tan
or brown granules in the nucleus and cytoplasm represented positive
RHEB protein expression.
Four different views were randomly observed with
high magnification (×400), and the number of total cells and cells
positive for nuclear staining were recorded. Based on the ratio of
positive cells, the staining was scored as 1 (0–10 cells), 2 (11–50
cells), 3 (51–75 cells) and 4 (76–100 cells). The staining
intensity was scored as 1 (negative staining), 2 (weak staining), 3
(moderate staining) and 4 (strong staining). The final score for
RHEB protein expression was calculated as the product of the
staining score and the intensity score. Based on the final score,
0–4 was (−), 5–8 was (+), 9–12 was (++) and 13–16 was (+++). For
the purpose of statistical evaluation, (−) and (+) represented
negative and weakly positive expression, respectively, while (++)
and (+++) represented highly positive expression. All results were
confirmed using a blind method by ≥2 pathologists.
Western blotting
After being cultured for 48–72 h, the adherent cells
were rinsed in chilled PBS, and lysis buffer was added to cover the
cells completely at room temperature. Then, the buffer was
collected and the cells were centrifuged at 600 × g/min for
5 min at 4°C. Upon determination of protein concentration with the
BCA Protein Assay kit (P0012; Beyotime Institute of Biotechnology),
the remaining supernatant was boiled and placed on ice for 5 min.
In total, 50 µg of sample was loaded in each well, and separated by
12% polyacrylamide gel electrophoresis at ~200 V. The separated
proteins were electrophoretically transferred onto nitrocellulose
membranes at 60 V. The blotted membrane was blocked with 5% skim
milk in Tris-buffered saline with Tween 20 (TBST), and then washed
with TBST. Anti-RHEB antibody (ab92313) was next added at a 1:1,000
dilution and incubated at 4°C overnight. The membrane was washed in
TBST, and horseradish peroxidase-conjugated immunoglobulin G
secondary antibody (7074; Sigma-Aldrich; Merck Millipore,
Darmstadt, Germany) was added at a 1:1,000 dilution and incubated
at room temperature for 2 h. The nitrocellulose membrane was
developed with a chemiluminescent solution (Kangwei, Beijing,
China), covered with plastic wrap and placed in a cassette.
Finally, the blot was analyzed upon exposure.
RNA interference (RNAi)
The nucleotide sequences were designed for the RHEB
mRNA sequence derived from the Human Gene Mutation Database
(http://www.hgmd.cf.ac.uk/ac/index.php) using the
Ambion RNAi software version 3 (Thermo Fisher Scientific, Inc.).
According to this nucleotide sequence, a pair of complementary
oligonucleotide chains encoding the corresponding short hairpin
(sh) small interfering (si) RNA that binds specifically to the RHEB
mRNA were synthesized. The sequences were as follows: Sense strand
5′-GAAAGACCUGCAUAUGGAAAGGGTG-3′ and antisense strand
5′-CACCCUUUCCAUAUGCAGGUCUUUCUU-3′. Without any sequences matched to
the human genome sequences, the siRNA-NC was used as the negative
control, with sense strand 5′-UUCUCCGAACGUGUACGUTT-3′ and antisense
strand 5′-ACGUGACACGUUCGGAGAATT-3′. The synthesis of these
oligonucleotide chains was performed by Bioneer Corporation
(Daejeon, Korea).
The pSilencer™ 2.1-U6neo plasmid (Ambion; Thermo
Fisher Scientific, Inc.), which was digested with BamHI and
HindIII endonucleases (New England BioLabs, Inc., Ipswich,
MA, USA), was collected and purified with a plasmid DNA extraction
kit (Qiagen GmbH, Hilden, Germany), according to the manufacturer's
protocol, to obtain the fragments of the target gene and carrier.
The eukaryotic expression vector pU-RHEB-siRNA was constructed by
ligating the oligonucleotide chains and carrier fragments into the
pSilencer™ 2.1-U6neo plasmid, and was sequenced with the Sanger
method by Thermo Fisher Scientific, Inc.
Prior to transfection (20 h), liver cancer cells
were plated in 6-well plates (Corning Incorporated, Corning, NY,
USA). Cells were transfected with the eukaryotic expression vector
pU-RHEB-siRNA using Lipofectamine™ 2000 (Invitrogen; Thermo Fisher
Scientific, Inc.) when the cells reached 80–90% confluency. Both
liver cancer cells, which were transfected with the empty vector,
and untreated liver cancer cells served as the control groups.
After transfection (24 h), the cells were subcultured in G418-free
DMEM at a ratio of 1:20, and selective medium with G418 (400 µg/ml)
(Sigma-Aldrich; Merck Millipore) was added the following day.
Cell cycle analysis
To analyze the cell cycle distribution, all the
collected cells were fixed in 70% ethanol at −20°C overnight,
stained with propidium iodide (36 mg/ml; Sigma-Aldrich; Merck
Millipore) for 30 min and analyzed by flow cytometry (Beckman
Coulter, Inc., Brea, CA, USA).
Statistical analyses
The association between RHEB expression and
clinicopathological features was evaluated with the χ2
test, or with the Fisher's exact test when the χ2 test
was not suitable. The correlation between RHEB overexpression (as
determined by immunohistochemistry and quantified by integral
optical density) and tumor-node-metastasis (TNM) stages was
evaluated by analysis of variance (ANOVA) and Spearman correlation
analysis. Cox proportional hazards regression analysis of multiple
factors was employed to assess the association between clinical
data and survival or recurrence. All statistical analyses were
performed using the SPSS version 12.0 software (SPSS, Inc.,
Chicago, IL, USA). P<0.05 was considered to indicate a
statistically significant difference.
Results
Aberrant expression of RHEB in liver
cancer cell lines and tissues
To investigate the RHEB expression in different
liver cancer cell lines, a total of six strains of liver cancer
cells were selected, including Hep3B, SMMC-7721, HepG2, MHCC-97-L,
MHCC-97-H and Huh-7. Western blot analysis demonstrated that RHEB
is overexpressed in all these cell lines, with the exception of
MHCC-97-L (Fig. 1A).
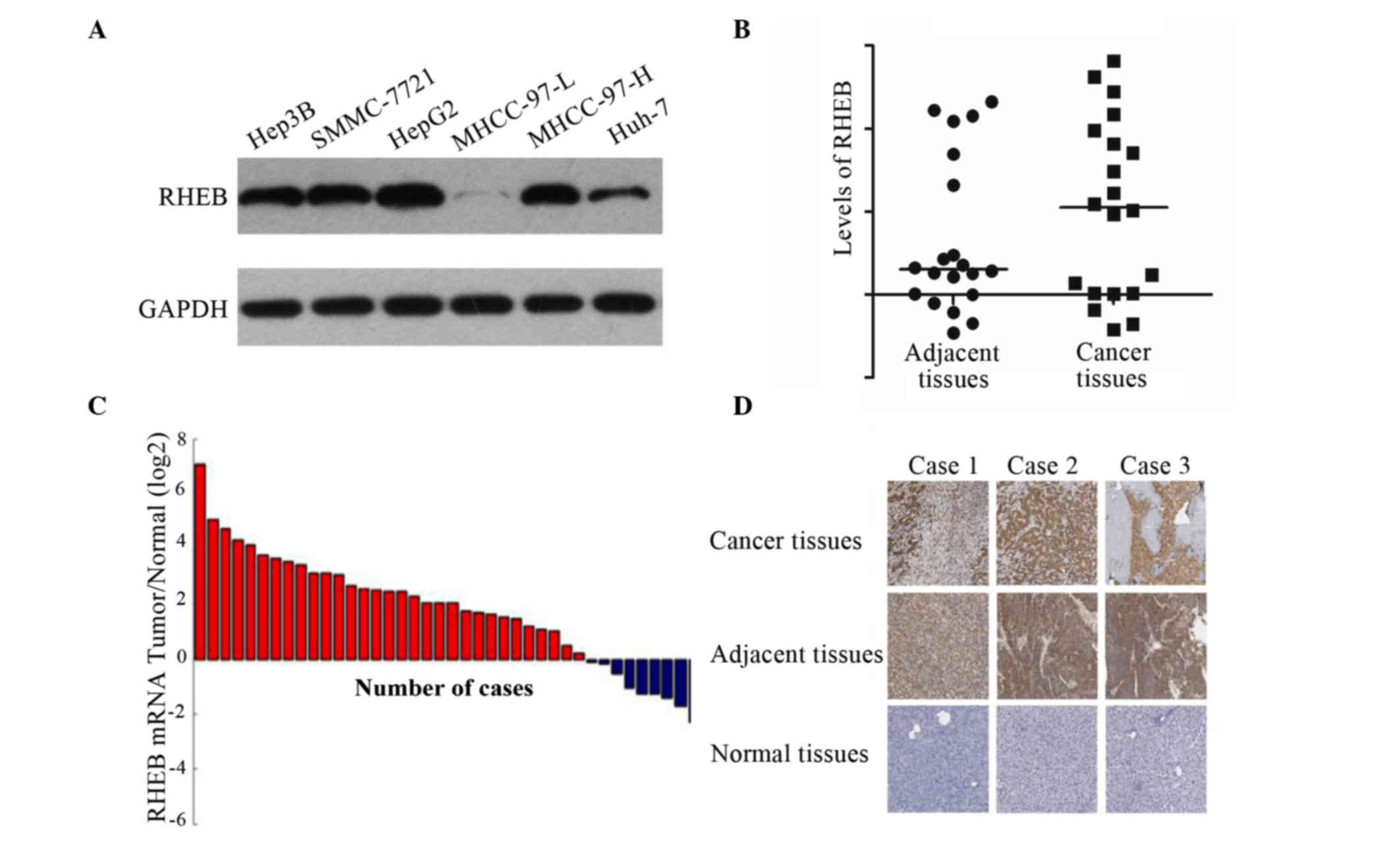 | Figure 1.RHEB expression in liver cancer cell
lines and tissues. (A) RHEB was overexpressed in Hep3B, SMMC-7721,
HepG2 and MHCC-97-H cells, but not in MHCC-97-L cells, as
demonstrated by western blot analysis. (B) RHEB expression in liver
cancer relative to the control gene GAPDH (5.00±0.34-fold) was
upregulated compared with that in adjacent tissues (2.00±0.27-fold)
and normal tissues (2.00±0.19-fold), as evaluated by gene
microarray analysis. (C) RHEB messenger RNA level in liver cancer
samples was upregulated compared with that in non-cancerous liver
samples, as demonstrated by reverse transcription-quantitative
polymerase chain reaction analysis. (D) RHEB was overexpressed in
liver cancer tissues, but little or no expression was detected in
normal liver tissues, as analyzed by immunohistochemistry.
Magnification, ×200. RHEB, Ras homolog enriched in brain; mRNA,
messenger RNA. |
To further study the RHEB expression in liver cancer
tissue in vivo, 20 paired liver cancer tissues and adjacent
tissue specimens were examined via gene microarray. The results
revealed that the expression of RHEB in liver cancer compared with
the control gene GAPDH (5.00±0.34-fold) was upregulated relative to
adjacent tissues (2.00±0.27-fold; P=0.025) and normal tissues
(2.00±0.19-fold; P=0.035) (Fig. 1B).
Furthermore, the RHEB mRNA expression in the liver cancer tissue
samples, as detected by RT-qPCR, was significantly upregulated
compared with non-cancerous liver tissues (Fig. 1C), which was consistent with the
results of gene microarray. The immunohistochemical results
revealed that RHEB was expressed primarily in the nucleus and
partially in the cytoplasm, and of 60 liver cancer tissues, 41
exhibited positive expression of RHEB, while little or no RHEB
expression was observed in the adjacent or normal tissues (Fig. 1D).
Relative analysis between RHEB
expression and clinicopathological features in patients with
HCC
To further investigate the role of RHEB in patients
with HCC, the association between the immunohistochemical results
and the clinicopathological data was evaluated. According to the
immunohistochemical results, of the 26 liver cancer samples at
stage T1, positive RHEB expression occurred in 17. Of the 15 liver
cancer samples at stage T2, positive RHEB expression occurred in 9.
Of the 19 liver cancer samples at stage T3-T4, positive RHEB
expression occurred in 13. Statistical analysis demonstrated that
the differences in positive RHEB expression between T1 and the
other stages were not significant (P=0.876). The differences in
RHEB expression between liver cancer groups with different TNM
stages were analyzed by ANOVA and were observed to be significant
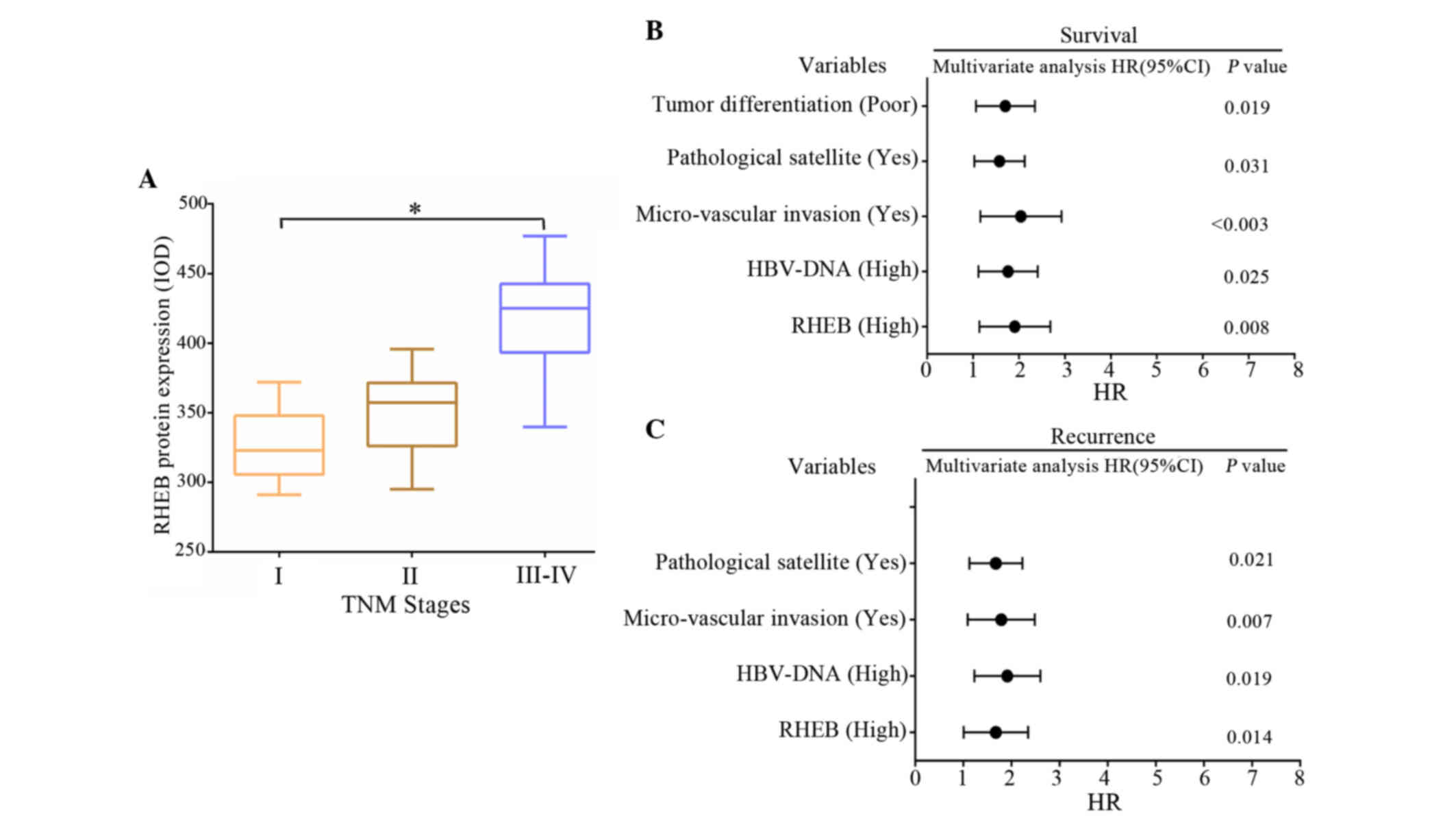
(P=0.020) (Fig. 2A). Spearman
correlation analysis demonstrated that high expression of RHEB
positively correlated with TNM staging in HCC (r=0.583, P=0.031).
Survival analysis revealed that the survival time of HCC patients
was closely correlated to RHEB expression level, hepatitis B virus
(HBV)-DNA titer, micro-vascular invasion, pathological satellites
and tumor differentiation (P<0.05) (Fig. 2B), and was not dependent on gender
(P=0.231), age (P=0.064) or tumor size (P=0.157). According to the
correlation analysis between clinicopathological data and
postoperative recurrence, postoperative recurrence was closely
correlated to RHEB expression level, HBV-DNA titer, micro-vascular
invasion and pathological satellites (P<0.05) (Fig. 2C), and was not dependent on gender
(P=0.165), age (P=0.073), tumor size (P=0.142) or tumor
differentiation (P=0.351).
RHEB silencing downregulates the
proliferation of a hepatoma cell line
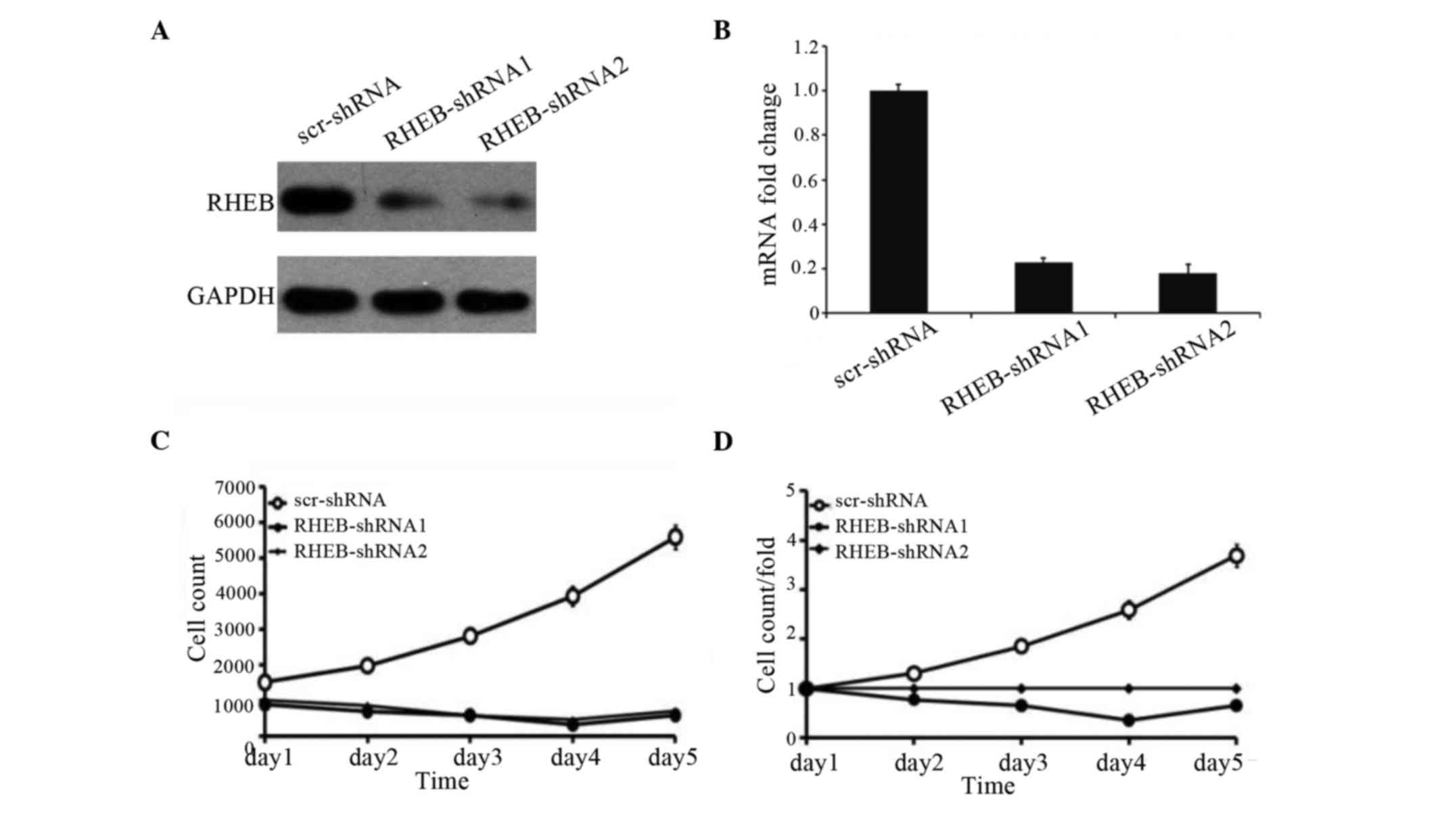
To identify the effects of RHEB expression on the
growth of HCC cells, the SMMC-7721 liver cancer cell line with RHEB
overexpression was selected and treated with shRNA-RHEB. shRNA-RHEB
downregulated RHEB expression in SMMC-7721 cells, and its
interference efficiency was detected by RT-qPCR and western
blotting (Fig. 3A), which
demonstrated 80% efficiency (P<0.0001; Fig. 3B). Upon downregulation of RHEB
expression, the proliferation of the knocked down cells was
significantly inhibited (P=0.025; Fig.
3C), and the inhibition was obvious on the third day
post-transfection (P=0.03; Fig. 3D),
which suggests that RHEB plays an important biological role in
HCC.
Influence of RHEB silencing on the
biological behavior of a liver cancer cell line
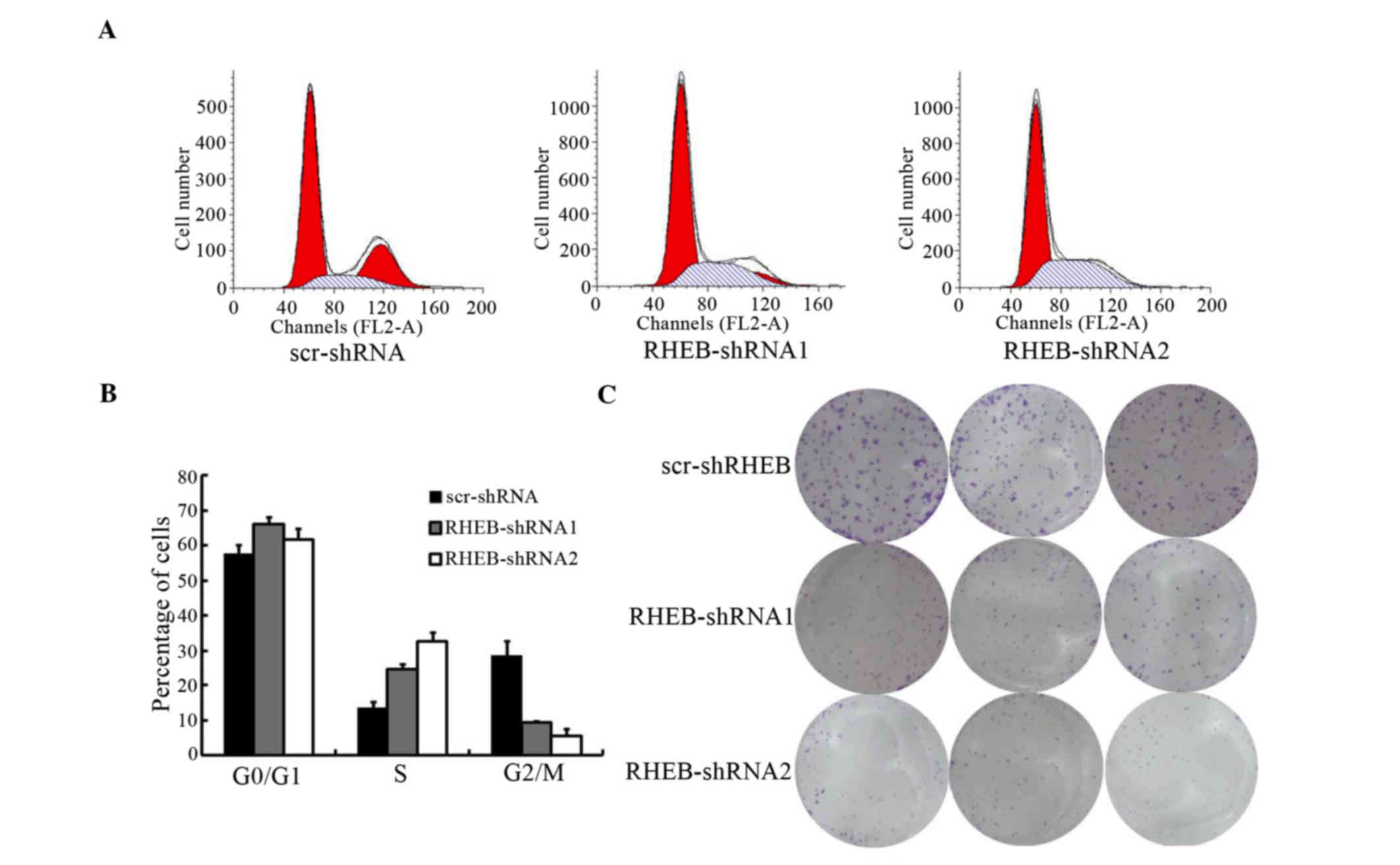
To evaluate the biological functions of RHEB, the
cell cycle of SMMC-7721 cells treated with shRNA-RBEB was analyzed,
and was observed to change significantly, according to the results
of flow cytometry[scramble (scr)-shRNA vs. RHEB-shRNA1, P=0.041 and
scr-shRNA vs. RHEB-shRNA2, P=0.021; Fig.
4A]. The number of S-phase cells increased significantly, while
the number of G2/M-phase cells decreased significantly (scr-shRNA
vs. RHEB-shRNA1, P=0.016 and scr-shRNA vs. RHEB-shRNA2, P=0.011),
with the majority of cells arrested in S phase (Fig. 4B). In soft agar colony-forming assay,
the colony-formation and proliferation abilities of the groups
treated with shRNA-RHEB decreased significantly by 6.0±0.5-fold
(scr-shRNA vs. RHEB-shRNA1, P=0.004 and scr-shRNA vs. RHEB-shRNA2,
P=0.003; Fig. 4C).
RHEB silencing suppresses the growth
of HCC cells in vivo
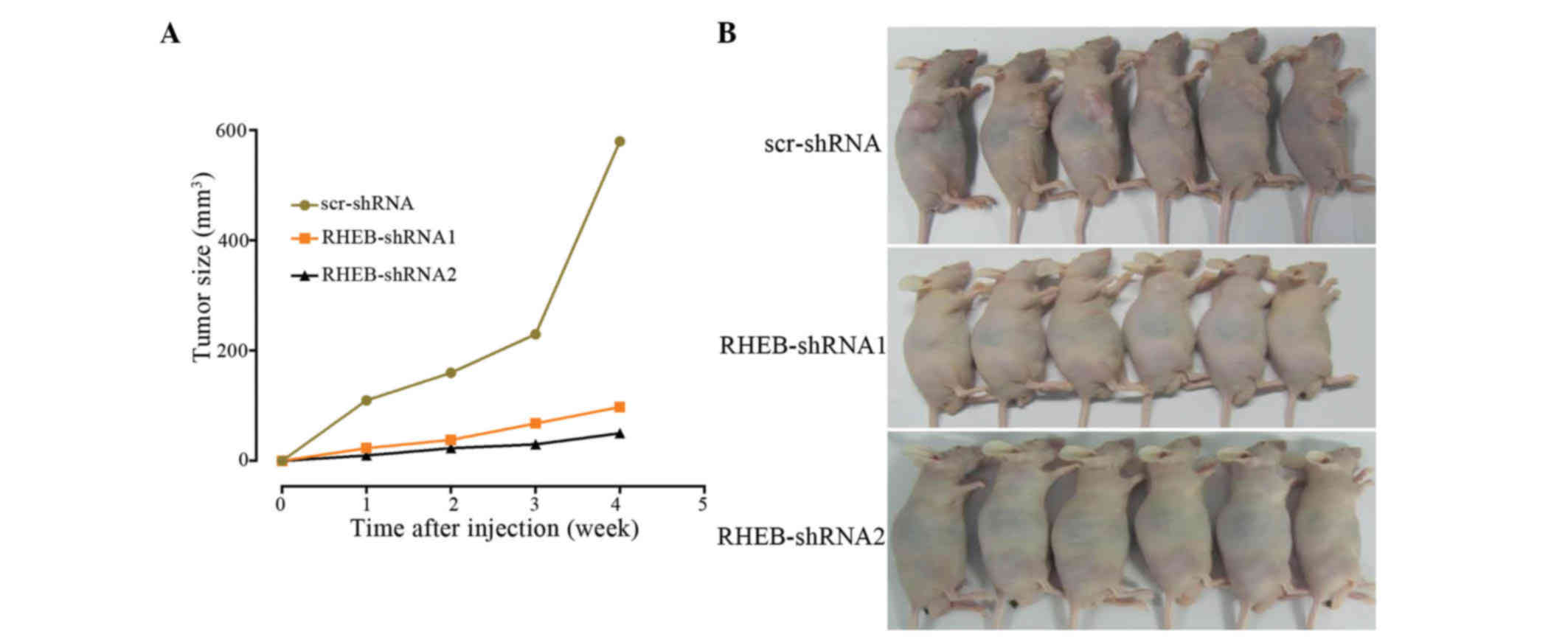
In the tumorigenicity assay, SMMC-7721 cells were
subcutaneously injected into nude mice, and knocked down RHEB in
SMMC-7721 cells was observed to decelerate cancer growth, with no
difference in tumor growth between the RHEB-shRNA1 and the
RHEB-shRNA2 groups (RHEB-shRNA1 vs. RHEB-shRNA2, P=0.620), although
there was a significant difference between the downregulated groups
and the scr-shRNA group (scr-shRNA vs. RHEB-shRNA1, P=0.003 and
scr-shRNA vs. RHEB-shRNA2, P=0.006; Fig.
5A). In addition, the tumorigenic ability of the control group
was higher than that of the RHEB-shRNA1 and RHEB-shRNA2 groups
(Fig. 5B), and the difference in
tumorigenic ability between the experimental and control groups was
significant (scr-shRNA vs RHEB-shRNA1, P=0.003 and scr-shRNA vs.
RHEB-shRNA2, P=0.006).
Discussion
The RHEB protein is an extremely important activator
in cell signal transduction mediated by insulin, and previous
studies have demonstrated that insulin elevates its GTP-binding
state (17,18). GTP-loaded RHEB binds directly to the
catalytic domain of mechanistic target of rapamycin (mTOR)to
activate its serine/threonine kinase activity and to increase
cancer cell growth, which can be blocked by rapamycin (17–19). In
numerous tissues, RHEB overexpression can promote cancer cell
growth and change the cell cycle kinetics through accelerating the
G1-S phase, while the cell division rates are not affected
(20).
In the present study, RHEB overexpression in liver
cancer tissues was observed to be significant, as demonstrated by
gene microarrays and verified by RT-qPCR. According to the results
of immunohistochemistry, RHEB protein displayed positive expression
in HCC tissues compared with normal liver tissues, and was
expressed in the cytoplasm of liver cancer cells. Furthermore, RHEB
overexpression was observed in the majority of liver cancer lines
in the present study, which suggests that RHEB serves an important
role in the occurrence and development of HCC. In addition,
analysis of the association between RHEB expression and
clinicopathological data in patients with HCC demonstrated that
RHEB expression increased with clinical staging, which further
suggests that RHEB is implicated in the development and progression
of HCC. These results are consistent with those from previous
studies on head and neck, breast and prostate cancer, which further
indicates that RHEB overexpression is common in the development and
progression of numerous cancers (14,15,16).
Survival analysis revealed that the overall survival
time was closely correlated to the RHEB expression level, and was
decreased in patients with high RHEB expression. Further analysis
of the association between clinicopathological data and recurrence
upon resection revealed that a high RHEB expression level was
closely correlated to overall survival time. Based on these
findings, overexpression of RHEB in patients with HCC appears to
result in increased rates of recurrence following resection and in
decreased overall survival time. These results further suggest that
RHEB is involved in the metastasis, recurrence and prognosis of
patients with HCC.
Due to its high RHEB expression, the liver cancer
cell line SMMC-7721 was selected and treated with siRNA-RHEB in the
present study. Inhibition of its cell growth could be observed,
with the majority of cells being blocked in S phase, which led to
an increase in the number of cells in S phase, while the number of
cells in G2/M phase was decreased, which suggests that RHEB is
involved in regulating the synthesis of DNA in S phase.
Additionally, the decrease in soft agar colony-forming ability
demonstrated a decrease in cancer cell invasiveness, which suggests
that RHEB may be involved in the regulation of invasiveness and
metastasis in patients with HCC. Tumor growth also decreased
subsequent to downregulation of RHEB expression in the animal
experiments, and the tumorigenesis of the nude mice used in the
study also decreased, which further suggests that the RHEB gene
participates in the regulation of tumor growth.
However, the critical role of RHEB in the
occurrence, development, metastasis and recurrence of HCC remains
unknown. Previous studies have demonstrated that RHEB can activate
the S6 kinase (S6K) transcription factor through the regulation of
the mTOR signaling pathway and androgen receptor trans-activity,
thus regulating the proliferation of prostate cancer cells
(15,21). RHEB is located downstream of the tumor
inhibitors tuberous sclerosis (Tsc)1 and Tsc2, and upstream of mTOR
in the mTOR-S6K signaling pathway (19). The GTP-bound activated state of RHEB
could activate the mTOR signaling pathway and increase the
phosphorylation of S6K and 4E-binding protein 1, which promotes
mRNA translation and protein synthesis, thus enhancing cancer cell
growth and inhibiting autophagy (19,22,23). A
similar function of RHEB in head and neck cancer, breast cancer and
lymphoma was also reported in previous studies (16,24,25), which
suggests that RHEB potentially promotes the proliferation,
invasiveness and metastasis of HCC via the mTOR signaling pathway,
thus affecting the development, progression and prognosis of
patients with HCC. Further studies on the underlying mechanism of
RHEB in HCC should be conducted.
RHEB overexpression also occurs in non-small cell
lung cancer and lymphoma (24,26).
Previous studies have suggested that RHEB is likely to be a
therapeutic target (24,26). The present study demonstrates the
significance of RHEB in HCC and provides a basis for non-operative
treatment of patients with HCC (27).
In conclusion, the present study demonstrated that
RHEB overexpression is closely correlated to the
clinicopathological features of patients with HCC, and is involved
in the proliferation, invasiveness, metastasis, recurrence and
prognosis of HCC. RHEB plays a significant role in the development
and progression of HCC, and is an important prognostic marker and
likely to be a therapeutic target for patients with HCC.
Acknowledgements
This work was supported by the grants from
International Science and Technology Cooperation Program of the
Ministry of Science and Technology (2011DFA32980), National Natural
Science Foundation of China (81772529, 81271694, 81372652,
81572336), The Innovation Program of Shanghai Municipal Education
Commission (2013ZZ060), One Hundred Person Project of the Shanghai
Health (XBR2013117) and National Key Basic Research Program of
China 973 Program (2012CB526706).
References
|
1
|
Ferlay J, Shin HR, Bray F, Forman D,
Mathers C and Parkin DM: Estimates of worldwide burden of cancer in
2008: GLOBOCAN 2008. Int J Cancer. 127:2893–2917. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Jemal A, Bray F, Center MM, Ferlay J, Ward
E and Forman D: Global cancer statistics. CA Cancer J Clin.
61:69–90. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
El-Serag HB and Rudolph KL: Hepatocellular
carcinoma: Epidemiology and molecular carcinogenesis.
Gastroenterology. 132:2557–2576. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Song P, Feng X, Zhang K, et al: Screening
for and surveillance of high-risk patients with HBV-related chronic
liver disease: Promoting the early detection of hepatocellular
carcinoma in China. Biosci Trends. 7:1–6. 2013.PubMed/NCBI
|
|
5
|
Tang ZY, Ye SL, Liu YK, Qin LX, Sun HC, Ye
QH, Wang L, Zhou J, Qiu SJ, Li Y, et al: A decade's studies on
metastasis of hepatocellular carcinoma. J Cancer Res Clin Oncol.
130:187–196. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Tanaka M, Katayama F, Kato H, Tanaka H,
Wang J, Qiao YL and Inoue M: Hepatitis B and C virus infection and
hepatocellular carcinoma in China: A review of epidemiology and
control measures. J Epidemiol. 21:401–416. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Forner A, Llovet JM and Bruix J:
Hepatocellular carcinoma. Lancet. 379:1245–1255. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Singhal A, Jayaraman M, Dhanasekaran DN
and Kohli V: Molecular and serum markers in hepatocellular
carcinoma: Predictive tools for prognosis and recurrence. Crit Rev
Oncol Hematol. 82:116–140. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Feng YX, Wang T, Deng YZ, Yang P, Li JJ,
Guan DX, Yao F, Zhu YQ, Qin Y, Wang H, et al: Sorafenib suppresses
postsurgical recurrence and metastasis of hepatocellular carcinoma
in an orthotopic mouse model. Hepatology. 53:483–492. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Wu KT, Wang CC, Lu LG, Zhang WD, Zhang FJ,
Shi F and Li CX: Hepatocellular carcinoma: Clinical study of
long-term survival and choice of treatment modalities. World J
Gastroenterol. 19:3649–3657. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Im E, von Lintig FC, Chen J, Zhuang S, Qui
W, Chowdhury S, Worley PF, Boss GR and Pilz RB: Rheb is in a high
activation state and inhibits B-Raf kinase in mammalian cells.
Oncogene. 21:6356–6365. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Aspuria PJ and Tamanoi F: The Rheb family
of GTP-binding proteins. Cell Signal. 16:1105–1112. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Inoki K, Li Y, Xu T and Guan KL: Rheb
GTPase is a direct target of TSC2 GAP activity and regulates mTOR
signaling. Genes Dev. 17:1829–1834. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Lu ZH, Shvartsman MB, Lee AY, Shao JM,
Murray MM, Kladney RD, Fan D, Krajewski S, Chiang GG, Mills GB and
Arbeit JM: Mammalian target of rapamycin activator RHEB is
frequently overexpressed in human carcinomas and is critical and
sufficient for skin epithelial carcinogenesis. Cancer Res.
70:3287–3298. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kobayashi T, Shimizu Y, Terada N, Yamasaki
T, Nakamura E, Toda Y, Nishiyama H, Kamoto T, Ogawa O and Inoue T:
Regulation of androgen receptor transactivity and mTOR-S6 kinase
pathway by Rheb in prostate cancer cell proliferation. Prostate.
70:866–874. 2010.PubMed/NCBI
|
|
16
|
Wazir U, Newbold RF, Jiang WG, Sharma AK
and Mokbel K: Prognostic and therapeutic implications of mTORC1 and
Rictor expression in human breast cancer. Oncol Rep. 29:1969–1974.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Avruch J, Hara K, Lin Y, Liu M, Long X,
Ortiz-Vega S and Yonezawa K: Insulin and amino-acid regulation of
mTOR signaling and kinase activity through the Rheb GTPase.
Oncogene. 25:6361–6372. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wullschleger S, Loewith R and Hall MN: TOR
signaling in growth and metabolism. Cell. 124:471–484. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Guertin DA and Sabatini DM: Defining the
role of mTOR in cancer. Cancer Cell. 12:9–22. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Saucedo LJ, Gao X, Chiarelli DA, Li L, Pan
D and Edgar BA: Rheb promotes cell growth as a component of the
insulin/TOR signalling network. Nat Cell Biol. 5:566–571. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Inoue T, Yoshida T, Shimizu Y, Kobayashi
T, Yamasaki T, Toda Y, Segawa T, Kamoto T, Nakamura E and Ogawa O:
Requirement of androgen-dependent activation of protein kinase
Czeta for androgen-dependent cell proliferation in LNCaP Cells and
its roles in transition to androgen-independent cells. Mol
Endocrinol. 20:3053–3069. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Stocker H, Radimerski T, Schindelholz B,
Wittwer F, Belawat P, Daram P, Breuer S, Thomas G and Hafen E: Rheb
is an essential regulator of S6K in controlling cell growth in
Drosophila. Nat Cell Biol. 5:559–565. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Hay N and Sonenberg N: Upstream and
downstream of mTOR. Genes Dev. 18:1926–1945. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Mavrakis KJ, Zhu H, Silva RL, Mills JR,
Teruya-Feldstein J, Lowe SW, Tam W, Pelletier J and Wendel HG:
Tumorigenic activity and therapeutic inhibition of Rheb GTPase.
Genes Dev. 22:2178–2188. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Wen ZH, Su YC, Lai PL, Zhang Y, Xu YF,
Zhao A, Yao GY, Jia CH, Lin J, Xu S, et al: Critical role of
arachidonic acid-activated mTOR signaling in breast carcinogenesis
and angiogenesis. Oncogene. 32:160–170. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Zheng H, Liu A, Liu B, Li M, Yu H and Luo
X: Ras homologue enriched in brain is a critical target of
farnesyltransferase inhibitors in non-small cell lung cancer cells.
Cancer Lett. 297:117–125. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Mazhab-Jafari MT, Marshall CB, Ho J,
Ishiyama N, Stambolic V and Ikura M: Structure-guided mutation of
the conserved G3-box glycine in Rheb generates a constitutively
activated regulator of mammalian target of rapamycin (mTOR). J Biol
Chem. 289:12195–12201. 2014. View Article : Google Scholar : PubMed/NCBI
|