Introduction
Colorectal cancer (CRC) is the 3rd most commonly
diagnosed cancer and the fourth leading cause of death worldwide
(1). The routine prognostic factors
for patient survival are histological grade and tumor staging
(1,2),
which is depending on the depth of tumor invasion, involvement of
regional lymph nodes, and metastatic spread to other organs
(3). Distinct molecular drivers and
prognosis may be implicated in the same stage of CRC patients.
Therefore, it is imperative to better understand the carcinogenic
and molecular markers of CRC and to identify new therapeutic
targets for the treatment of this disease.
The flavonoid monooxygenase family (FMO) includes a
large variety of endogenous and exogenous substrates (4). FMO-catalyzed oxygenation usually
produces polar and relatively stable, nontoxic products that are
easily excreted from the body, allowing FMOs to become an important
part of metabolic detoxification (5).
Flavin-containing monooxygenase 5 (FMO5) is classified as an
oxidoreductase, and it is possible that its effects are mediated
through regulation of the cellular redox state. The ability of FMO5
to catalyze a Baeyer-Villiger oxidation reaction was demonstrated
in a study of an anticancer therapeutic (6). Bièche et al found that
overexpression of FMO5 significantly associated with longer
relapse-free survival (RFS) among postmenopausal patients with
breast carcinoma (7). Miller et
al showed that the overexpression of FMO5 upregulated the
expression of progesterone receptor B; Consequently, progesterone
enhanced the carcinogenicity of tamoxifen in breast cancer
(8). However, the roles of FMO5 in
CRC remain to be explored and discussed.
This study aimed to detect the expression of FMO5 in
CRC and investigate the correlations between FMO5 expression and
clinicopathological characteristics, including prognosis.
Materials and methods
Tissue samples
Tissue microarray (TMA, no. CO2161) was purchased
from Alenabio Biotechnology Co., Ltd. (Xian, China), which was
constructed from 208 histologically confirmed CRC samples and 8
normal colon tissues. The clinical information of these samples was
collected. All human tissues were collected under IRB and HIPPA
approved protocols. All samples had tested negative for HIV and
hepatitis B and were approved for commercial product development.
In addition, we used a publically available dataset: The Cancer
Genome Atlas (TCGA) dataset with 192 primary CRC tissues and mRNA
sequences.
Immunohistochemistry analysis
Dako Envision Systems (Dako Diagnostics AG, Zug,
Switzerland) were applied for immunohistochemistry (IHC) analysis.
In short, TMA specimens were blocked with proteolytic digestion and
using peroxidase, followed by incubation with 1:50 anti-FMO5
monoclonal antibody (ab189516; Abcam, Cambridge, MA, USA) at 4°C
overnight. After washing in PBS, peroxidase-labeled antibodies and
substrate chromogen applied to visualize the staining of the target
proteins.
Evaluation of immunostaining
TMA slides were scanned using a ScanScope and
analyzed using ImageScope v11 software (Aperio Technologies, Vista,
CA, USA). Immunostaining was scored by two independent experienced
pathologists, who were blinded to the clinicopathological data and
clinical outcomes of the patients. Antigen expression was evaluated
in a semi-quantitative manner. Each specimen was assessed for
staining intensity as follows: Non-significant brown, weak brown,
moderate brown, and strong brown staining intensities were scored
as 0, 1, 2, and 3, respectively. The percentage of immunoreactive
cells was rated as follows: 1 points, <25%; 2 point, 25–50%; 3
points, 50–75%; 4 points, >75%. The immunoreactivity score (IRS)
was determined by multiplying intensity and and extent of
positivity scores of stained cells. The final scores from the
pathologists were compared, and any inconsistencies were were
resolved by discussion between these two pathologists. The samples
were classified as high (based on an IRS value >4) and low
(based on an IRS value ≤4) levels per FMO5 protein expression (Wang
et al, 2013).
Statistical analysis
Statistical analyses were performed by means of SPSS
21.0 software (SPSS, Inc., Chicago, IL, USA). Statistical analysis
was performed using Pearson's Chi-squared and Fisher's exact tests
to compare the relativity of FMO5 expression with
clinicopathological characteristics. The Kaplan-Meier method was
used to calculate the actual survival rate and to plot survival
curves, followed by the log-rank test for clinical and histological
variables. Univariate and multivariate Cox proportional hazards
models were performed to obtain prognostic factors and
corresponding hazard ratios (HRs) for different factors with 95%
confidence intervals (CIs). P<0.05 was considered to indicate a
statistically significant difference.
Results
High FMO5 protein was associated with
aggressive CRC
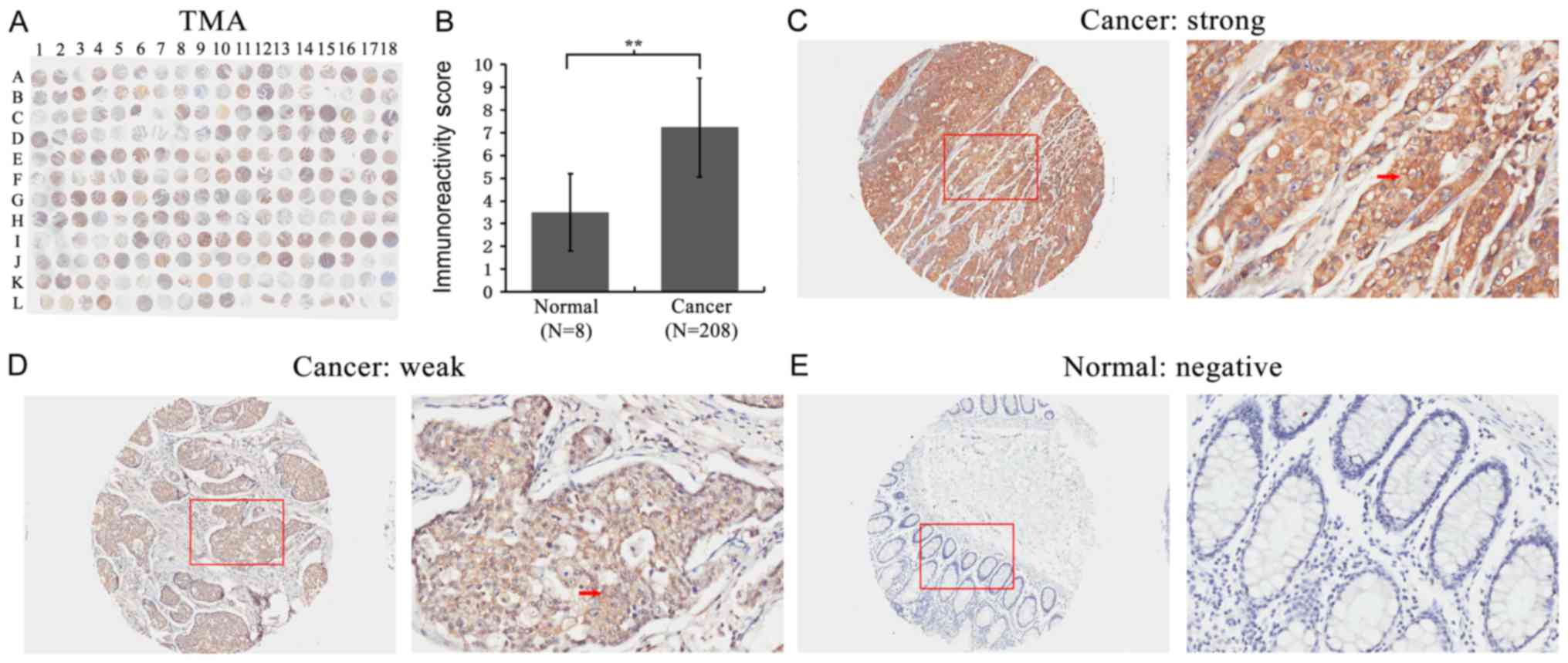
First, FMO5 expression was detected in a TMA using
IHC staining (Fig. 1A). We found that
the expression of FMO5 significantly increased in CRC compared to
that in normal colon tissues (P<0.001) (Fig. 1B). The staining of FMO5 was localized
within the cytoplasm of colorectal and normal glandular epithelial
cells (Fig. 1C-E).
The study further analyzed the correlation between
FMO5 expression and clinical or pathological characteristics of the
tissues in patients with CRC. Table I
showed that the high FMO5 protein expression was associated with
older age (P=0.015), advanced clinical stage (P=0.018) and lymph
node metastasis (P=0.030). However, no relationship was observed
between FMO5 expression and other clinical features, such as
gender, pathological grade, tumor invasion, and distant metastasis
(all P>0.05). These findings indicated that high levels of FMO5
expression correlated with aggressive clinical features in CRC.
 | Table I.Correlation of FMO5 expression with
clinico-pathological characteristics of colon cancer. |
Table I.
Correlation of FMO5 expression with
clinico-pathological characteristics of colon cancer.
|
| TMA | TCGA |
|---|
|
|
|
|
|---|
| Clinical
features | Case | Low, n (%) | High, n (%) | P-value | Case | Mean ± SD | P-value |
|---|
| Tissue |
|
|
|
|
|
|
|
|
Cancer | 208 | 44 (21.2) | 164 (78.8) | <0.001 | 192 | 297.70±218.89 | – |
|
Normal |
8 | 7
(87.5) |
1 (12.5) |
| – |
|
|
| Age (years) |
|
|
|
|
|
|
|
|
<25 |
2 | 10 (37.0) | 17
(63.0) | 0.015 |
0 | – | 0.529 |
|
25–40 | 27 | 23 (22.6) | 70
(77.4) |
|
3 | 204.00±26.47 |
|
|
41–60 | 93 | 10 (11.6) | 76
(88.4) |
| 38 | 52.36±205.20 |
|
|
>60 | 86 | 10 (37.0) | 17
(63.0) |
| 151 | 306.00±224.02 |
|
| Sex |
|
|
|
|
|
|
|
| Male | 118 | 23 (19.5) | 95
(80.5) | 0.501 | 94 | 288.88±213.95 | 0.586 |
|
Female | 90 | 21 (23.3) | 69
(76.7) |
| 98 | 306.16±224.30 |
|
| Pathological
grade |
|
|
|
|
|
|
|
| ≤2 | 150 | 26 (17.3) | 124 (82.7) | 0.090 | – | – | – |
|
>2 | 41 | 12 (29.3) | 29
(70.7) | – | – | – |
|
| Clinical stage |
|
|
|
|
|
|
|
| I–II | 150 | 38 (25.3) | 112 (74.7) | 0.018 | 108 | 326.62±246.68 | 0.047 |
|
III–IV | 58 | 6
(10.3) | 52
(89.7) |
| 81 | 262.37±173.24 |
|
| Tumor invasion |
|
|
|
|
|
|
|
|
T1-T2 | 23 | 4
(17.4) | 19
(82.6) | 0.790 | 172 | 298.73±256.84 | 0.988 |
|
T3-T4 | 185 | 40 (21.6) | 145 (78.4) |
|
4 | 298.15±208.42 |
|
| Lymph node
metastasis |
|
|
|
|
|
|
|
| N0 | 153 | 38 (24.8) | 115 (75.2) | 0.030 | 114 | 324.77±242.47 | 0.045 |
|
N1–2 | 55 | 6
(10.9) | 49
(89.1) |
| 77 | 260.16±173.12 |
|
| Distant
metastasis |
|
|
|
|
|
|
|
| M0 | 197 | 44 (22.3) | 153 (77.7) | 0.125 | 158 | 313.67±230.88 | 0.030 |
| M1 | 11 | 0
(0.0) |
11 (100.0) |
| 30 | 218.50±131.43 |
|
Overexpression of FMO5 mRNA was
associated with aggressive CRC in TCGA
FMO5 mRNA expression data of 192 primary CRC
patients from TCGA was used to validate the findings of TMA. As
shown in Table I, FMO5 was
upregulated in CRC with advanced clinical stage (P=0.047), lymph
node metastasis (P=0.045), and distant metastasis (P=0.030).
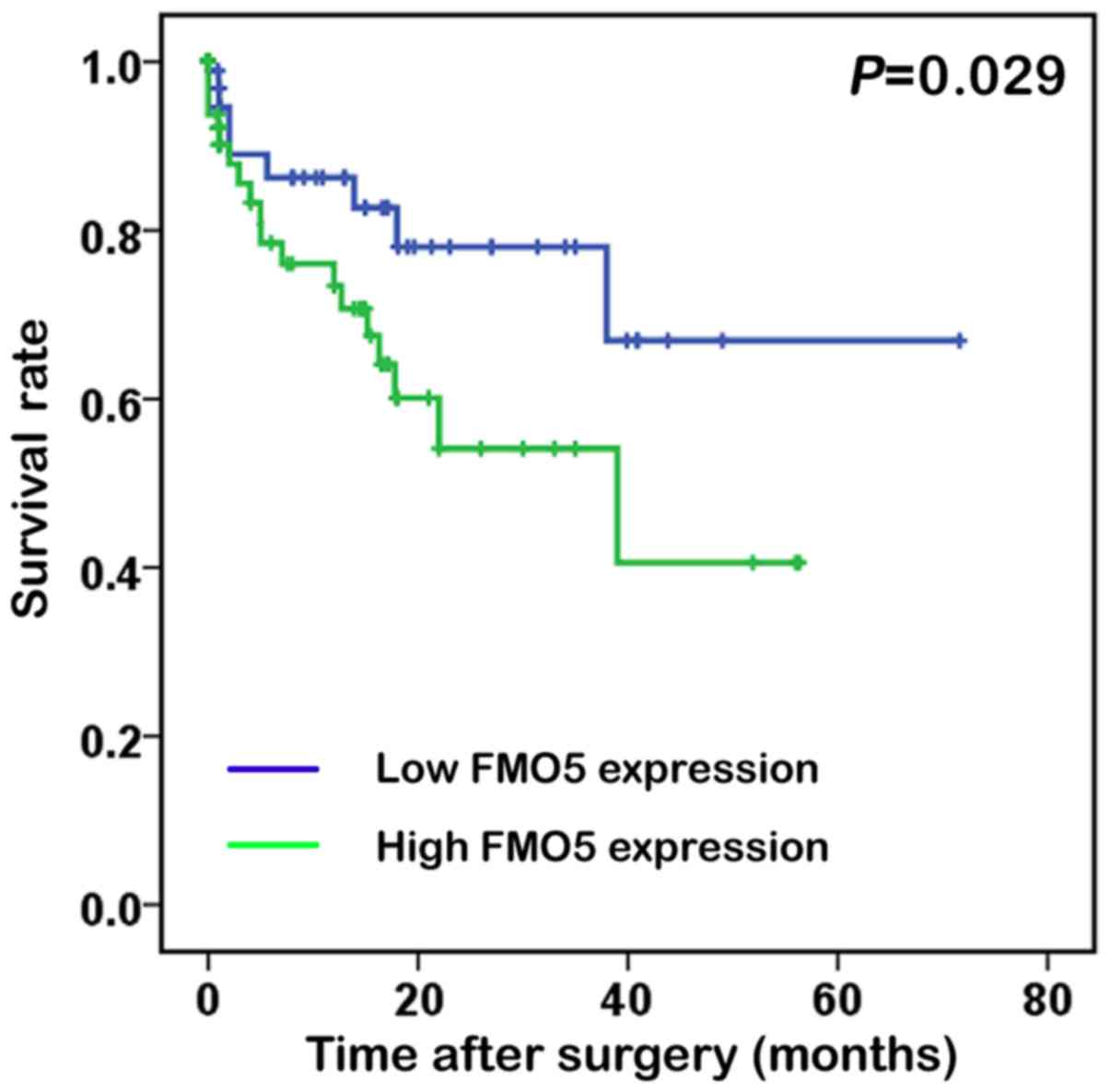
To reveal whether high FMO5 mRNA expression was a
prognostic factor in patients with CRC, the Kaplan-Meier survival
analysis was performed and K-M curve was plotted for groups
classified by high and low FMO5 expression levels (Fig. 2). The mean FMO5 mRNA level in the TCGA
dataset was used as a cutoff to segregate patients into high- and
low-FMO5 expression groups (9).
Interestingly, the overall survival after surgery in the high-FMO5
expression groups were 7.81±12.5 months while in the low-FMO5
expression groups were 8.21±14.1 months. Actually, high FMO5
expression in CRC patients indicated a shorter survival compared
with those having low expression (log rank=4.776; P=0.029).
FMO5 was an independent prognostic
factor for the survival of patients with CRC
The study analyzed whether FMO5 expression could
predict the prognosis of patients with CRC. Univariate analysis
revealed that FMO5 could serve as a valuable prognostic factor for
the overall survival rate of patients with CRC (HR, 2.326; 95% CI,
1.062–5.091; P=0.035). Multivariate analysis further revealed that
high FMO5 expression served as independent prognostic factor for
the overall survival rate of patients with CRC (HR, 2.865; 95% CI,
1.116–7.355; P=0.029), as shown in Table
II.
 | Table II.Prognostic value of FMO5 expression
in OS. |
Table II.
Prognostic value of FMO5 expression
in OS.
|
| OS |
|---|
|
|
|
|---|
| Variables | HR (95% CI) | P-value |
|---|
| Univariate
analysis |
|
|
| Sex
(male vs. female) | 0.722
(0.294–1.770) | 0.476 |
| Age,
years (≥60 vs. <60) | 2.698
(0.625–11.652) | 0.184 |
|
Clinical stage (I–II vs.
III–IV) | 1.809
(0.735–4.453) | 0.197 |
| T stage
(T1-2 vs. T3-4) | 2.408
(0.555–10.437) | 0.240 |
| N stage
(N0 vs. N1-2) | 1.633
(0.674–3.956) | 0.278 |
| M stage
(M0 vs. M1) | 2.706
(1.023–7.162) | 0.045 |
| FMO5
expression (low vs. high) | 2.326
(1.062–5.091) | 0.035 |
| Multivariate
analysis |
|
|
| Sex
(male vs. female) | 0.573
(0.231–1.426) | 0.231 |
| Age,
years (≥60 vs. <60) | 2.451
(0.710–8.464) | 0.156 |
| FMO5
expression (low vs. high) | 2.865
(1.116–7.355) | 0.029 |
Discussion
Research has helped us accumulate extensive
knowledge about FMOs since they were founded by Ziegler in the
early 1970s (10). FMOs family
consists of a group of important drug-metabolizing enzymes, which
catalyze oxidation reactions that are complementary to cytochrome
P450-mediated biotransformations (11). Among all the isoforms, FMO3 is the
primary enzyme in human hepatic metabolism and helps to the
metabolism of a variety of common drugs (12). However, recent studies have shown that
FMO5 mRNA is almost the same as FMO3 in adult liver and occupy the
leading position in the small intestine in humans (13). FMO5 is classified as an
oxidoreductase, whose effects are quite likely to be mediated by
means of adjusting the cellular redox state. Although highly
expression of FMO5 is found in the liver of mice (14) and humans (15), but very little is known about the
function of this protein. The knowledge on FMO5 substrates is
limited (16–18): Known catalysis of FMO5 are the
N-oxygenation of short-chain aliphatic primary amines such as
N-octylamine (19) and the
S-oxygenation of S-methyl-esonarimod, an active metabolite
of the antirheumatic esonarimod (17,20).
Sandra found that interindividual variation in FMO5 expression
(8,16,21,22) might
make contribution to diversity in fat deposits and plasma
cholesterol, and some therapeutic agents induced FMO5 expression
may have an adverse effect on the patient's metabolic health
(23,24). The development of CRC is known to be
closely related to diet, lifestyle, and metabolic syndrome.
However, the relationship between FMO5 and CRC remains unclear.
This study showed that FMO5 was primarily located in
the cytoplasm of CRC epithelial cells, and upregulated in CRC
tissues in comparison with that in normal colon tissues by a human
TMA containing 208 primary CRCs and 8 normal colon tissues.
Moreover, it was found that FMO5 upregulation was associated with
advanced clinical stage and lymph node metastasis. Further, these
detections were acknowledged in the TCGA dataset, in which
significant correlations between FMO5 overexpression and advanced
clinical stage, lymph node involvement and distant metastasis were
procured. However, no association was found between high FMO5
protein expression and distant metastasis by TMA, which may due to
the small sample size as there were only 11 M1 patients. In
general, these findings strongly recommended that FMO5 was
associated with CRC progression.
Moreover, the correlation between FMO5 mRNA
expression and overall survivals of patients were analyzed. The
results indicated that patients with high FMO5 expression were
found to have a shorter overall survival (P=0.029). The Cox
proportional hazards model further showed that overexpression of
FMO5 was an independent prognostic factor of poor prognosis for
patients with CRC.
The expression of FMO5 should be compared among
normal, benign and cancer tissues, which will do help to better and
further explore the roles of FMO5 in CRC. And we also expect to
investigate the correlations of FMO5 with other putative markers
like Kras, p53, Braf, TS. However, these data were unavailable, and
we could not investigate these features and relationships. In our
future study, we will further investigate such aspects and the
molecular characteristics of FMO5.
In conclusion, high FMO5 was associated with
aggressive CRC, and may predict shorter survival for patients with
CRC. The molecular activities of FMO5 in CRC still require further
investigation.
Acknowledgements
This study was supported by the grants from National
Natural Science Foundation of China (81272556), Science and
Technology Project of Guangdong Province (2014A020212614) and
Science and Technology Program of Guangzhou, China
(2014Y2-00137).
Glossary
Abbreviations
Abbreviations:
|
FMO5
|
flavin-containing monooxygenase 5
|
|
CRC
|
colorectal cancer
|
|
TMA
|
tissue microarray
|
|
TCGA
|
The Cancer Genome Atlas
|
References
|
1
|
Global Burden of Disease Cancer
Collaboration, ; Fitzmaurice C, Dicker D, Pain A, Hamavid H,
Moradi-Lakeh M, MacIntyre MF, Allen C, Hansen G, Woodbrook R, et
al: The global burden of cancer 2013. JAMA Oncol. 1:505–527. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Eyking A, Reis H, Frank M, Gerken G,
Schmid KW and Cario E: miR-205 and miR-373 are associated with
aggressive human mucinous colorectal cancer. PLoS One.
11:e01568712016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Compton CC: Colorectal carcinoma:
Diagnostic, prognostic, and molecular features. Mod Pathol.
16:376–388. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Lai WG, Farah N, Moniz GA and Wong YN: A
Baeyer-Villiger oxidation specifically catalyzed by human
flavin-containing monooxygenase 5. Drug Metab Dispos. 39:61–70.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Siddens LK, Krueger SK, Henderson MC and
Williams DE: Mammalian flavin-containing monooxygenase (FMO) as a
source of hydrogen peroxide. Biochem Pharmacol. 89:141–147. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Henderson MC, Siddens LK, Krueger SK,
Stevens JF, Kedzie K, Fang WK, Heidelbaugh T, Nguyen P, Chow K,
Garst M, et al: Flavin-containing monooxygenase S-oxygenation of a
series of thioureas and thiones. Toxicol Appl Pharmacol. 278:91–99.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Biéche I, Girault I, Urbain E, Tozlu S and
Lidereau R: Relationship between intratumoral expression of genes
coding for xenobiotic-metabolizing enzymes and benefit from
adjuvant tamoxifen in estrogen receptor alpha-positive
postmenopausal breast carcinoma. Breast Cancer Res. 6:R252–R263.
2004. View
Article : Google Scholar : PubMed/NCBI
|
|
8
|
Miller MM, James RA, Richer JK, Gordon DF,
Wood WM and Horwitz KB: Progesterone regulated expression of
flavin-containing monooxygenase 5 by the B-isoform of progesterone
receptors: Implications for tamoxifen carcinogenicity. J Clin
Endocrinol Metab. 82:2956–2961. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Wang GH, Yao L, Xu HW, Tang WT, Fu JH, Hu
XF, Cui L and Xu XM: Identification of MXRA5 as a novel biomarker
in colorectal cancer. Oncol Lett. 5:544–548. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Cai C, Chen JY, Han ZD, He HC, Chen JH,
Chen YR, Yang SB, Wu YD, Zeng YR, Zou J, et al: Down-regulation of
dual-specificity phosphatase 5 predicts poor prognosis of patients
with prostate cancer. Int J Clin Exp Med. 8:4186–4194.
2015.PubMed/NCBI
|
|
11
|
Pettit FH, Orme-Johnson W and Ziegler DM:
The requirement for flavin adenine dinucleotide by a liver
microsmal oxygenase catalyzing the oxidation of alkylaryl amines.
Biochem Biophys Res Commun. 16:444–448. 1964. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Uetrecht J and Trager W: Drug metabolism
chemical and enzymatic aspects. New York: Crc Press; 2007,
View Article : Google Scholar
|
|
13
|
Zhang J and Cashman JR: Quantitative
analysis of FMO gene mRNA levels in human tissues. Drug Metab
Dispos. 34:19–26. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Krueger SK and Williams DE: Mammalian
flavin-containing monooxygenases: Structure/function, genetic
polymorphisms and role in drug metabolism. Pharmacol Ther.
106:357–387. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Janmohamed A, Hernandez D, Phillips IR and
Shephard EA: Cell-, tissue-, sex- and developmental stage-specific
expression of mouse flavin-containing monooxygenases (Fmos).
Biochem Pharmacol. 68:73–83. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Cashman JR and Zhang J: Human
flavin-containing monooxygenases. Annu Rev Pharmacol Toxicol.
46:65–100. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Gonzalez Malagon SG, Melidoni AN,
Hernandez D, Omar BA, Houseman L, Veeravalli S, Scott F, Varshavi
D, Everett J, Tsuchiya Y, et al: The phenotype of a knockout mouse
identifies flavin-containing monooxygenase 5 (FMO5) as a regulator
of metabolic ageing. Biochem Pharmacol. 96:267–277. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zhang J, Cerny MA, Lawson M, Mosadeghi R
and Cashman JR: Functional activity of the mouse flavin-containing
monooxygenase forms 1, 3 and 5. J Biochem Mol Toxicol. 21:206–215.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Motika MS, Zhang J, Ralph EC, Dwyer MA and
Cashman JR: pH dependence on functional activity of human and mouse
flavin-containing monooxygenase 5. Biochem Pharmacol. 83:962–968.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Lang DH, Yeung CK, Peter RM, Ibarra C,
Gasser R, Itagaki K, Philpot RM and Rettie AE: Isoform specificity
of trimethylamine N-oxygenation by human flavin-containing
monooxygenase (FMO) and P450 enzymes: Selective catalysis by FMO3.
Biochem Pharmacol. 56:1005–1012. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Ohmi N, Yoshida H, Endo H, Hasegawa M,
Akimoto M and Higuchi S: S-oxidation of S-methyl-esonarimod by
flavin-containing monooxygenases in human liver microsomes.
Xenobiotica. 33:1221–1231. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Rae JM, Johnson MD, Lippman ME and
Flockhart DA: Rifampin is a selective, pleiotropic inducer of drug
metabolism genes in human hepatocytes: Studies with cDNA and
oligonucleotide expression arrays. J Pharmacol Exp Ther.
299:849–857. 2001.PubMed/NCBI
|
|
23
|
Krusekopf S and Roots I: St. John's wort
and its constituent hyperforin concordantly regulate expression of
genes encoding enzymes involved in basic cellular pathways.
Pharmacogenet Genomics. 15:817–829. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Overby LH, Carver GC and Philpot RM:
Quantitation and kinetic properties of hepatic microsomal and
recombinant flavin-containing monooxygenases 3 and 5 from humans.
Chem Biol Interact. 106:29–45. 1997. View Article : Google Scholar : PubMed/NCBI
|