Introduction
Acute leukemia (AL) is the most common type of
malignancy in children and adolescents; it includes B-cell acute
lymphoblastic leukemia (B-ALL), T-cell ALL (T-ALL) and acute
myeloid leukemia, and comprises 25–35% of all childhood cancer
cases (1). The peak prevalence of AL
incidence occurs between 2 and 5 years of age (1). B-ALL is the most common type of acute
leukemia in children but, owing to risk stratification and improved
supportive care, the survival rate of patients diagnosed with
pediatric B-ALL has improved from 60 to 90% between 2000–2005
(2,3).
However, the intensive and long-term cytotoxic treatment for B-ALL
is inevitably accompanied by a large number of adverse effects.
Identification of novel prognostic markers and therapeutic targets
would assist in minimizing these effects by providing personalized
treatment.
Aberrant purine synthesis leads to significant
pathological defects in humans (4–8), thus the
de novo purine biosynthesis pathway is a target for
currently available cancer chemotherapy agents with an increasing
number of novel agents continuously being developed. The
phosphoribosyl pyrophosphate synthetase 1 gene (PRPS1), which
encodes a rate-limiting purine biosynthesis enzyme, has been
demonstrated to be associated with gouty arthritis, Arts syndrome,
Charcot-Marie-Tooth disease 5 and hearing loss (9–14).
However, a previous study indicated that PRPS1 also serves an
important role in ALL relapse by reducing the feedback inhibition
of de novo purine biosynthesis and competitive inhibition of
thiopurine activation (15). All
patients with ALL in the present study who harbored certain PRPS1
mutations relapsed early during treatment, and the number of ALL
clones expressing this mutant protein exponentially expanded prior
to clinical relapse.
To analyze the role of PRPS1 in pediatric B-cell
acute lymphoblastic leukemia further, the expression of PRPS1 was
measured in biopsy samples from children with B-ALL; it was
revealed that elevated expression of PRPS1 was able to predict an
adverse prognosis. Furthermore, the overexpression of PRPS1 in the
B-ALL Sup-B15 and Raji cell lines induced B-cell lymphoma 2 (Bcl-2)
overexpression, and a subsequent anti-apoptotic effect in these
cells. These results indicate that PRPS1 is a potential therapeutic
target for the treatment of B-ALL.
Materials and methods
Clinical samples and data
This study enrolled 71 pediatric patients who were
newly diagnosed with B-ALL between March 2012 and November 2015 at
the Children's Hospital of Chongqing Medical University (Chongqing,
China). Among all patients, there were 42 males and 29 females with
a median age of 50 months (4 years 2 months) and ranged from 8
months to 17 years and 2 months. The diagnosis of AL was performed
using a standard French-American-British morphological analysis
(16) and the morphology, immunology,
cytogenetics and molecular biology criteria from the World Health
Organization Classification of Tumors of Hematopoietic and Lymphoid
tissues in 2008 (17), and the
immunological phenotype was determined for all patients. For all
patients enrolled in this study, the risk stratification and
treatment options followed the Children's Cancer & Leukemia
Group-ALL2008 therapy guidelines (18). All the patients enrolled in this study
were categorized into 3 risk groups: Standard risk, intermediate
risk and high risk. They were categorized after the induction
chemotherapy following the risk stratification standard in this
guideline considering the risk factors at diagnosis, including
peripheral white blood cell count, age, specific recurrent
chromosomal abnormalities, and the response to initial therapy.
There were 31 patients who achieved complete remission from AL and
were enrolled as the remission group. Concurrently, 21 sex- and
age-matched patients without a malignant hematological disorder
were enrolled as a control group in the present study. The details
of the included subjects are listed in Table I. The present study was approved by
the Ethics Committee of Children's Hospital of Chongqing Medical
University and followed all principles set by the Declaration of
Helsinki. Informed consent was obtained from the parents or
guardians of all participants. The basic information of all groups
of patients is shown in Table I.
 | Table I.Details of patient samples in the
present study. |
Table I.
Details of patient samples in the
present study.
| Disease | Patients, no. | Age, years
(range) | Sex,
male/female |
|---|
| B-ALL | 71 | 4.0 (2.0–9.0) | 42/29 |
| B-ALL-CR | 31 | 3.2 (2.2–8.7) | 20/13 |
| Control | 21 | 6.2
(2.4-12-8) |
9/12 |
Bone marrow (BM) samples (1–2 ml) of patients in
each group were collected and treated with EDTA anticoagulant upon
preliminary diagnosis, and following the achievement of complete
remission. Mononuclear cells from the BM (BMMNC) were isolated by
applying a density gradient centrifugation over Ficoll solution,
later identified via light microscopy, and stored at −80°C until
further use.
Cell culture
The two human acute B-ALL cell lines (Sup-B15 and
Raji, ATCC, Manassas, VA, USA) used in this study were obtained
from the Ministry of Education Key Laboratory of Child Development
and Disorders (Chongqing, China). Sup-B15 and Raji cells were
cultured in RPMI-1640 supplemented with 10% heat-inactivated fetal
bovine serum (both from Gibco; Thermo Fisher Scientific, Inc.,
Waltham, MA, USA) and 1 ml glutamine, and were maintained at 37°C
in a humidified atmosphere containing 5% CO2. The cells
were grown in suspension, the medium was changed every 2–3 days and
the experiments were conducted with cells in the logarithmic growth
phase.
Lentivirus infection
Lentivirus plasmids, used for the overexpression of
PRPS1, and a negative control plasmid were purchased from Suzhou
GenePharma Co., Ltd. (Suzhou, China). The cDNA sequence used for
PRPS1 expression was matched to RefSeq NM_002764.3, from the
National Center for Biotechnology Information GenBank (19). The viral titer was ~1×109
transducing units in 1 ml RPMI-1640 medium. Prior to infection,
3×106 Sup-B15 or Raji cells/ml were cultured in
RPMI-1640 medium containing no serum. The cells were divided into
two groups: The control group (infected with lentivirus produced
from transfection with empty vector) and the PRPS1+ group (infected
with lentivirus produced from transfection of the
PRPS1-overexpressing vector). Once the cells were infected at
multiplicity of infection of 3, they were cultured at 37°C in 5%
CO2. The mRNA and protein expression levels of PRPS1
were identified by reverse transcription-quantitative polymerase
chain reaction (RT-qPCR) and western blotting, respectively.
Cell counting Kit-8 (CCK-8) cell
growth assay
Sup-B15 and Raji cells were cultured at a density of
5×103 cells/well in 96-well microtiter plates, and their
proliferative abilities were evaluated at 0, 24, 48 and 96 h using
CCK-8 (Nanjing KeyGen Biotech Co., Ltd., Nanjing, China), according
to the manufacturer's protocol. Once the cells had been treated
with CCK-8 at 37°C for 1 h, the absorbance at 450 nm was measured
in each well using a microplate reader. All assays were repeated
three times in parallel.
Flow cytometry analysis of cell
apoptosis
The apoptotic rate was measured via flow cytometry
using a FACSCalibur flow cytometer (BD Biosciences, Franklin Lakes,
NJ, USA) with the Annexin V-Allophycocyanin
(APC)/7-aminoactinomycin (7AAD) Apoptosis kit (Nanjing KeyGen
Biotech Co., Ltd., Nanjing China), following the manufacturer's
protocol. The three aforementioned groups of Sup-B15 and Raji cells
were harvested, and washed twice with ice-cold PBS. A total of
1×106 cells/condition were resuspended in 500 µl 1X
binding buffer, incubated with 5 µl annexin V-APC and 5 µl 7AAD
solution for 10 min at room temperature in the dark. The prepared
samples were sorted using a flow cytometer and were analyzed by BD
FACSDiva software v8.0.1 (BD Biosciences). All assays were repeated
three times in parallel.
RT-qPCR
The expression of PRPS1 in BMMNCs from patients with
either B-ALL or non-malignant hematological disease, as well as the
expression of PRPS1, MYC proto-oncogene, bHLH transcription factor
(c-Myc), cyclin E1, Bcl-2 and cyclin-dependent kinase 2 (CDK2) in
Sup-B15 and Raji cells, were analyzed by RT-qPCR. Total RNA from
~1×106 cells was extracted by using TRIzol (Invitrogen;
Thermo Fisher Scientific, Inc.) following the manufacturer's
protocol. Reverse transcription was performed using the
PrimeScript™ RT reagent kit. The primer sequences for PCR, designed
based on cDNA sequence from GenBank database (https://www.ncbi.nlm.nih.gov/genbank/),
are listed in Table II, with the
β-actin gene used as an endogenous control. Quantitect
SYBR® Green RT-PCR kit (Qiagen GmbH, Hilden, Germany)
was employed for the amplification reactions using the Applied
Biosystems® StepOnePlus™ Real-time PCR system (Applied
Biosystems; Thermo Fisher Scientific, Inc.) under following
condition: 3 min pre-denaturation at 95°C, followed by 40 cycles of
denaturation at 95°C for 10 sec, annealing and extension at 60°C
for 30 sec. The Cq value of each sample was obtained via the PCR
system. The data were analyzed using the 2−ΔΔCq relative
quantity algorithm (20). Each assay
was repeated three times in parallel.
 | Table II.List of primers for reverse
transcription-quantitative polymerase chain reaction. |
Table II.
List of primers for reverse
transcription-quantitative polymerase chain reaction.
| Gene | Sequences | Template | Size, bp |
|---|
| PRPS1 |
| NM_001204402.1 | 127 |
|
Forward |
5′-GATGGCATAAACTCTGGTGGC-3′ |
|
|
|
Reverse |
5′-GGTGCTTGTGGGAGATGTGAA-3′ |
|
|
| c-Myc |
| NM_002467.4 | 180 |
|
Forward |
5′-CATTCTCTGCTCTCCTCGAC-3′ |
|
|
|
Reverse |
5′-TCCAGACTCTGACCTTTGC-3′ |
|
|
| Cyclin E1 |
| NM_001238.3 | 359 |
|
Forward |
5′-CTGGATGTTGACTGCCTTGA-3′ |
|
|
|
Reverse |
5′-CCGCTGCTCTGCTTCTTAC-3′ |
|
|
| CDK2 |
| NM_001798.4 | 395 |
|
Forward |
5′-CCTTGTTTGTCCCTTCTAC-3′ |
|
|
|
Reverse |
5′-CAAATCCACCCACTATGA-3′ |
|
|
| Bcl-2 |
| NM_000633.2 | 119 |
|
Forward |
5′-CTGCACCTGACGCCCTTCACC-3′ |
|
|
|
Reverse |
5′-CACATGACCCCACCGAACTCAAAGA-3′ |
|
|
| β-actin |
| NM_001101.3 | 191 |
|
Forward |
5′-AAGATGACCCAGATCATGTTTGAGACC-3′ |
|
|
|
Reverse |
5′-GCCAGGTCCAGACGCAGGAT-3′ |
|
|
Western blot analysis
The antibodies used for western blotting, targeting
PRPS1 (cat. no. ab137577), c-Myc (cat. no. ab32072), CDK2 (cat. no.
ab32147), Bcl-2 (cat. no. ab32124), cyclin E1 (cat. no. ab33911)
and caspase-3 (cat. no. ab32042), were purchased from Abcam
(Cambridge, MA, USA) and the β-actin (cat. no. TA-09) antibody was
acquired from Santa Cruz Biotechnology, Inc. (Dallas, TX, USA). All
primary antibodies were diluted at a ratio of 1:1,000. Sup-B15 and
Raji cells were harvested, and extracted using
radioimmunoprecipitation assay buffer (Beyotime Institute of
Biotechnology, Haimen, China), proteinase inhibitor cocktail (cat.
no. P8340; Sigma-Aldrich; Merck KGaA, Darmstadt, Germany), PMSF (1
mM) and phosphatase inhibitor cocktail (cat. no. 04906845001; Roche
Diagnostics GmbH, Mannheim, Germany). The protein concentration was
measured using a bicinchoninic acid protein assay (Pierce; Thermo
Fisher Scientific, Inc.). Approximately 100 ng total protein/lane
was separated on 8% SDS-PAGE gels and were transferred onto
polyvinylidene fluoride membranes followed by blocking in 5%
skimmed dry milk at room temperature for 1 h. Next, the membranes
were incubated with primary antibodies overnight at 4°C. The
membranes were rinsed three times with TBS-T (Tris-buffered saline
with 0.1% Tween-20) and incubated with either horseradish
peroxidase (HRP)-conjugated goat anti-rabbit (cat. no. ab6721;
Abcam) or HRP-conjugated goat anti-mouse (cat. no. ab6789; Abcam)
IgG (H+L) secondary antibody at a dilution of 1:2,000 at room
temperature for 2 h. The blots were visualized using a Clarity Max™
Western ECL Substrate kit (cat. no. 1705062; Bio-Rad Laboratories,
Inc., Hercules, CA, USA), according to the manufacturer's protocol
and each assay was repeated three times in parallel. The amount of
protein in each band was quantified using Quantity One 4.6.2
software (Bio-Rad Laboratories, Inc., Hercules, CA, USA).
Immunohistochemical (IHC)
staining
BMMNCs smears were uniformly spread on poly-lysine
coated slides at 2×105 cells/slide, and were fixed with
4% paraformaldehyde at room temperature for 20 min prior to being
washed with PBS 3 times. The slides were incubated with 0.3%
tritron-100 at room temperature for 30 min to permeabilize the
cells. Following washing with PBS for 3 times, the slides were
blocked with 10% bovine serum albumin at room temperature for a
further 30 min. Slides were then incubated with a rabbit anti-human
PRPS1 polyclonal antibody (dilution, 1:200; cat. no. ab137577;
Abcam) at 4°C overnight followed by a peroxidase-conjugated goat
anti-rabbit secondary antibody (dilution, 1:50; cat. no. TA140003;
OriGene Technologies, Inc., Rockville, MD, USA) at room temperature
for 30 min. The slides were then stained with diaminobenzidine at
room temperature for 2 min and counterstained with hematoxylin at
room temperature for 40 sec. Images were acquired using a light
microscope with Olympus BX51 system (Olympus Corporation, Tokyo,
Japan) at ×100 magnification.
Statistical analysis
Non-Gaussian numerical data were presented as the
median with 25th and 75th quartiles, whereas either normally
distributed data or data from parallel repeated experiments were
presented as the mean ± standard deviation. Statistical differences
in each assay were analyzed via SPSS 20.0 (IBM Corp., Armonk, NY,
USA). Significance was determined by applying an unpaired Student's
t-test for Gaussian-distributed data from two groups, the
Mann-Whitney test for data from two groups that did not follow
Gaussian distribution and one-way analysis of variance, followed by
the Bonferroni correction post hoc test, was used for data from
more than two groups. P<0.05 was considered to indicate a
statistically significant difference.
Results
Expression of PRPS1 in pediatric
patients with B-cell acute lymphoblastic leukemia
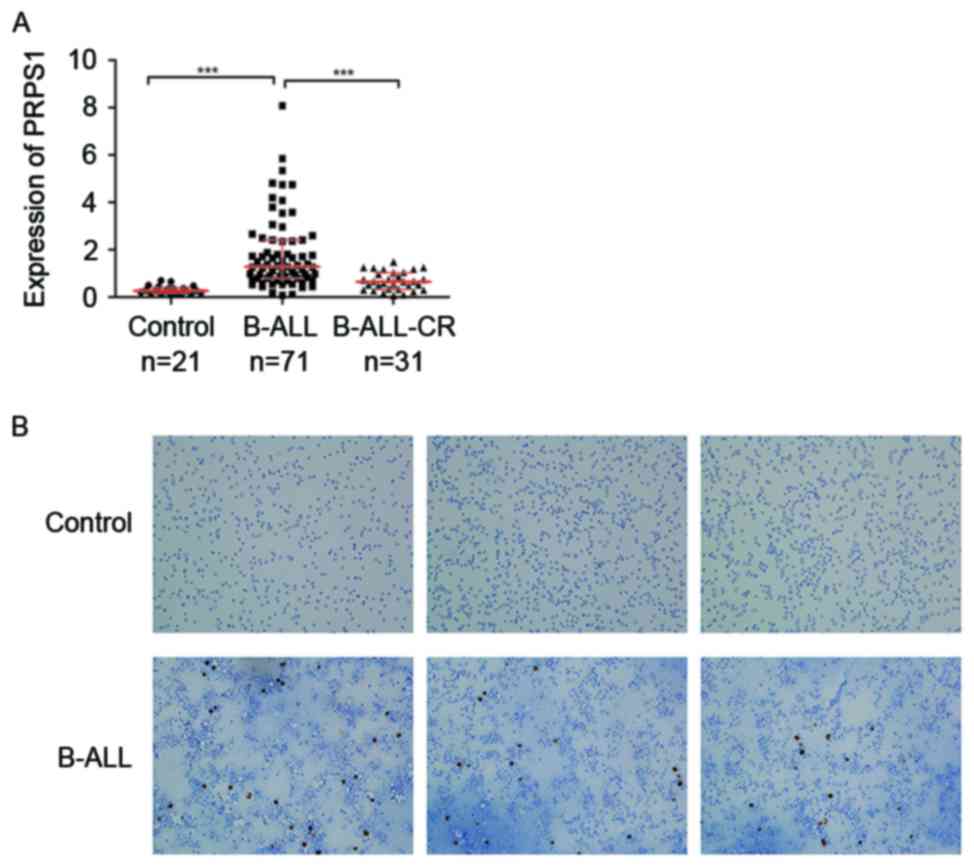
The expression of PRPS1, as determined by RT-qPCR,
was significantly higher in patients newly diagnosed with B-ALL
compared with those in the control group or those that had achieved
complete remission (Fig. 1A and
Table III).
 | Table III.Association of PRPS1 expression with
clinical manifestations in AL. |
Table III.
Association of PRPS1 expression with
clinical manifestations in AL.
| Characteristic | Patients, no. | PRPS1
expression | P-value |
|---|
| Sex |
|
| 0.472 |
|
Male | 42 | 1.37
(0.83–2.38) |
|
|
Female | 29 | 1.05
(0.66–2.58) |
|
| Age, years |
|
| 0.124 |
|
1–10 | 52 | 1.14
(0.68–2.37) |
|
| <1
or ≥10 | 19 | 1.67
(0.89–2.67) |
|
| WBC count |
|
| 0.020 |
|
<50×109/l | 47 | 0.99
(0.69–1.73) |
|
|
≥50×109/l | 24 | 1.79
(1.27–2.87) |
|
| MRD |
|
| 0.026 |
|
Positive | 8 | 3.69
(1.03–4.750) |
|
|
Negative | 63 | 1.27
(0.71–1.88) |
|
| Relapse |
|
| 0.112 |
|
Yes | 8 | 1.80
(1.21–3.46) |
|
| No | 63 | 1.27
(0.71–2.37) |
|
| Risk
stratification |
|
|
|
| SR
group | 27 | 0.79
(0.53–1.07) | 0.0017a |
| IR
group | 21 | 1.28
(0.91–2.11) | 0.0033b |
| HR
group | 23 | 2.42
(1.65–3.41) | 0.0000c |
IHC staining to detect the protein expression and
distribution of PRPS1 in B-ALL and control patients revealed that
the staining for PRPS1 in the BM was stronger in the B-ALL patients
compared with that in the control group (Fig. 1B), which was consistent with the
RT-qPCR data.
Increased PRPS1 expression is
associated with high-risk stratification and poor prognosis in
patients with B-ALL
The risk factors for B-ALL patients include an
elevated peripheral white blood cell (WBC ≥50×109/l)
count, age (infant or ≥10 years old), recurrent chromosomal
abnormalities and poor response to initial therapy (2). Depending on these risk factors, the
patients were categorized into the standard risk, intermediate risk
and high-risk groups, according to the CCLG ALL2008 guidelines
(18), and were treated with
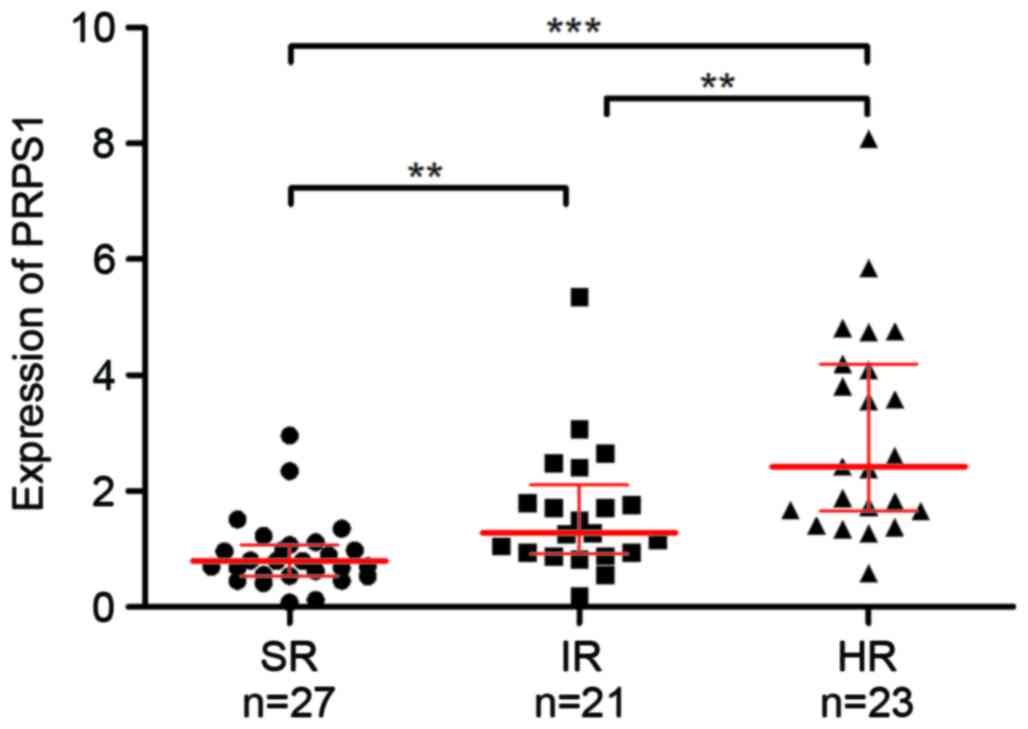
chemotherapy accordingly. Analysis of PRPS1 in these subgroups
revealed a significant difference in PRPS1 levels between each of
the three risk groups of patients with B-ALL (Fig. 2 and Table
III), and PRPS1 expression was significantly increased in
patients with higher risk stratification. These results indicated
that PRPS1 expression was associated with risk stratification in
B-ALL.
To understand the association of PRPS1 expression
with the clinical manifestations of B-ALL patients further, the
PRPS1 expression levels were compared with the clinical
manifestations of children with B-ALL (Table III). The expression of PRPS1 was
observed to be higher in samples from patients with a peripheral
WBC count >50×109/l and from patients with minimal
residual disease. The expression level of PRPS1 was not associated
with any other clinical manifestations in patients with B-ALL,
including sex, age and the incidence of relapse. Although no
significant difference in relapse was identified, patients who
experienced relapse tended to have elevated PRPS1 expression levels
(P=0.112) compared with those who did not experience relapse.
Overall, these results indicated that increased expression of PRPS1
was able to predict a poor prognosis of children with B-ALL.
PRPS1 overexpression increases the
growth and inhibits apoptosis of B-ALL cell lines
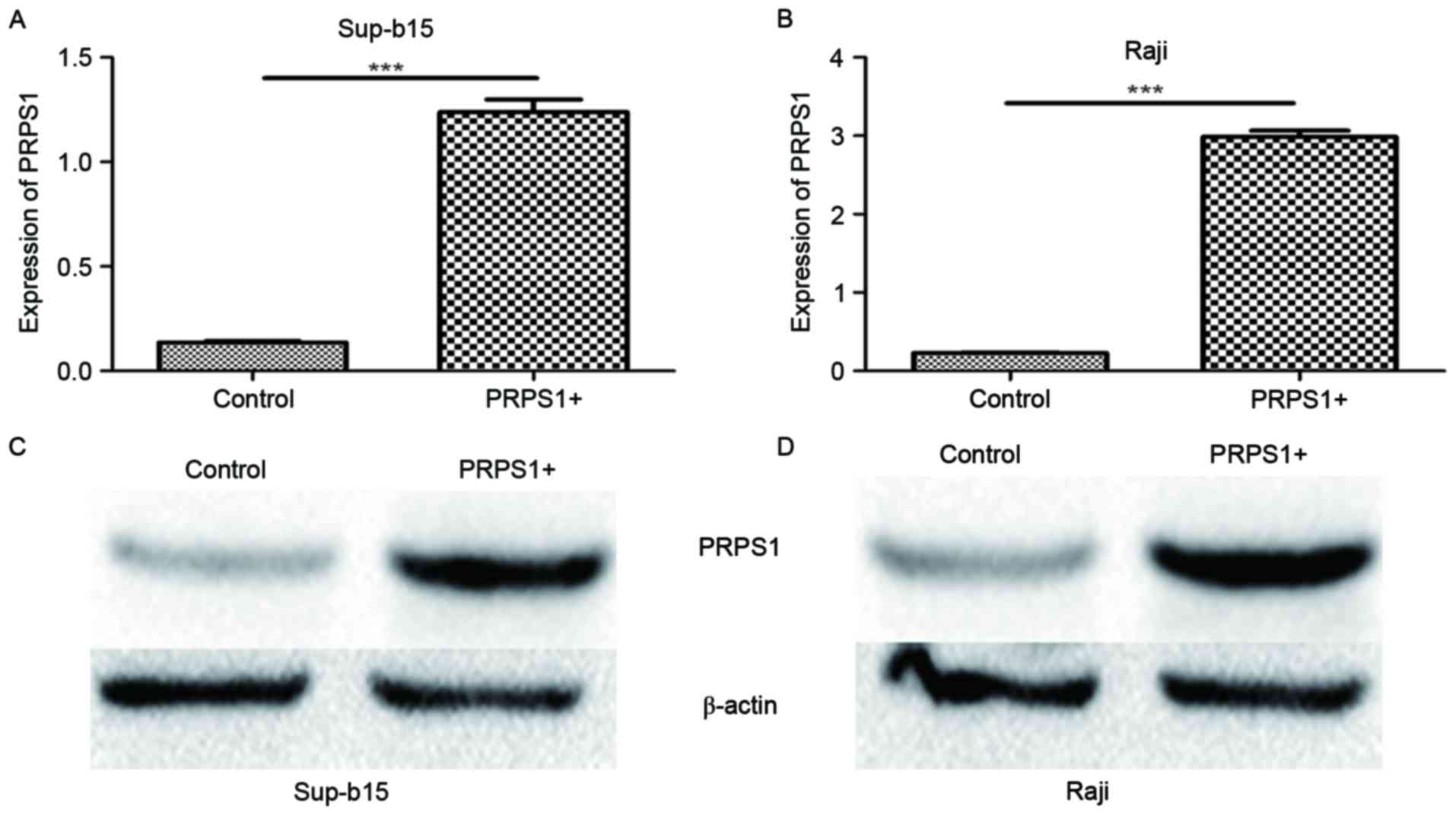
To investigate the function of PRPS1 in the
leukemogenesis of B-ALL further, the B-ALL cell lines Sup-B15 and
Raji were transfected with lentivirus particles containing the
PRPS1-overexpressing vector. The increased PRPS1 expression was
confirmed by RT-qPCR and western blot analysis to be significant in
these cells (P<0.05; Fig. 3).
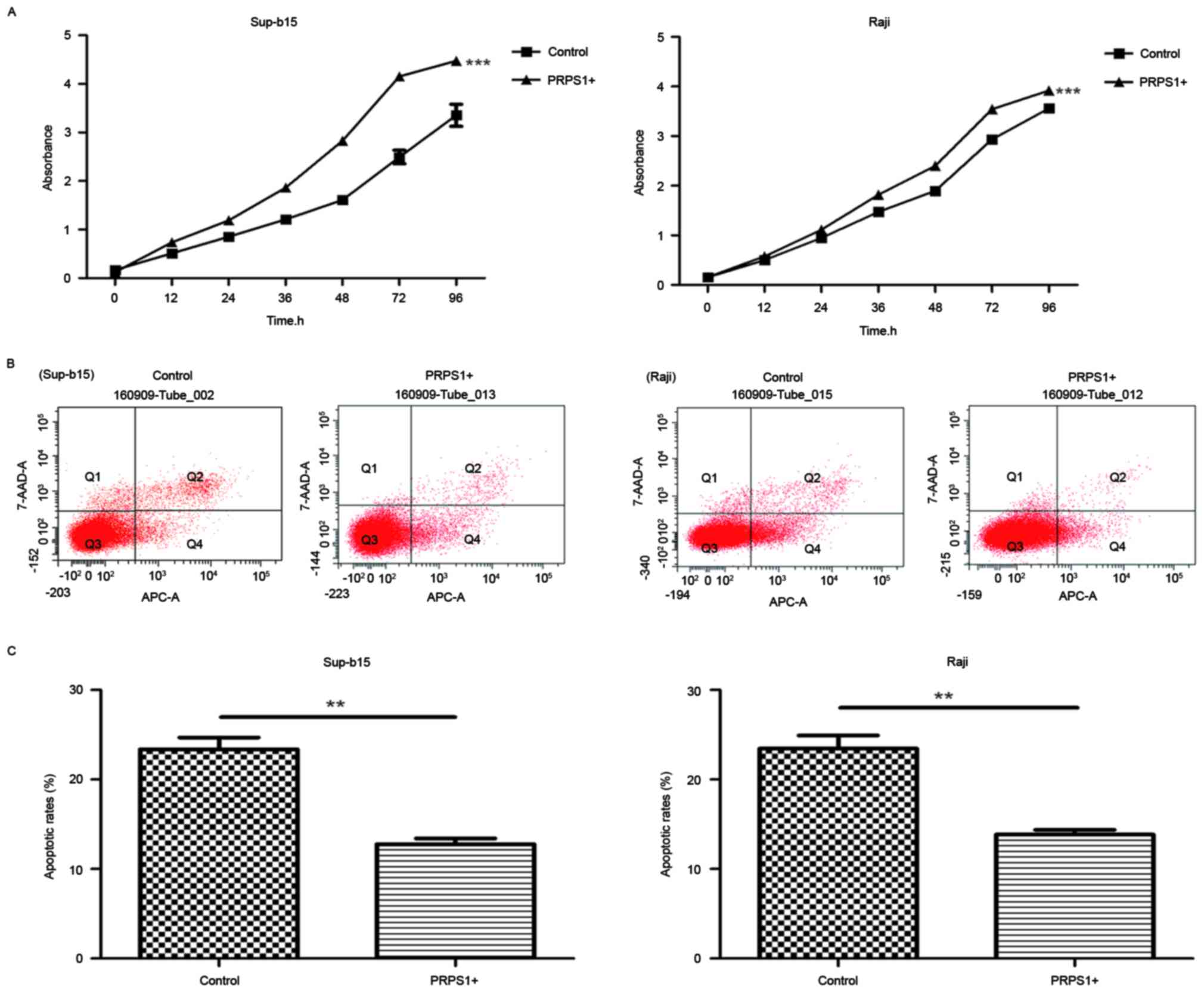
To examine the effect of PRPS1 overexpression on ALL
cell behaviors, cell proliferation (Fig.
4A) and annexin V-APC/7AAD staining assays (Fig. 4B and C) were performed on Sup-B15 and
Raji cells. The results of the cell proliferation assay revealed
that the proliferation of cells overexpressing PRPS1 was
significantly increased compared with cells that did not
overexpress PRPS1 (P<0.05; Fig.
4A), which indicated that PRPS1 overexpression significantly
increased the proliferation of B-ALL cells. Furthermore, the
results of the annexin V-APC/7AAD flow cytometry assay (Fig. 4B) demonstrated that, compared with the
control groups, cells overexpressing PRPS1 exhibited a decrease in
apoptosis (P<0.05; Fig. 4B and C).
The phase distributions of the cell cycle were detected; however,
the results did not show any significant differences between the
cells overexpressing PRPS1 and the control cells at any of the
phases (Table IV). When accounting
for the results in all assessed cell lines, PRPS1 overexpression
decreased the apoptosis of B-ALL cell lines and thus inhibited the
growth of cells, but this exogenous expression did not affect the
distribution of cells in different phases of the cell cycle.
 | Table IV.Cell cycle distribution in Sup-b15
and Raji cells. |
Table IV.
Cell cycle distribution in Sup-b15
and Raji cells.
| Cell line |
G0/G1 phase | P-value | S phase | P-value | G2/M
phase | P-value |
|---|
| Sup-b15 |
| 0.129 |
| 0.086 |
| 0.855 |
|
PRPS1+ |
37.53±4.40 |
|
42.85±2.63 |
|
19.62±1.88 |
|
| VC |
43.30±3.98 |
|
37.36±3.79 |
|
19.34±1.85 |
|
| Raji |
| 0.793 |
| 0.464 |
| 0.924 |
|
PRPS1+ |
32.64±3.55 |
|
44.92±0.45 |
|
22.44±3.59 |
|
| VC |
32.04±1.09 |
|
45.77±1.75 |
|
22.19±2.09 |
|
PRPS1 increases the expression of
Bcl-2, inducing an anti-apoptotic effect in B-ALL cell lines
To investigate the anti-apoptotic effect induced by
PRPS1 overexpression in B-ALL cell lines, the expression levels of
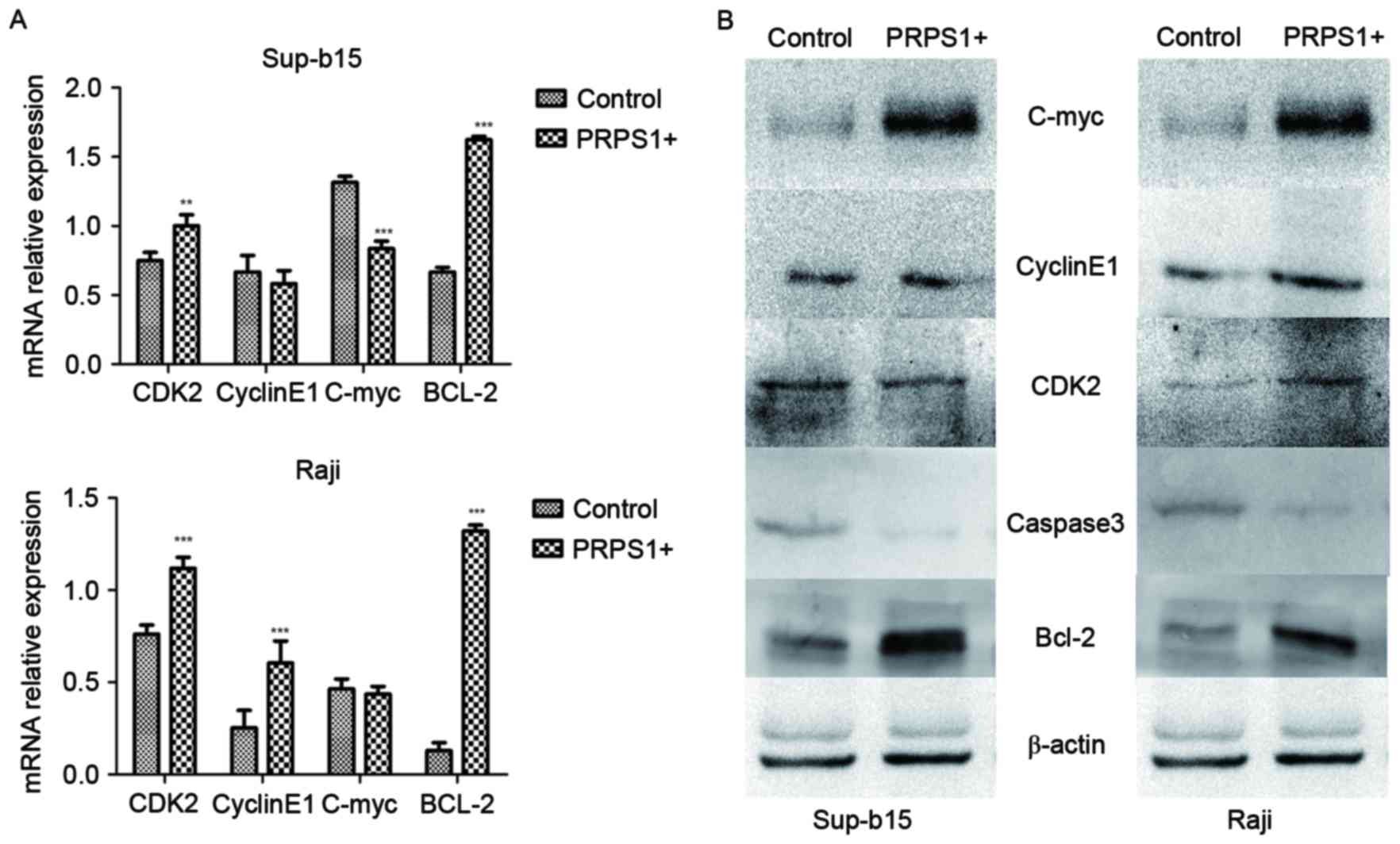
c-Myc, cyclin E1, Bcl-2, CDK2 and caspase-3 were evaluated in
Sup-B15, and Raji cells by RT-qPCR (Fig.
5A) and western blot analysis (Fig.
5B). The results revealed that Bcl-2, which is a key inhibitor
of the mitochondrial associated apoptosis pathway, was
significantly upregulated (P<0.05) in B-ALL cells overexpressing
PRPS1. Protein expression of other apoptotic or cell cycle
regulators, including CDK2, c-Myc, cyclin E1 and caspase-3, did not
exhibit any significant changes in the assays in the two cell
lines. These results indicate that Bcl-2 may be the effector that
implements the anti-apoptotic function downstream of PRPS1.
Discussion
Evasion of apoptosis is an important mechanism for
tumorigenesis (21), the death
receptor pathway (22), the
mitochondrial associated apoptosis pathway (23), and the crosstalk between these
pathways (24) has been identified to
serve important roles during apoptosis in cancer cells. The present
study revealed that the purine synthetase PRPS1 was highly
expressed in the BM samples of children suffering from B-ALL, and
was able to induce an anti-apoptotic effect by increasing the
expression of Bcl-2, which is an essential anti-apoptotic gene in
the mitochondrial pathway (25).
Compared with samples from the control group, PRPS1 was highly
expressed in the samples from patients with B-ALL; furthermore,
samples from children newly diagnosed with B-ALL exhibited
significantly higher expression levels of PRPS1 compared with
patients who achieved complete remission. These findings indicate a
likelihood of increased PRPS1 expression in B-ALL leukemic cells,
but not in normal hematopoietic cells in this type of malignancy.
Overexpression of PRPS1 in established B-ALL cell lines decreased
the apoptotic rate in these cells, and a small but insignificant
change was induced in other processes, including cell proliferation
and phase distribution in the cell cycle. Determining changes in
the expression of apoptotic genes revealed the increased expression
of the key anti-apoptotic gene Bcl-2 in cells overexpressing PRPS1.
Multiple enzymes in the purine synthesis pathway are targets for
nucleotide analog resistance inducers owing to their nucleotide
metabolic activity; however, this novel function of PRPS1 provides
another possibility in influencing tumorigenesis more directly by
inhibiting apoptosis.
PRPS1 is a first-step enzyme that catalyzes the
phosphoribosylation of ribose 5-phosphate to
5-phosphoribosyl-1-pyrophosphate, which is necessary in the de
novo pathway for purine biosynthesis (26,27).
Defects in this gene are a cause of phosphoribosyl pyrophosphate
synthetase superactivity, Charcot-Marie-Tooth disease X-linked
recessive type 5 and Arts syndrome (26,28).
However, the role of PRPS1 in tumorigenesis has been seldom
mentioned in the literature.
Recently, Li et al (15) identified PRPS1 mutations in pediatric
B-ALL as specific relapse markers and identified mutated PRPS1 as a
driver for drug resistance. The present study overexpressed normal
PRPS1 in B-ALL cell lines and investigated the function of
unmutated PRPS1 during leukemogenesis. It was revealed that PRPS1
was able to increase the expression of Bcl-2 and induce an
anti-apoptotic effect in both B-ALL cell lines. These results
indicate PRPS1 acts as an anti-apoptotic inducer in ALL and
therefore, the results of the present study suggest a potential
mechanism through which PRPS1 may affect the leukemic cells in the
relapse process. Bcl-2, a key gene in the mitochondrial associated
apoptosis pathway, is highly expressed in multiple types of
malignancies and commonly induces the anti-apoptotic effect
observed in tumorigenesis (29–32). The
present study indicates that Bcl-2 is a downstream effector of
PRPS1, owing to the response of its expression to the PRPS1
overexpression, which implies that PRPS1 exerts an anti-apoptotic.
The Bcl-2-family inhibitor obatoclax has been identified as having
potent cytotoxicity against MLL-rearranged and infant ALL cells in
Phase III clinical trials (33).
Furthermore, the results of the present study may indicate that
Bcl-2 inhibitors represent a treatment option for pediatric
patients with B-ALL who have increased levels of Bcl-2 or
PRPS1.
Although the present study revealed a role of Bcl-2
downstream of PRPS1, how PRPS1 regulates the expression of Bcl-2
remains unclear. As the elevated expression of PRPS1 was able to
increase Bcl-2 expression at the RNA and protein level, PRPS1 may
exert a transcriptional effect on Bcl-2, which indicates the
possibility of PRPS1 functioning as a transcription factor to
regulate Bcl-2. Kaida et al (34) identified PRPS1 as a P300-specific, but
not a CREB-binding-protein binding protein in 293 cells, which
indicates that PRPS1 possibly exerts transcription factor
activities. Thus, further study of the regulatory mechanism of
Bcl-2 expression by PRPS1 should be conducted.
The present study identified the purine synthetase
PRPS1 as a leukemogenesis driver by increasing Bcl-2 expression and
subsequently inducing an anti-apoptotic effect in B-ALL cells.
Acknowledgements
The present study was supported by funding from the
Clinical Research Project of the Children's Hospital of Chongqing
Medical University (grant no. lcyj2014-12) and the Medical Research
Project of the Health and Family Planning Commission of Chongqing,
China (grant nos. 2015MSXM042 and 2016ZDXM015).
Glossary
Abbreviations
Abbreviations:
|
AL
|
acute leukemia
|
|
B-ALL
|
B-cell acute lymphoblastic
leukemia
|
|
T-ALL
|
T-cell lymphoblastic leukemia
|
|
BMMNC
|
bone marrow mono-nucleic cells
|
|
WBC
|
white blood cells
|
|
SR
|
standard risk
|
|
IR
|
intermediate risk
|
|
HR
|
high risk
|
References
|
1
|
Brisson GD, Alves LR and Pombo-de-Oliveira
MS: Genetic susceptibility in childhood acute leukaemias: A
systematic review. Ecancermedicalscience. 9:5392015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Inaba H, Greaves M and Mullighan CG: Acute
lymphoblastic leukaemia. Lancet. 381:1943–1955. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Hunger SP, Lu X, Devidas M, Camitta BM,
Gaynon PS, Winick NJ, Reaman GH and Carroll WL: Improved survival
for children and adolescents with acute lymphoblastic leukemia
between 1990 and 2005: A report from the children's oncology group.
J Clin Oncol. 30:1663–1669. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Zhang F, Patel DM, Colavita K, Rodionova
I, Buckley B, Scott DA, Kumar A, Shabalina SA, Saha S, Chernov M,
et al: Arginylation regulates purine nucleotide biosynthesis by
enhancing the activity of phosphoribosyl pyrophosphate synthase.
Nature Commun. 6:75172015. View Article : Google Scholar
|
|
5
|
Turner RN, Aherne GW and Curtin NJ:
Selective potentiation of lometrexol growth inhibition by
dipyridamole through cell-specific inhibition of hypoxanthine
salvage. Br J Cancer. 76:1300–1307. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Sessa C, de Jong J, D'Incalci M, Hatty S,
Pagani O and Cavalli F: Phase I study of the antipurine antifolate
lometrexol (DDATHF) with folinic acid rescue. Clin Cancer Res.
2:1123–1127. 1996.PubMed/NCBI
|
|
7
|
Laohavinij S, Wedge SR, Lind MJ, Bailey N,
Humphreys A, Proctor M, Chapman F, Simmons D, Oakley A, Robson L,
et al: A phase I clinical study of the antipurine antifolate
lometrexol (DDATHF) given with oral folic acid. Invest New Drugs.
14:325–335. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Wedge SR, Laohavinij S, Taylor GA, Boddy
A, Calvert AH and Newell DR: Clinical pharmacokinetics of the
antipurine antifolate (6R)-5,10-dideaza-5,6,7,8-tetrahydrofolic
acid (Lometrexol) administered with an oral folic acid supplement.
Clin Cancer Res. 1:1479–1486. 1995.PubMed/NCBI
|
|
9
|
Pei W, Xu L, Varshney GK, Carrington B,
Bishop K, Jones M, Huang SC, Idol J, Pretorius PR, Beirl A, et al:
Additive reductions in zebrafish PRPS1 activity result in a
spectrum of deficiencies modeling several human PRPS1-associated
diseases. Sci Rep. 6:299462016. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Maruyama K, Ogaya S, Kurahashi N, Umemura
A, Yamada K, Hashiguchi A, Takashima H, Torres RJ and Aso K: Arts
syndrome with a novel missense mutation in the PRPS1 gene: A case
report. Brain Dev. 38:954–958. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Mittal R, Patel K, Mittal J, Chan B, Yan
D, Grati M and Liu XZ: Association of PRPS1 mutations with disease
phenotypes. Dis Markers. 2015:1270132015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Gandia M, Fernández-Toral J, Solanellas J,
Domínguez-Ruiz M, Gómez-Rosas E, Del Castillo FJ, Villamar M,
Moreno-Pelayo MA and Del Castillo I: Mutations in PRPS1 causing
syndromic or nonsyndromic hearing impairment: Intrafamilial
phenotypic variation complicates genetic counseling. Pediatr Res.
78:97–102. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Almoguera B, He S, Corton M, Fernandez-San
Jose P, Blanco-Kelly F, López-Molina MI, García-Sandoval B, Del Val
J, Guo Y, Tian L, Liu X, et al: Expanding the phenotype of PRPS1
syndromes in females: neuropathy, hearing loss and retinopathy.
Orphanet J Rare Dis. 9:1902014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Robusto M, Fang M, Asselta R, Castorina P,
Previtali SC, Caccia S, Benzoni E, De Cristofaro R, Yu C, Cesarani
A, et al: The expanding spectrum of PRPS1-associated phenotypes:
Three novel mutations segregating with X-linked hearing loss and
mild peripheral neuropathy. Eur J Hum Genet. 23:766–773. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Li B, Li H, Bai Y, Kirschner-Schwabe R,
Yang JJ, Chen Y, Lu G, Tzoneva G, Ma X, Wu T, et al: Negative
feedback-defective PRPS1 mutants drive thiopurine resistance in
relapsed childhood ALL. Nat Med. 21:563–571. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Hassan K, Bukhari KP, Zafar A, Malik MZ
and Akhtar MJ: Acute leukaemia in children-French-American-British
(FAB) classification and its relation to clinical features. J Pak
Med Assoc. 42:29–31. 1992.PubMed/NCBI
|
|
17
|
Vardiman JW, Thiele J, Arber DA, Brunning
RD, Borowitz MJ, Porwit A, Harris NL, Le Beau MM,
Hellström-Lindberg E, Tefferi A and Bloomfield CD: The 2008
revision of the world health organization (WHO) classification of
myeloid neoplasms and acute leukemia: Rationale and important
changes. Blood. 114:937–951. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Cui L, Gao C, Zhang RD, Jiao Y, Li WJ,
Zhao XX, Liu SG, Yue ZX, Zheng HY, Deng GR, et al: Low expressions
of ARS2 and CASP8AP2 predict relapse and poor prognosis in
pediatric acute lymphoblastic leukemia patients treated on China
CCLG-ALL 2008 protocol. Leuk Res. 39:115–123. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
He M, Chao L and You YP: PRPS1 silencing
reverses cisplatin resistance in human breast cancer cells. Biochem
Cell Biol. 95:385–393. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Hanahan D and Weinberg RA: Hallmarks of
cancer: The next generation. Cell. 144:646–674. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Peter ME, Hadji A, Murmann AE, Brockway S,
Putzbach W, Pattanayak A and Ceppi P: The role of CD95 and CD95
ligand in cancer. Cell Death Differ. 22:885–886. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Lopez J and Tait SW: Mitochondrial
apoptosis: Killing cancer using the enemy within. Br J Cancer.
112:957–962. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Ola MS, Nawaz M and Ahsan H: Role of Bcl-2
family proteins and caspases in the regulation of apoptosis. Mol
Cell Biochem. 351:41–58. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Murphy KM, Ranganathan V, Farnsworth ML,
Kavallaris M and Lock RB: Bcl-2 inhibits Bax translocation from
cytosol to mitochondria during drug-induced apoptosis of human
tumor cells. Cell Death Differ. 7:102–111. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Becker MA: Phosphoribosylpyrophosphate
synthetase and the regulation of phosphoribosylpyrophosphate
production in human cells. Prog Nucleic Acid Res Mol Biol.
69:115–148. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Yamada Y, Yamada K, Nomura N, Yamano A,
Kimura R, Naiki M, Fukushi D, Wakamatsu N, Taniguchi A, Yamaoka N,
et al: Molecular analysis of X-linked inborn errors of purine
metabolism: HPRT1 and PRPS1 mutations. Nucleosides Nucleotides
Nucleic Acids. 30:1272–1275. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
de Brouwer AP, van Bokhoven H, Nabuurs SB,
Arts WF, Christodoulou J and Duley J: PRPS1 mutations: four
distinct syndromes and potential treatment. Am J Hum Genet.
86:506–518. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Kim S, Nam SJ, Kwon D, Kim H, Lee E, Kim
TM, Heo DS, Park SH, Kim CW and Jeon YK: MYC and BCL2
overexpression is associated with a higher class of memorial
sloan-kettering cancer center prognostic model and poor clinical
outcome in primary diffuse large B-cell lymphoma of the central
nervous system. BMC Cancer. 16:3632016. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Cabrera Ortega AA, Gonçalves Vde P,
Guimarães MR, Rossa Junior C and Spolidorio LC: Overexpression of
Bcl-2, SOCS 1, 3 and Cdh 1, 2 are associated with the early
neoplasic changes in modified 4-nitroquinoline 1-oxide-induced
murine oral cancer model. J Oral Pathol Med. 45:573–580. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Jager R, Herzer U, Schenkel J and Weiher
H: Overexpression of Bcl-2 inhibits alveolar cell apoptosis during
involution and accelerates c-myc-induced tumorigenesis of the
mammary gland in transgenic mice. Oncogene. 15:1787–1795. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Hsu SY, Lai RJ, Finegold M and Hsueh AJ:
Targeted overexpression of Bcl-2 in ovaries of transgenic mice
leads to decreased follicle apoptosis, enhanced folliculogenesis,
and increased germ cell tumorigenesis. Endocrinology.
137:4837–4843. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Urtishak KA, Edwards AY, Wang LS, Hudome
A, Robinson BW, Barrett JS, Cao K, Cory L, Moore JS, Bantly AD, et
al: Potent obatoclax cytotoxicity and activation of triple death
mode killing across infant acute lymphoblastic leukemia. Blood.
121:2689–2703. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Kaida A, Ariumi Y, Baba K, Matsubae M,
Takao T and Shimotohno K: Identification of a novel
p300-specific-associating protein, PRS1
(phosphoribosylpyrophosphate synthetase subunit 1). Biochem J.
391:239–247. 2005. View Article : Google Scholar : PubMed/NCBI
|