Introduction
Lung cancer is the most common cause of
cancer-associated mortality worldwide (1). The incidence of lung cancer is
increasing in China, leading to ~250,000 incidences of mortality
each year (2). Although surgical
resection is the primary treatment for lung cancer, only 10–15% of
patients underwent surgical resection as a result of late diagnosis
(3,4).
Chemotherapy remains the first-line treatment for patients with
lung cancer (5); however, lung cancer
exhibits intrinsic multidrug resistance (6). Therefore, the efficacy of chemotherapy
is often hampered by multidrug resistance (MDR), which leads to the
loss of activity of agents against cancer cells (7).
MDR is common in human cancer, and is a key
hindrance to the effectiveness of chemotherapy (8,9). Although
the exact mechanisms of resistance remain unclear, a reduction in
the intracellular accumulation of drugs was observed in cancer
cells exhibiting MDR, which was mediated by increasing drug efflux
(8). The phenomenon of drug efflux
results from the high expression of ATP-binding cassette (ABC)
proteins, which are a drug transporter family that includes
P-glycoprotein (P-gp) and multidrug resistance-associated protein
(MRP1) (10). P-gp and MRP1 affect
the intracellular drug concentration and contribute to the MDR of
cancer cells via the alteration of drug influx or efflux. It has
been reported that P-gp regulates the efflux of various anticancer
drugs from cells (11,12). Furthermore, P-gp expression is
associated with a poorer prognosis in patients with several types
of human tumor including leukemia and lung, bladder, ovarian and
breast cancer (13–15). Additionally, the expression of MRP1 is
associated with the chemosensitivity of anticancer drugs including
paclitaxel and anthracyclines (16).
The lung resistance protein (LRP), a major vault protein, mediates
MDR via molecular pumping molecules or exocytotic vesicles to
increase the drug efflux from intracellular drug targets (16,17).
Previous study has reported that LRP serves important functions in
the MDR of lung cancer (16).
The ginsenosides are active chemical components
extracted from Panax ginseng (18,19).
Ginsenosides Rg1, Re, Rc, and Rd were identified to prevent MDR in
mouse lymphoma cells by inhibiting the drug efflux pump and
increasing drug accumulation (20).
20(S)-ginsenoside Rg3 (Rg3) is a ginsenoside that has attracted
attention owing to its inhibitory effect on several types of tumor,
including lung, gallbladder, liver, and ovarian cancer (21–24).
Furthermore, it has been reported that Rg3 regulates MDR of
numerous tumors cells, including human acute myeloid leukemia
cells, KBV20C cells and murine leukemia P388 cells (8,25).
However, the effect of Rg3 on the MDR of lung cancer has not been
studied.
The aim of this study is to investigate the effect
of Rg3 on the MDR of A549 lung cancer cells. A cell viability assay
was used to detect the effect of Rg3 on cisplatin (DDP)-induced
A549/DDP cells cytotoxicity. The antitumor function of Rg3 was
tested in A549/DDP xenograft mice. Western blot analysis was
performed to detect the expression of MDR-mediated proteins,
including P-gp, MPR1 and LPR1, in the tumor tissue of A549/DDP
xenograft mice with or without Rg3 treatment.
Technetium-99m-labeled hexakis-2-methoxyisobutylisonitrile
(99mTc-MIBI) single-photon emission computed tomography
(SPECT) was used to monitor the effect of Rg3 on cisplatin
sensitivity of A549/DDP xenograft tumors. Our results suggested
that Rg3 increased chemosensitivity of the DDP-resistant lung
cancer A549/DDP cell line via the downregulation of P-gp, MRP1 and
LRP.
Materials and methods
Cell line and cell culture
The human lung cancer A549 and DDP-resistant
A549/DDP cell lines were purchased from the Type Culture Collection
of the Chinese Academy of Sciences (Shanghai, China) and cultured
in RPMI-1640 medium (Gibco; Thermo Fisher Scientific, Inc.,
Waltham, MA, USA) supplemented with 10% fetal bovine serum (Gibco;
Thermo Fisher Scientific, Inc.) at 37°C in a humidity incubator
with 5% CO2. To maintain drug resistance, DDP-resistant
A549 cells were cultured with 5 µg/ml DDP (Sigma-Aldrich; Merck
KGaA, Darmstadt, Germany) and then cultured further in DDP-free
RPMI-1640 medium for two days prior to starting the experiment.
Chemicals and drug preparations
Ginsenoside Rg3 was purchased from Zhejiang Yatai
Pharmaceutical Co., Ltd. (Shaoxing, China). Purity quotient of Rg3
was ≥99.5%. Cisplatin (DDP) was obtained from Sigma-Aldrich (Merck
KGaA). The compounds were dissolved in dimethyl sulfoxide to make
stock solution (Rg3, 15 mM; and DDP, 100 mM). These two compounds
were kept at −80°C as aliquots.
Cell viability assay
For the cell viability assay, 5×103
A549/DDP cells were seeded into each well of the 96-well microplate
overnight. The cells were treated with various concentrations of
DDP with or without Rg3 (40 µM). The final concentrations were
0.02, 0.2, 2, and 20 µg/ml, which are 10 times of the human peak
serum doses for DDP, as previously demonstrated (26). The peak plasma concentration of DDP
was 2.0 µg/ml (27). At ~48 h after
addition of DDP and Rg3, MTT (20 µl; 5 mg/ml; Sigma-Aldrich; Merck
KGaA) was added into each well, and culture was sustained for 4 h
at 37°C in an incubator with 5% CO2 according to the
manufacturer's protocol. Samples were read on a microplate reader
(SpectraMAX Plus, Molecular Devices, Sunnyvale, CA, USA) at 490 nm.
The half-maximal inhibitory concentration (IC50) of DDP
was then calculated. The reversed effect of Rg3 on IC50
was presented as reversal fold, [Reversal fold=IC50
(resistant cells)/IC50 (reversal cells)].
Tumor xenografts
Animal studies were approved by the Institutional
Animal Care and Use Committee of Yunnan Provincial Tumor Hospital
(Kunming, China). A total of 20 male BALB/c nude mice, age of 7–8
weeks, weight of 18–22 g, 10 mice/group (Beijing Vital River
Laboratory Animal Technology Co., Ltd.) were used. Mice were housed
in laminar airflow cabinets under pathogen-free conditions with a
12/12 h light/dark schedule and fed autoclaved semipurified diet
(AIN-93) and water. Mice were injected subcutaneously into the
flank with 5×106 A549/DDP cells. When the tumor volume
reached 100 mm3, mice were randomly assigned to two
groups; the time was defined as day 1, which was the starting point
for treatment. DDP (7.5 mg/kg in 0.2 ml normal saline) with or
without Rg3 (15 mg/kg) were intraperitoneally injected,
twice-weekly for 4 weeks. Once each week, mice were weighed, and
tumor volume was measured using the following formula: Tumor
volume=1/2 × (width)2 × length. At the end of 8 weeks,
mice were euthanized via CO2 inhalation, and tumor
tissues were dissected and measured Once tumors reached ~2,000
mm3 mice were sacrificed via CO2
inhalation.
99mTc-MIBI scintigraphy and
SPECT imaging
A549/DDP-xenograft mice underwent
99mTc-MIBI SPECT imaging. After 4 weeks of treatment,
mice were intravenously injected with 740 MBq 99mTc-MIBI
(Daiichi Sankyo, Ltd., Tokyo, Japan) and SPECT dual-phase imaging
was performed with standard parameters after 10, 60 and 120 min
using SPECT (E. CAM; Siemens AG, Munich, Germany). The SPECT
component of the study was then acquired in 120 projections, with a
3° angle step, in a 256×256 matrix, and at 20 sec/view. Image
reconstruction was performed using a 2-dimensional Butterworth
filter and corrected for attenuation using an order subset
expectation maximization algorithm.
SPECT image analysis
SPECT images were analyzed and quantified using the
AMIDE Medical Image Data Examiner software (version 0.9.1; Stanford
University, Stanford, CA, USA) (28)
by a team of three nuclear medicine physicians from The Department
of Nuclear Medicine, Yunnan Provincial Tumor Hospital (Kunming,
China). A distinct focus of increased or separate MIBI uptake was
designated to be positive on visual analysis. Tumor uptake ratios
were obtained from a transverse image on early (15 min) and delayed
(120 min) SPECT images. The regions of interest (ROIs) were
annotated over the tumor, and another ROI of the same size was
drawn over the lung using the modified method described by Wang
et al (7). The tumor uptake
ratio was calculated using the following formula: Tumor uptake
ratio=(mean count of the ROI in the tumor/mean count of the ROI in
the lung). Early tumor uptake ratio (ER, 15 min) and delayed tumor
uptake ratio (DR, 120 min) were also calculated. The washout rate
(WR%) of 99mTc-MIBI from the tumor was calculated using
the formula: WR%=(ER-DR)/ER ×100% (29).
Flow cytometry
Following 99mTc-MIBI SPECT analyses,
tumor tissue was isolated and digested. The tissues were digested
with 2 mg/ml collagenase I (cat. no. C0130; Sigma-Aldrich; Merck
KGaA) and 2 mg/ml hyalurinidase (cat. no. H3506; Sigma-Aldrich;
Merck KGaA) in 37°C for 3 h. Cells were filtered, washed with PBS
for three times, and followed by Percoll gradient centrifugation.
Mouse IgG2bk-FITC (cat. no. 11-4732; 1:1,000 dilution; eBioscience;
Thermo Fisher Scientific, Inc.) and mouse IgG2ak-PE (cat. no.
12-4724, 1:1,000 dilution; eBioscience; Thermo Fisher Scientific,
Inc.) were used to remove mouse cells. The primary tumor cells were
blocked with 10% goat serum (Thermo Fisher Scientific, Inc.) at
room temperature for 30 min, followed by incubation with anti-P-gp
(cat. no. sc-71557; 1:1,000 dilution; Santa Cruz Biotechnology,
Inc., Dallas, TX, USA) in ice for 60 min, and then with
APC-conjugated goat anti-mouse secondary antibody (cat. no. F0117;
1:1,000 dilution; R&D Systems, Inc., Minneapolis, MN, USA),
mouse IgG2bk-FITC and mouse IgG2ak-PE in ice and dark for 25 min,
then were washed with PBS. To measure the proportion of mouse
IgG2bk-FITCnegative/IgG2ak-PEnegative/P-pgpositive
cells, at least 30,000 events were acquired using the CellQuest™
software (version 3.2) of the FACSCalibur™ flow cytometer (BD
Biosciences, Franklin Lakes, NJ, USA). The data was analyzed by
FlowJo software (version 7.6; FlowJo LLC., Ashland, OR, USA).
Protein extraction and western
blot
Protein extraction and western blot analysis were
performed as previously described (30). Briefly, total proteins were extracted
from tumor tissue using a radioimmunoprecipitation assay lysis
buffer (Beyotime Institute of Biotechnology, Haimen, China)
supplemented with Complete EDTA-free Protease Inhibitor cocktail
tablets (Roche Applied Science, Penzberg, Germany), according to
the manufacturer's protocol. Protein concentrations were tested
with a Bicinchoninic Acid Protein Assay kit (Wuhan Boster
Biological Technology, Ltd., Wuhan, China). A total of ~40 µg
protein per lane from each group was isolated using 8% SDS-PAGE,
and transferred onto polyvinylidene fluoride membranes. After
blocking using 5% non-fat milk for 1 h at room temperature,
membranes were incubated with primary antibodies against P-gp,
MRP1, LRP1 and GAPDH, at 4°C overnight. The membrane was incubated
with horseradish peroxidase-conjugated anti-rabbit secondary
antibody for 1 h at room temperature following three washes with
Tris-buffered saline with 0.1% Tween-20 (Ameresco, Inc.,
Framingham, MA, USA). Signal detection was performed using Super
ECL Plus Detection reagent (Applygen Technologies Inc., China) and
densitometry anaylsis were performed using ImageJ software (version
1.48; National Institutes of Health, Bethesda, MD, USA). Relative
fold-change of protein expressions was calculated by normalization
to GAPDH levels.
Antibodies used in western blot analysis: Anti-P-gp
(cat. no. Sc-71557, 1:1,000 dilution); anti-MRP1 (cat. no.
Sc-136447, 1:2,000 dilution); anti-LRP1 (cat. no. Sc-16168, 1:500
dilution); and anti-GAPDH (cat. no. sc-293335, 1:1,000 dilution);
all were purchased from Santa Cruz Biotechnology, Inc. The
horseradish peroxidase-conjugated goat anti-rabbit IgG (cat. no.
12–348, 1:5,000 dilution) and goat anti-mouse IgG (cat. no. 12-349,
1:5,000 dilution) secondary antibodies were purchased from
Sigma-Aldrich (Merck KGaA).
RNA extraction and reverse
transcription-quantitative polymerase chain reaction (RT-qPCR)
Total RNA was extracted from A549/DDP tumor tissues
was using TRIzol® reagent (Life Technologies; Thermo
Fisher Scientific, Inc.), according to the manufacturer's protocol.
cDNA was acquired from 1 µg RNA using reverse transcription for 1 h
at 37°C (Omniscript RT kit, Qiagen) with oligo (dT) primers (Oligo
(dT)12-18 primer; Invitrogen; Thermo Fisher Scientific,
Inc.), according to the manufacturers protocol. Following RT, qPCR
was conducted using 2X TransStart Green qPCR SuperMix (TransGen
Biotech Co., Beijing, China) on an ABI 7500 instrument, as
previously described (31). PCR
thermocycling parameters included: Initial denaturation for 3 min
at 94°C; 40 total cycles of denaturation 30 sec at 94°C, annealing
for 30 sec at 60°C, extension for 30 sec at 72°C; and a final
extension step of 2 min at 72°C. The primers as previously
described (32,33) were as follows: P-gp forward,
5′-AAAAAGATCAACTCGTACCACTC-3′ and reverse,
5′-GCACAAAATACACCAACAA-3′; MRP1 forward, 5′-TCAAATACCTGCTGTTCGG-3′
and reverse, 5′-TGAAGTCCTGTCCTGATGCCAT-3′; LRP1 forward,
5′-GTGGTGGTAGGAGATGAGTG-3′ and reverse, 5′-CCAGATGTCCACGAGGAGG-3′;
and β-actin forward, 5′-GCCAACCGTGAAAAGATGACC-3′ and reverse
5′-CCCTCGTAGATGGGCACAGT-3′. The relative expression quantity of
P-gp, MRP1 and LRP1 mRNA were calculated with the 2−ΔΔCq
method (34) and normalized to the
expression of β-actin. All the above assays were repeated three
times.
Statistical analyses
The data are presented as the mean ± standard
deviation. Semi-quantitative analysis of 99mTc-MIBI
SPECT was compared with the expression of P-gp. The Pearson's
correlation coefficient was used for statistical analysis.
Student's t-test was performed to assess differences between two
groups. One-way analysis of variance and the post-hoc Bonferroni
test was conducted to assess differences among multiple groups. All
statistical calculations were performed using GraphPad Prism
software (version 6; GraphPad Software, Inc., La Jolla, CA, USA)
and P<0.05 was considered to indicate a statistically
significant difference.
Results
Rg3 increases the sensitivity to DDP
in A549/DDP cells
The present study determined the effect of Rg3 on
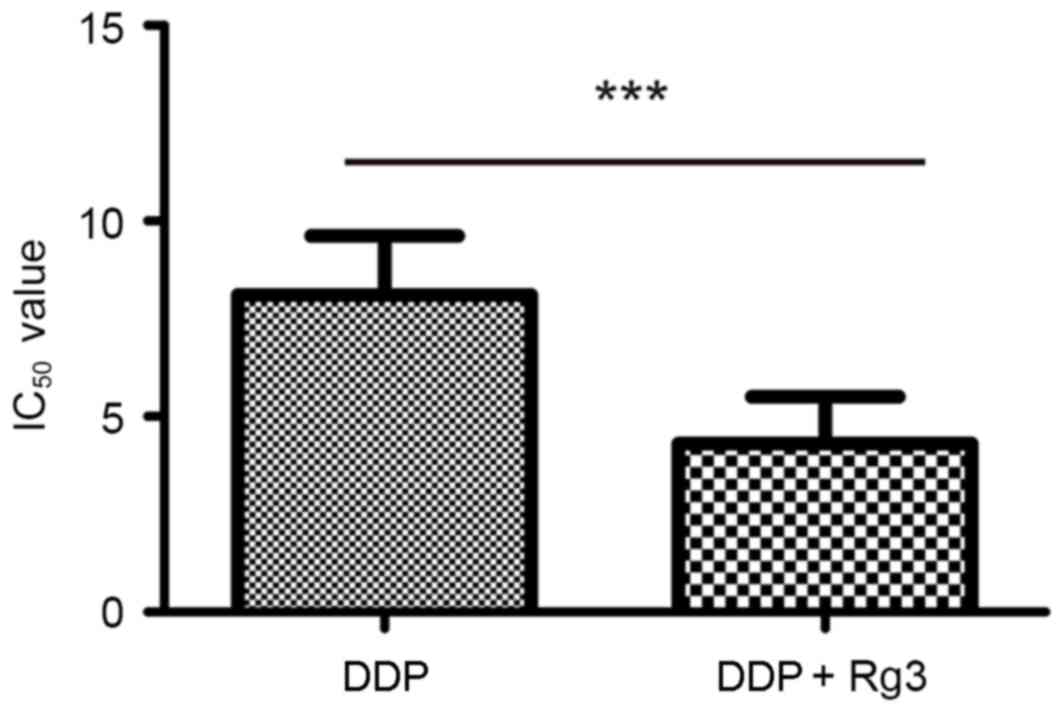
the sensitivity of the cells to DDP. As presented in Fig. 1, the IC50 value of the Rg3
treated cells was lower than that of the parental A549/DDP cells
(IC50, 8.14±0.59 vs. 11.97±0.71 µg/ml; P<0.01). The
reversal fold of Rg3 treatment was 1.29-fold. Thus, these results
indicated that Rg3 is able to increase the DDP sensitivity of the
A549/DDP cells.
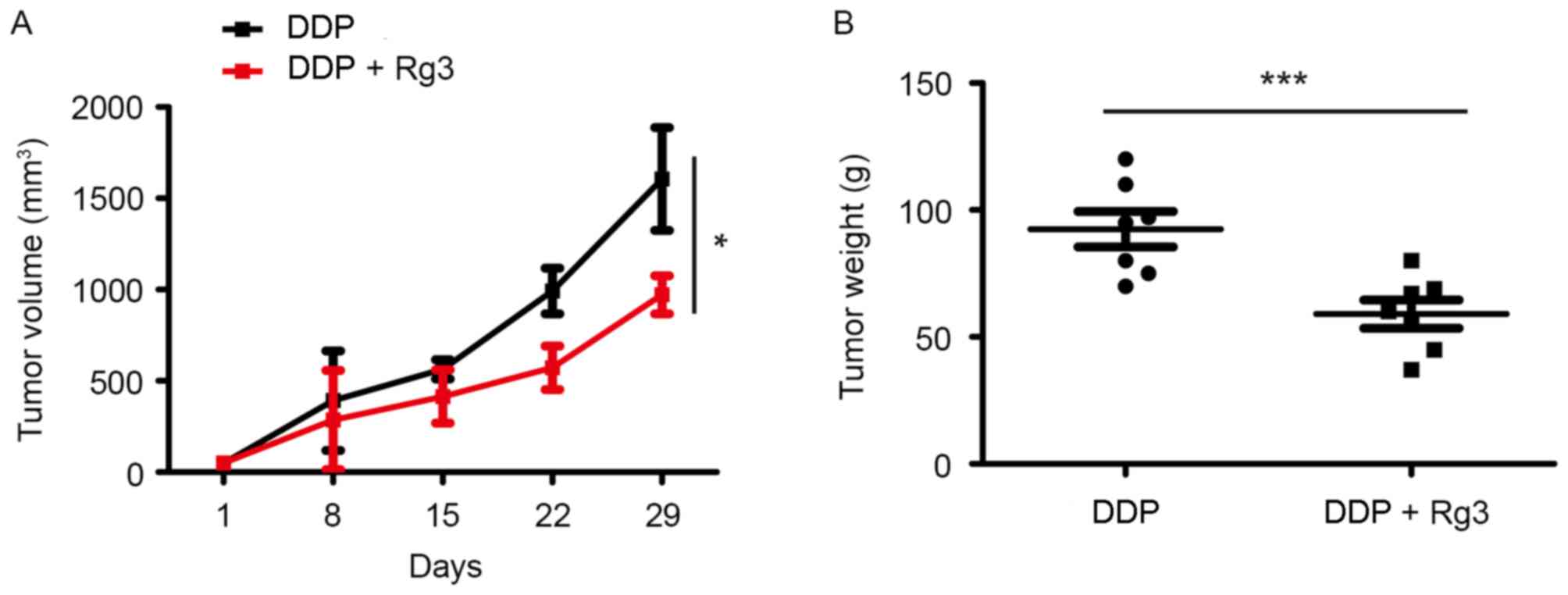
Rg3 increases the antitumor effect of
DDP on A549/DDP xenograft mice
A549/DDP xenograft mice were used to assess whether
the addition of Rg3 increased the sensitivity of A549/DDP cells to
DDP in vivo. Mice were intraperitoneally injected twice
weekly for 4 weeks with DDP (7.5 mg/kg in 0.2 ml of normal saline)
and/or Rg3 (15 mg/kg). The mice that received DDP-Rg3 combination
therapy exhibited significantly reduced tumor volumes on day 29
(39.5% reduction; P=0.0006) compared with animals treated using DDP
alone (Fig. 2). Furthermore, the
weight of tumors in the DDP-Rg3 combination group was decreased
compared with that of the DDP group (85% reduction; P=0.003). Taken
together, these results indicated that Rg3 can increase the
sensitivity of lung cancer cells to DDP in vivo.
99mTc-MIBI SPECT monitoring
of the effect of Rg3 on the sensitivity of A549/DDP to DDP
99mTc-MIBI SPECT imaging was used to
monitor the implication of Rg3 on the DDP sensitivity of A549/DDP.
99mTc-MIBI data are summarized in Table I. The results revealed that ER and DR
in the DDP-Rg3 combination therapy group were higher than that of
DDP group (ER, 1.90±0.53 vs. 1.08±0.08; P<0.001; DR, 1.91±0.54
vs. 1.04±0.06; P<0.001). The WR did not differ significantly
between the groups. These results indicated that Rg3 treatment
increased uptake of 99mTc-MIBI.
 | Table I.ER, DR and WR value at each time
point of imaging in the DDP and DDP + Rg3 groups. |
Table I.
ER, DR and WR value at each time
point of imaging in the DDP and DDP + Rg3 groups.
| Variable | DDP | DDP + Rg3 | P-value |
|---|
| ER | 1.08±0.08 | 1.90±0.53 | <0.001 |
| DR | 1.04±0.06 | 1.91±0.54 | <0.001 |
| WR | 0.03±0.07 | −0.07±0.40 | 0.465 |
The expression of the MDR-associated protein P-gp
was analyzed using flow cytometry analysis. Compared with the
control group, the proportion of P-gp-positive cells was
significantly reduced in the DDP/Rg3 group (71.62±10.52 vs.
45.40±8.65; P<0.01). Moreover, the proportion of P-gp positive
cells was positively associated with WR values (Table II), which suggested that the drug
clearance detected by 99mTc-MIBI imaging is positively
correlated with P-gp expression in A549/DDP xenograft mice. Thus,
99mTc-MIBI SPECT can reveal the MDR state of tumors and
also dynamically monitor the effect of Rg3 on the reversion of MDR
of A549/DDP-xenograft mice.
 | Table II.Percentage of positive cells
expressing P-gp in the DDP and DDP + Rg3 group and their Pearson's
correlation coefficients with the ER, DR and WR values. |
Table II.
Percentage of positive cells
expressing P-gp in the DDP and DDP + Rg3 group and their Pearson's
correlation coefficients with the ER, DR and WR values.
| Variable | ERa | DRa | WRa | P-gp-positive
cellsa, % |
|---|
| DDP group | 1.08±0.08 | 1.04±0.06 | 0.03±0.07 |
71.62±10.52c |
|
Pearson's correlation
coefficientb | 0.555 | −0.323 | 0.780 |
|
|
P-value | 0.096 | 0.363 | 0.008 |
|
| DDP + Rg3
group | 1.90±0.53 | 1.91±0.54 | −0.07±0.40 |
45.40±8.65e |
|
Pearson's correlation
coefficientd | 0.145 | −0.129 | 0.858 |
|
|
P-value | 0.690 | 0.722 | 0.002 |
|
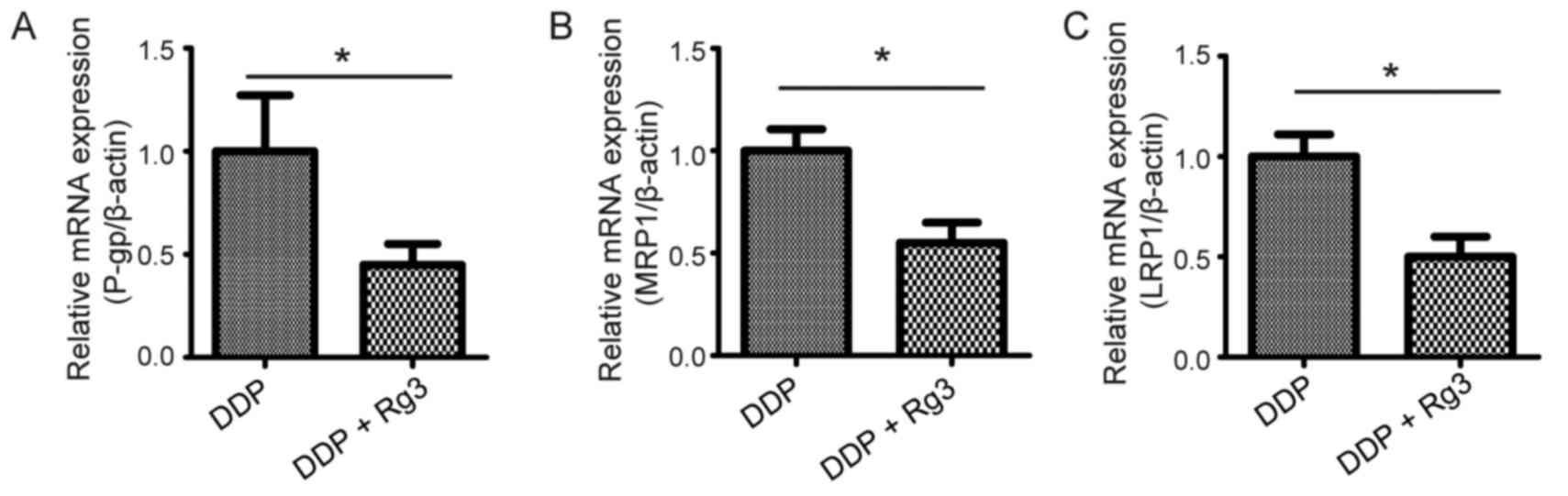
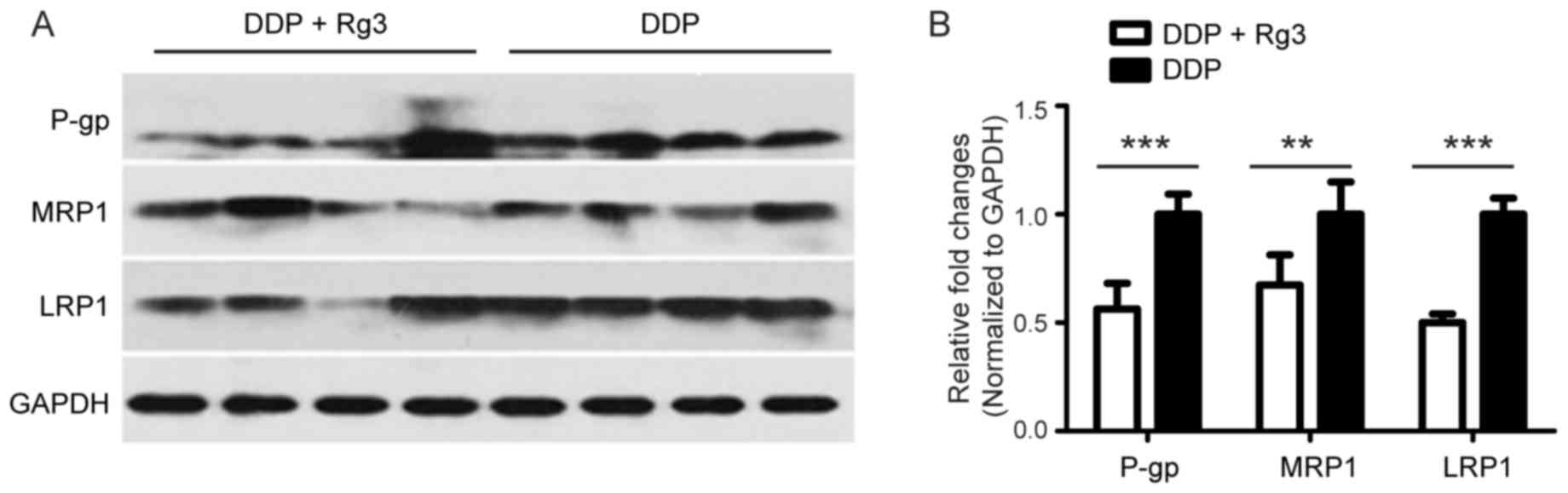
Rg3 reverses MDR by downregulating the
expression of MRP1, LRP and P-gp
To identify the mechanism by which Rg3 reversed MDR,
the mRNA expression levels of multidrug resistance-associated
proteins including MRP1, LRP, and P-gp in tumor tissue of A549/DDP
xenograft mice treated with DDP and/or Rg3. The results revealed
that the mRNA and protein levels of MRP1, LRP, and P-gp in mice
treated with the DDP-Rg3 combination therapy were significantly
downregulated compared with animals treated using DDP alone
(Figs. 3 and 4). Taken together, these results indicated
that Rg3 reversed MDR in vivo by downregulating the
expression of MDR-associated genes.
Discussion
Lung cancer represents a considerable threat to
global health and human life (35).
Over half of patients with lung cancer are diagnosed late,
resulting in a minimal opportunity for patients to undergo surgical
resection (36). Currently,
chemotherapy is the primary therapeutic treatment (37,38). DDP
is the most common chemotherapy drug used, which causes the death
of tumor cells by induction of DNA damage (39). DDP-based combination chemotherapies
are widely used for patients with lung cancer (40). However, the occurrence of chemotherapy
resistance in lung cancer prevents successful treatment (41). Thus, MDR in lung cancer is a
widespread problem that remains unresolved.
Rg3 is a pharmaceutical ingredient extracted from
the Chinese herb P. ginseng. It has been reported that
ginsenosides such as Rg1, Re, Rc and Rd exert an inhibitory effect
on drug efflux pumps, thus preventing MDR in lymphoma (20). In the present study, a cell viability
assay revealed the increased DDP cytotoxicity of A549/DDP cells via
when treated with Rg3, which indicated that Rg3 may increase the
sensitivity of lung cancer cells to DDP in vitro.
Furthermore, Rg3 increases the antitumor effect of DDP on A549/DDP
xenograft mice. These results indicated that Rg3 may reverse MDR of
lung cancer. Consistent with the results of the present study,
previous studies reported that Rg3 was able to reverse MDR in oral
cancer KBV20C cells in a dose-dependent manner (9,25).
The efflux of drugs from cancer cells is one of the
mechanisms involved in drug resistance (42,43).
Increases of drug efflux from cancer cells are mediated by the
upregulation of ABC proteins, including the membrane transporters
P-gp and MRP1, in MDR cells (44–46). LPR
is also an important MDR-mediated protein (47). It has also been reported that Rg3
treatment is able to decrease MDR by altering the function of P-gp
(9). In the present study, the
expression of these proteins was evaluated in A549/DDP cells
treated with Rg3. The results revealed that Rg3 treatment
significantly decreased the mRNA and protein levels of P-gp, MRP1
and LRP in A549/DDP cells. Accordingly, a previous study reported
that Rg3 treatment reversed the MDR of A549/DDP cells by the
downregulation of membrane transporters (48).
99mTc-MIBI, a well-documented
tumor-seeking radiotracer, is a substrate of P-gp, which is widely
used for in vivo imaging, including cardiac, breast,
thyroid, bones, central nervous system and lung visualization
(7,29,49,50). The
accumulation of 99mTc-MIBI in tumor cells is increased
by preventing the efflux transport function (51). In the present study,
99mTc-MIBI SPECT was used to monitor the reversed effect
of Rg3 on P-gp-associated MDR in A549/DDP xenograft mice. The
uptake ratio detected by 99mTc-MIBI imaging is
positively associated with P-glycoprotein (P-gp) expression in
A549/DDP xenograft tumors, which indicated that
99mTc-MIBI SPECT may be a valuable diagnostic imaging
technique for assessing P-gp-associated MDR.
The study of MDR is valuable for identifying novel
strategies to overcome the treatment failure of lung cancer. In the
present study, Rg3 increased the sensitivity of A549/DDP cells to
DDP via downregulation of MDR-mediated proteins including P-gp,
MRP1 and LRP. These results indicating that Rg3 may possess the
potential to be used in reversing the MDR of lung cancer in
patients experiencing treatment failure.
Acknowledgements
This project was funded by the Basic Research Joint
Project of Yunnan Provincial Science and Technology Department
(grant no. 2013FB170), Scientific research foundation projects of
Yunnan Provincial Department of Education (grant no. 2017zzx197)
and Key Laboratory of Tumor Immunological Prevention and Treatment
of Yunnan Province (grant no. 2017DG004).
References
|
1
|
Siegel R, Naishadham D and Jemal A: Cancer
statistics, 2013. CA Cancer J Clin. 63:11–30. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
She J, Yang P, Hong Q and Bai C: Lung
cancer in China: Challenges and interventions. Chest.
143:1117–1126. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Thatcher N and Heighway J: Maintenance and
consolidation therapy in patients with unresectable stage III/IV
non-small cell lung cancer. Oncologist. 15:1034–1042. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Sereno M, Rodriguez-Esteban I,
Gómez-Raposo C, Merino M, López-Gómez M, Zambrana F and Casado E:
Lung cancer and peritoneal carcinomatosis. Oncol Lett. 6:705–708.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Hellmann MD, Li BT, Chaft JE and Kris MG:
Chemotherapy remains an essential element of personalized care for
persons with lung cancers. Ann Oncol. 27:1829–1835. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Wang J, Zhang J, Zhang L, Zhao L, Fan S,
Yang Z, Gao F, Kong Y, Xiao GG and Wang Q: Expression of P-gp, MRP,
LRP, GST-pi and TopoIIalpha and intrinsic resistance in human lung
cancer cell lines. Oncol Rep. 26:1081–1089. 2011.PubMed/NCBI
|
|
7
|
Wang XS, Zhang YJ, Liu XL, Zhou ZR, Hu CS
and Eisbruch A: The role of technetium-99m methoxyisobutyl
isonitrile scintigraphy in predicting the therapeutic effect of
chemotherapy against nasopharyngeal carcinoma. Cancer.
117:2435–2441. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kim SS, Seong S and Kim SY: Synergistic
effect of ginsenoside Rg3 with verapamil on the modulation of
multidrug resistance in human acute myeloid leukemia cells. Oncol
Lett. 7:1265–1269. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kwon HY, Kim EH, Kim SW, Kim SN, Park JD
and Rhee DK: Selective toxicity of ginsenoside Rg3 on multidrug
resistant cells by membrane fluidity modulation. Arch Pharm Res.
31:171–177. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Sadava D and Kane SE: Silibinin reverses
drug resistance in human small-cell lung carcinoma cells. Cancer
Lett. 339:102–106. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Gottesman MM, Fojo T and Bates SE:
Multidrug resistance in cancer: Role of ATP-dependent transporters.
Nature Rev Cancer. 2:48–58. 2002. View
Article : Google Scholar
|
|
12
|
Sharom FJ: The P-glycoprotein multidrug
transporter. Essays Biochem. 50:161–178. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Polgar O and Bates SE: ABC transporters in
the balance: Is there a role in multidrug resistance? Biochem Soc
Trans. 33:241–245. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Steinbach D and Legrand O: ABC
transporters and drug resistance in leukemia: Was P-gp nothing but
the first head of the Hydra? Leukemia. 21:1172–1176. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zhu WY, Hunag YY, Liu XG, He JY, Chen DD,
Zeng F, Zhou JH and Zhang YK: Prognostic evaluation of CapG,
gelsolin, P-gp, GSTP1 and Topo-II proteins in non-small cell lung
cancer. Anat Rec (Hoboken). 295:208–214. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Borst P, Evers R, Kool M and Wijnholds J:
A family of drug transporters: The multidrug resistance-associated
proteins. J Natl Cancer Inst. 92:1295–1302. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Scheffer GL, Wijngaard PL, Flens MJ,
Izquierdo MA, Slovak ML, Pinedo HM, Meijer CJ, Clevers HC and
Scheper RJ: The drug resistance-related protein LRP is the human
major vault protein. Nat Med. 1:578–582. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Attele AS, Wu JA and Yuan CS: Ginseng
pharmacology: Multiple constituents and multiple actions. Biochem
Pharmacol. 58:1685–1693. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Gillis CN: Panax ginseng pharmacology: A
nitric oxide link? Biochem Pharmacol. 54:1–8. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Molnar J, Szabo D, Pusztai R, Mucsi I,
Berek L, Ocsovszki I, Kawata E and Shoyama Y: Membrane associated
antitumor effects of crocine-, ginsenoside- and cannabinoid
derivates. Anticancer Res. 20:861–867. 2000.PubMed/NCBI
|
|
21
|
Geng L, Fan J, Gao QL, Yu J and Hua BJ:
Preliminary study for the roles and mechanisms of 20(R)-ginsenoside
Rg3 and PEG-PLGA-Rg3 nanoparticles in the Lewis lung cancer mice.
Beijing Da Xue Xue Bao Yi Xue Ban. 48:496–501. 2016.(In Chinese).
PubMed/NCBI
|
|
22
|
Zhang F, Li M, Wu X, Hu Y, Cao Y, Wang X,
Xiang S, Li H, Jiang L, Tan Z, et al: 20(S)-ginsenoside Rg3
promotes senescence and apoptosis in gallbladder cancer cells via
the p53 pathway. Drug Des Devel Ther. 9:3969–3987. 2015.PubMed/NCBI
|
|
23
|
Cheong JH, Kim H, Hong MJ, Yang MH, Kim
JW, Yoo H, Yang H, Park JH, Sung SH, Kim HP and Kim J:
Stereoisomer-specific anticancer activities of ginsenoside Rg3 and
Rh2 in HepG2 cells: Disparity in cytotoxicity and
autophagy-inducing effects due to 20(S)-epimers. Biol Pharm Bull.
38:102–108. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Liu T, Zhao L, Zhang Y, Chen W, Liu D, Hou
H, Ding L and Li X: Ginsenoside 20(S)-Rg3 targets HIF-1α to block
hypoxia-induced epithelial-mesenchymal transition in ovarian cancer
cells. PLoS One. 9:e1038872014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Kim SW, Kwon HY, Chi DW, Shim JH, Park JD,
Lee YH, Pyo S and Rhee DK: Reversal of P-glycoprotein-mediated
multidrug resistance by ginsenoside Rg (3). Biochem Pharmacol.
65:75–82. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Zhu W, Xu H, Zhu D, Zhi H, Wang T, Wang J,
Jiang B, Shu Y and Liu P: miR-200bc/429 cluster modulates multidrug
resistance of human cancer cell lines by targeting BCL2 and XIAP.
Cancer Chemother Pharmacol. 69:723–731. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Yamaue H, Tanimura H, Noguchi K, Hotta T,
Tani M, Tsunoda T, Iwahashi M, Tamai M and Iwakura S:
Chemosensitivity testing of fresh human gastric cancer with highly
purified tumour cells using the MTT assay. Br J Cancer. 66:794–799.
1992. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Loening AM and Gambhir SS: AMIDE: A free
software tool for multimodality medical image analysis. Mol
Imaging. 2:131–137. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Duan XY, Wang JS, Liu M and Guo YM:
Technetium-99m-hexakis-2-methoxyisobutylisonitrile scintigraphy and
multidrug resistance-related protein expression in human primary
lung cancer. Ann Nucl Med. 22:49–55. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Wang H, Wang L, Song L, Zhang YW, Ye J, Xu
RX, Shi N and Meng XM: TNNI3K is a novel mediator of myofilament
function and phosphorylates cardiac troponin I. Braz J Med Biol
Res. 46:128–137. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Wang L, Wang H, Ye J, Xu RX, Song L, Shi
N, Zhang YW, Chen X and Meng XM: Adenovirus-mediated overexpression
of cardiac troponin I-interacting kinase promotes cardiomyocyte
hypertrophy. Clin Exp Pharmacol Physiol. 38:278–284. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Gao Y, Li W, Liu X, Gao F and Zhao X:
Reversing effect and mechanism of soluble resistance-related
calcium-binding protein on multidrug resistance in human lung
cancer A549/DDP cells. Mol Med Rep. 11:2118–2124. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Wang H, Chen Y, Lu XA, Liu G, Fu Y and Luo
Y: Endostatin prevents dietary-induced obesity by inhibiting
adipogenesis and angiogenesis. Diabetes. 64:2442–2456. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Zhou W and Christiani DC: East meets west:
Ethnic differences in epidemiology and clinical behaviors of lung
cancer between East Asians and caucasians. Chin J Cancer.
30:287–292. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Li K, Chen B, Xu L, Feng J, Xia G, Cheng
J, Wang J, Gao F and Wang X: Reversal of multidrug resistance by
cisplatin-loaded magnetic Fe3O4 nanoparticles in A549/DDP lung
cancer cells in vitro and in vivo. Int J Nanomedicine. 8:1867–1877.
2013.PubMed/NCBI
|
|
37
|
Yan LH, Wei WY, Cao WL, Zhang XS, Xie YB
and Xiao Q: Overexpression of E2F1 in human gastric carcinoma is
involved in anti-cancer drug resistance. BMC Cancer. 14:9042014.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Kang JH, Lee SI, Lim DH, Park KW, Oh SY,
Kwon HC, Hwang IG, Lee SC, Nam E, Shin DB, et al: Salvage
chemotherapy for pretreated gastric cancer: A randomized phase III
trial comparing chemotherapy plus best supportive care with best
supportive care alone. J Clin Oncol. 30:1513–1518. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Montagnani F, Turrisi G, Marinozzi C,
Aliberti C and Fiorentini G: Effectiveness and safety of
oxaliplatin compared to cisplatin for advanced, unresectable
gastric cancer: A systematic review and meta-analysis. Gastric
Cancer. 14:50–55. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Ohe Y, Ohashi Y, Kubota K, Tamura T,
Nakagawa K, Negoro S, Nishiwaki Y, Saijo N, Ariyoshi Y and Fukuoka
M: Randomized phase III study of cisplatin plus irinotecan versus
carboplatin plus paclitaxel, cisplatin plus gemcitabine and
cisplatin plus vinorelbine for advanced non-small-cell lung cancer:
Four-Arm cooperative study in Japan. Ann Oncol. 18:317–323. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Chung AS, Wu X, Zhuang G, Ngu H, Kasman I,
Zhang J, Vernes JM, Jiang Z, Meng YG, Peale FV, et al: An
interleukin-17-mediated paracrine network promotes tumor resistance
to anti-angiogenic therapy. Nat Med. 19:1114–1123. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Kawakami M, Nakamura T, Okamura N, Komoto
C, Markova S, Kobayashi H, Hashimoto N, Okumura K and Sakaeda T:
Knock-down of sorcin induces up-regulation of MDR1 in HeLa cells.
Biol Pharm Bull. 30:1065–1073. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Akazawa Y, Kawaguchi H, Funahashi M,
Watanabe Y, Yamaoka K, Hashida M and Takakura Y: Effect of
interferons on P-glycoprotein-mediated rhodamine-123 efflux in
cultured rat hepatocytes. J Pharm Sci. 91:2110–2115. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Toner AP, McLaughlin F, Giles FJ, Sullivan
FJ, O'Connell E, Carleton LA, Breen L, Dunne G, Gorman AM, Lewis JD
and Glynn SA: The novel toluidine sulphonamide EL102 shows
pre-clinical in vitro and in vivo activity against prostate cancer
and circumvents MDR1 resistance. Br J Cancer. 109:2131–2141. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Tajitsu Y, Ikeda R, Nishizawa Y, Mataki H,
Che XF, Sumizawa T, Nitta M, Yamaguchi T, Yamamoto M, Tabata S, et
al: Molecular basis for the expression of major vault protein
induced by hyperosmotic stress in SW620 human colon cancer cells.
Int J Mol Med. 32:703–708. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Keppler D: Multidrug resistance proteins
(MRPs, ABCCs): Importance for pathophysiology and drug therapy.
Handb Exp Pharmacol. 1–323. 2011.
|
|
47
|
Tognon G, Bernasconi S, Celli N, Faircloth
GT, Cuevas C, Jimeno J, Erba E and D'Incalci M: Induction of
resistance to Aplidin in a human ovarian cancer cell line related
to MDR expression. Cancer Biol Ther. 4:1325–1330. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Jin L, Xu M, Luo XH and Zhu XF: Stephania
tetrandra and ginseng-containing chinese herbal formulation NSENL
reverses cisplatin resistance in lung cancer xenografts. Am J Chin
Med. 45:385–401. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Mubashar M, Harrington KJ, Chaudhary KS,
Lalani el-N, Stamp GW, Sinnett D, Glass DM and Peters AM:
99mTc-sestamibi imaging in the assessment of toremifene as a
modulator of multidrug resistance in patients with breast cancer. J
Nucl Med. 43:519–525. 2002.PubMed/NCBI
|
|
50
|
Saggiorato E, Angusti T, Rosas R,
Martinese M, Finessi M, Arecco F, Trevisiol E, Bergero N,
Puligheddu B, Volante M, et al: 99mTc-MIBI imaging in the
presurgical characterization of thyroid follicular neoplasms:
Relationship to multidrug resistance protein expression. J Nucl
Med. 50:1785–1793. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Piwnica-Worms D, Chiu ML, Budding M,
Kronauge JF, Kramer RA and Croop JM: Functional imaging of
multidrug-resistant P-glycoprotein with an organotechnetium
complex. Cancer Res. 53:977–984. 1993.PubMed/NCBI
|