Introduction
High-grade gliomas (HGGs), including glioblastoma
(GBM, World Health Organization, WHO, grade IV) and anaplastic
glioma (WHO grade III), are the most frequently primary brain
tumors (1). Current therapeutic
approaches include surgery, chemotherapy, radiotherapy, and
molecular targeted therapy, however, the overall prognosis of the
disease remains poor (2,3). Therefore, it is critical for developing
novel and effective molecular makers to assist early diagnosis and
accurate prediction of prognosis in patients with glioma.
Microarray and sequencing techniques have provided
better approaches for screening effective prognostic markers for
human cancers (4,5). It is well known that nuclear factor κB
(NF-κB) plays a significant role in inflammatory reaction and
tumorigenesis (6–8). Recently, their key regulatory protein,
relB, has been reported to overexpressed and associated with
pathogenesis of malignancies (9).
However, the molecular features and clinical signature of gliomas
with relB expression remain poorly understood.
In this study, we evaluated the relB expression in
the RNA sequencing of Diffuse Gliomas data and found the expression
of relB was associated with glioma grade and showed a mesenchymal
subtype preference. In vitro experiments demonstrated that
relB reduction inhibited glioma cell migration and invasion by
regulating MMPs, MMP2 and MMP9 especially (10,11).
Further more, some researchers find that relB/NF-κB links cell
cycle transition and proliferation to tumorigenesis (12–14). These
data demonstrate that relB drives malignant behavior of gliomas,
and it may be a novel prognostic biomarker in glioma.
Materials and methods
Human glioma tissues and cell
lines
The raw sequencing data for 325 gliomas was
downloaded from the Chinese Glioma Genome Atlas (CGGA) data portal
(http://www.cgga.org.cn/portal.php).
Human LN229 glioma cell line was obtained from the Chinese Academy
of Sciences cell bank. Glioma cell line was cultured in Dulbecco's
modified Eagle's medium (DMEM) (Thermo Fisher Scientific, Inc.,
Waltham, MA, USA), which was supplemented with 10% fetal bovine
serum (Thermo Fisher Scientific, Inc.), 100 units/ml penicillin,
and 100 ng/ml streptomycin (Abcam, Shanghai, China). All cells were
incubated at 37°C in an atmosphere of 5% CO2.
Oligonucleotides and cell
transfection
The relB-siRNA, MMP9-siRNA, and MMP2-siRNA
oligonucleotides were designed and synthesized by GenePharma and
Gene chem (Shanghai, China). An siRNA that was unrelated to any
human sequence was used as a negative control (NC). The plasmid
containing the ORF of relB, was generated from Abcam, Shanghai,
China. Blank vector was used as an NC. Then transfection complexes
were formed from oligonucleotides using Lipofectamine 2000 (Thermo
Fisher Scientific, Inc., Waltham, MA, USA). Transfection complexes
have been added to glioma cells and incubated for 6–8 h before the
medium was changed.
Cell proliferation assay
Cells in the logarithmic phase of growth were seeded
at 3,000 per well in 96-well plates and cultured. Cell
proliferation was assayed at the indicated time points using a CCK8
kit (Beyotime Institute of Biotechnology, Haimen, China) according
to the manufacturer's instructions.
Invasion assay
Cell invasion assays were performed using transwell
membranes coated with Matrigel (BD Biosciences, Franklin Lakes, NJ,
USA). Transfected cells were plated at a density of
3×104 cells per well in the upper chamber and in
serum-free medium. The lower chamber was filled with 20% FBS as a
chemo-attractant. After 24 h of incubation, cells remaining in the
upper chamber of each well were carefully removed with cotton
swabs, and invading cells were fixed with 3% paraformaldehyde
(Abcam, Shanghai, China), stained with crystal violet (Abcam,
Shanghai, China), and counted from three independent fields (×100
magnification).
Wound healing assay
Cells were cultured until reached 90% confluence in
6-well plates. Cell layers were scratched using a 20-µl tip to form
wound gaps, washed twice with PBS and cultured. The wound healing
was photographed at different time points and wounded gaps were
analyzed by measuring the distance of migrating cells for three
different areas for each wound.
Western blot analysis
Equal amounts of protein per lane were separated by
8% SDS-polyacrylamide gel and transferred to Polyvinylidene
difluoride (PVDF) membrane. The membrane was blocked in 5% skim
milk for 2 h and then incubated with diluted primary antibody in 5%
w/v BSA, 1X TBS, 0.1% Tween20 at 4°C with gentle shaking,
overnight. The antibodies used in this study were: relB (1:1,000;
10544, Cell Signaling Technology, Danvers, MA, USA), MMP2 (1:1,000;
87809, Cell Signaling Technology, Danvers, MA, USA), MMP9 (1:1,000;
13667, Cell Signaling Technology, Danvers, MA, USA) and cyclin A
(1:1,000; 554175, BD Pharmingen, San Diego, CA, USA). The antibody
against GAPDH (glyceraldehyde-3-phosphate dehydrogenase) (Abcam,
Shanghai, China) was used as a control. The specific protein was
detected by using a SuperSignal protein detection kit (Abcam,
Shanghai, China). The band densities of specific proteins were
quantified after normalization with the density of GAPDH. Secondary
antibody is HRP Goat Anti-rabbit Ig (1:1,000; 7074, Cell Signaling
Technology, Danvers, MA, USA) and HRP Goat Anti-Mouse Ig (1:1,000;
554002, BD Pharmingen, San Diego, CA, USA).
Statistical analysis
Each value was obtained from at least three
independent experiments and presented as means ± SD. Significant
differences were calculated using one-way ANOVA followed by S-N-K
method for three-group comparisons and t-test for two-group
comparisons. The SPSS 22.0 software package was employed (SPSS,
Inc., Chicago, IL, USA). Pearson correlation analysis was performed
using MATLAB software (The MathWorks, Inc., Natick, Massachusetts,
USA). A probability value of <0.05 was considered statistically
significant.
Results
RelB expression was associated with
glioma grade and showed a mesenchymal subtype preference
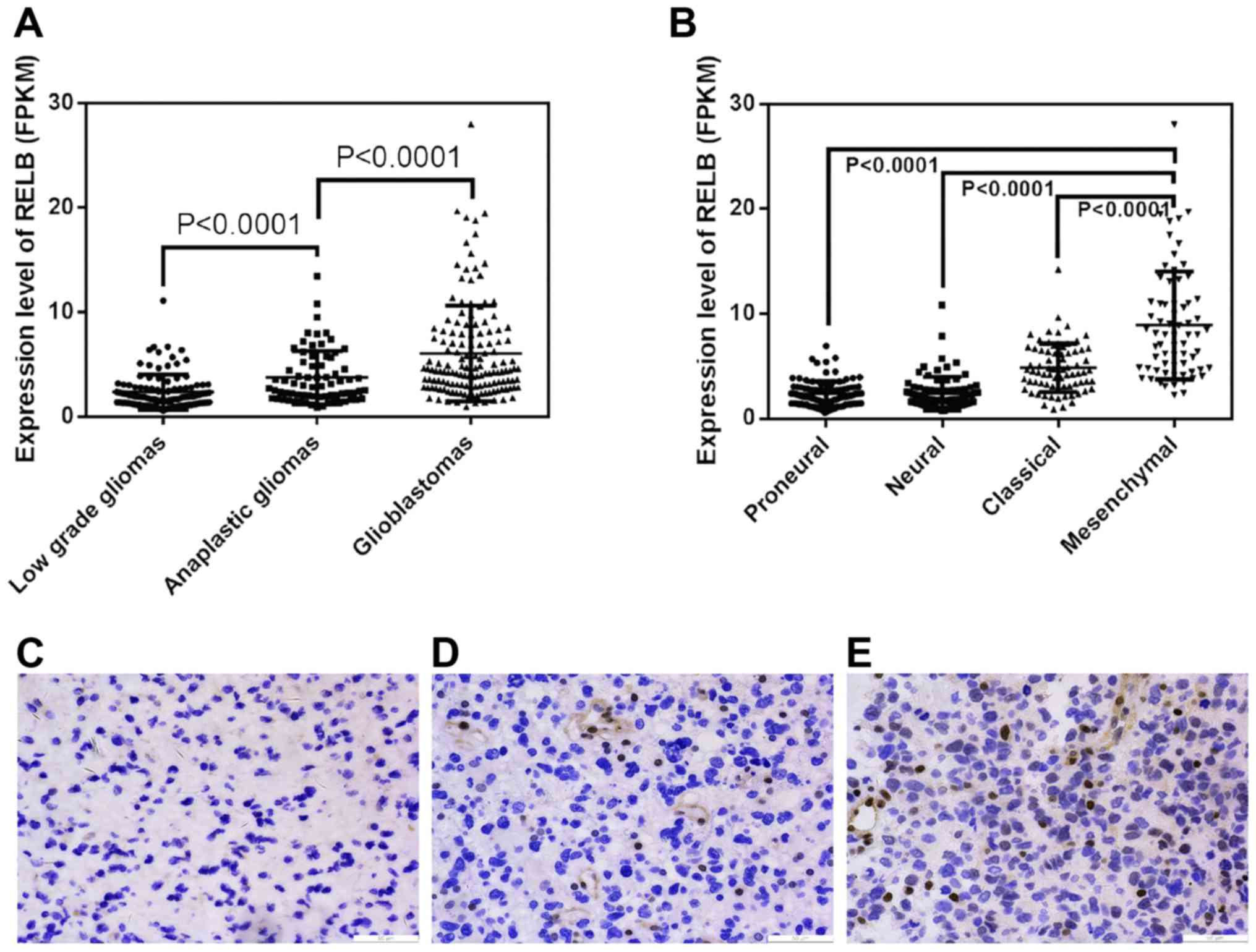
Using the RNA sequencing of Diffuse Gliomas data, we
initially explored relB expression patterns in different grades of
glioma tissues and found that the level of relB mRNA increased
markedly in gliomas of increasing malignancy grades (Fig. 1A). Moreover, we evaluated relB
expression in TCGA molecular subtypes of gliomas and found that
relB showed a mesenchymal subtype preference (Fig. 1B). We further validated the protein
expression level of relB in an independent group of 32 glioma
patients by Immunohistochemistry (IHC) (Fig. 1C). Similar to the findings above, relB
were up-regulated with ascending malignancy grades.
Low expression of relB was a better
prognostic marker in anaplastic gliomas and glioblastomas
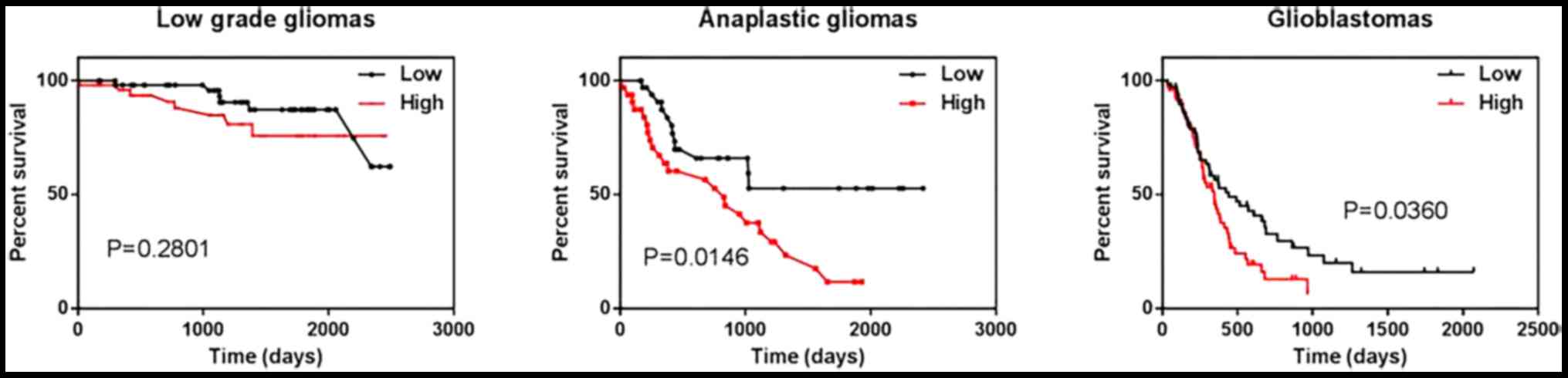
Next we investigated the correlation between relB
expression and overall survival using Kaplan-Meier survival curve
analysis. We found that expressing higher than mean levels of relB
were associated with decreased survival relative to those with relB
levels lower than the mean in anaplastic glioma and GBM patients
(Fig. 2). Therefore, Low expression
of relB was a better prognostic marker in anaplastic gliomas and
glioblastomas.
relB-associated genes were mainly
involved in cell-cycle and migration biological process
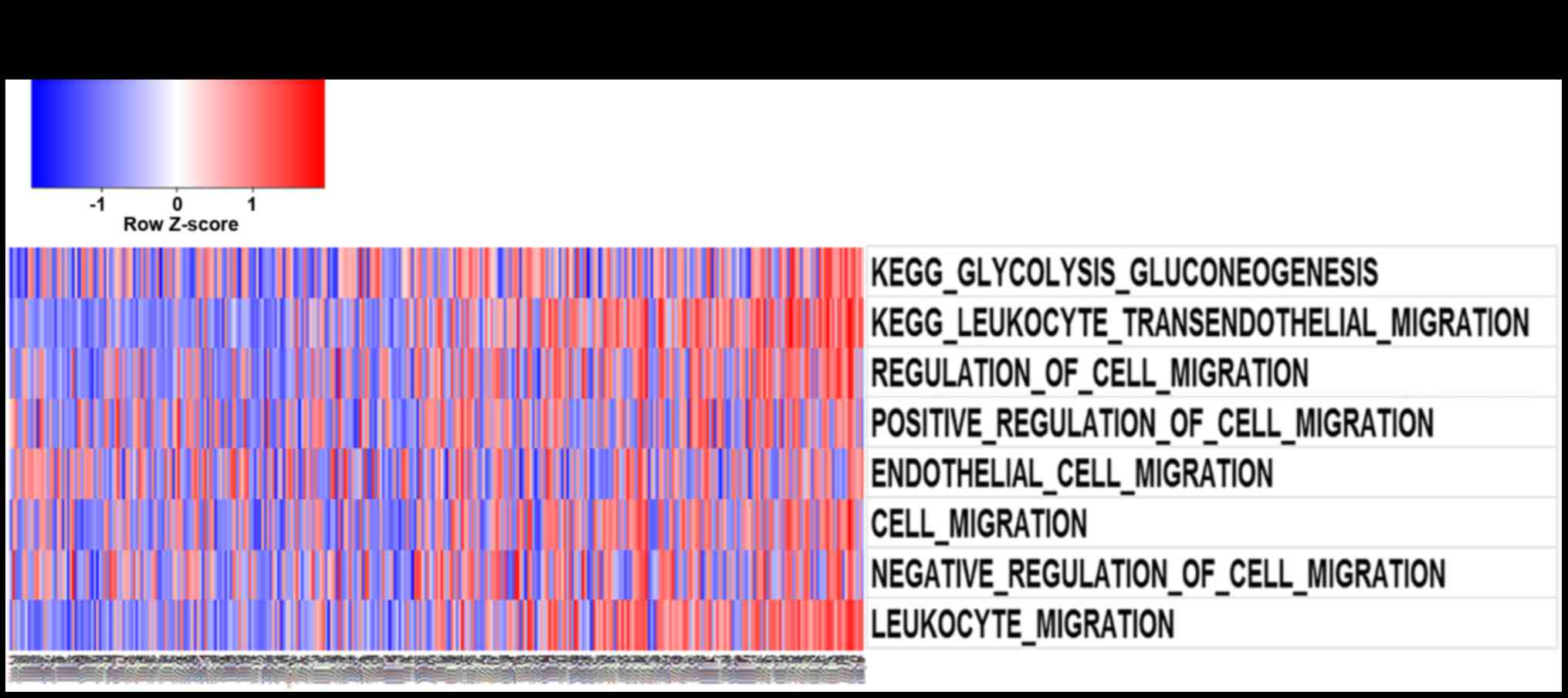
After Pearson correlation analysis of the RNA
sequencing data, the significantly positively correlated genes were
used for GO analysis. Gene set variation analysis with relB
expression was analyzed by GSVA package of R.Gene list was obtained
from GSVA date package (15). As
illustrated in Fig. 3A and B,
function annotation of relB which was performed by GO analysis and
GSVA revealed its correlation with cell-cycle and migration
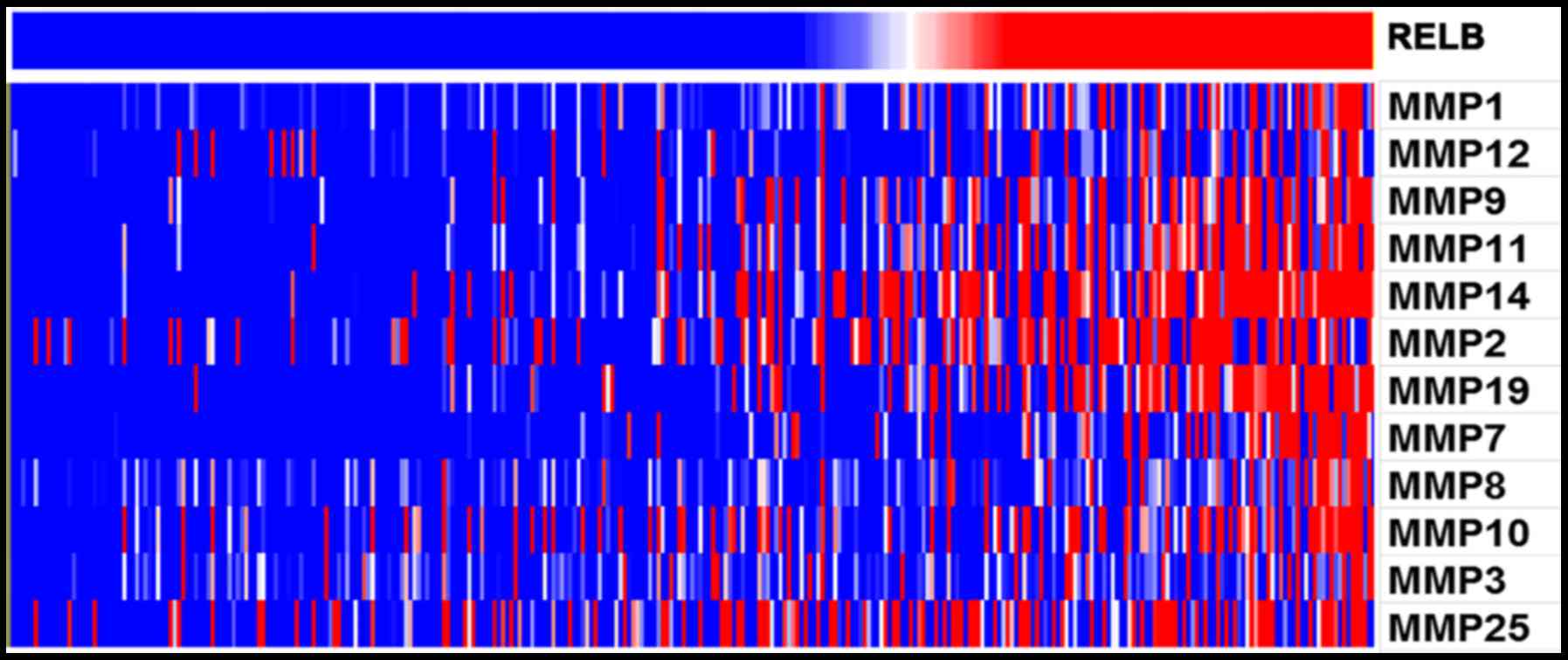
biological process. Furthermore, With the increase of relB
expression, the expression of MMP family genes increased
(P<0.05). MMP family genes were found to positively correlate
with relB expression (Fig. 4). In
particular, MMP2 and MMP9 were known to promote cell invasion and
migration in cancers. Overall, relB may involves in glioma
invasion, migration, and cell-cycle biological process.
Repression of relB induces glioma cell
cycle arrest
We then constructed a lentivirus containing a siRNA
sequence targeting relB to verify whether relB plays a potentially
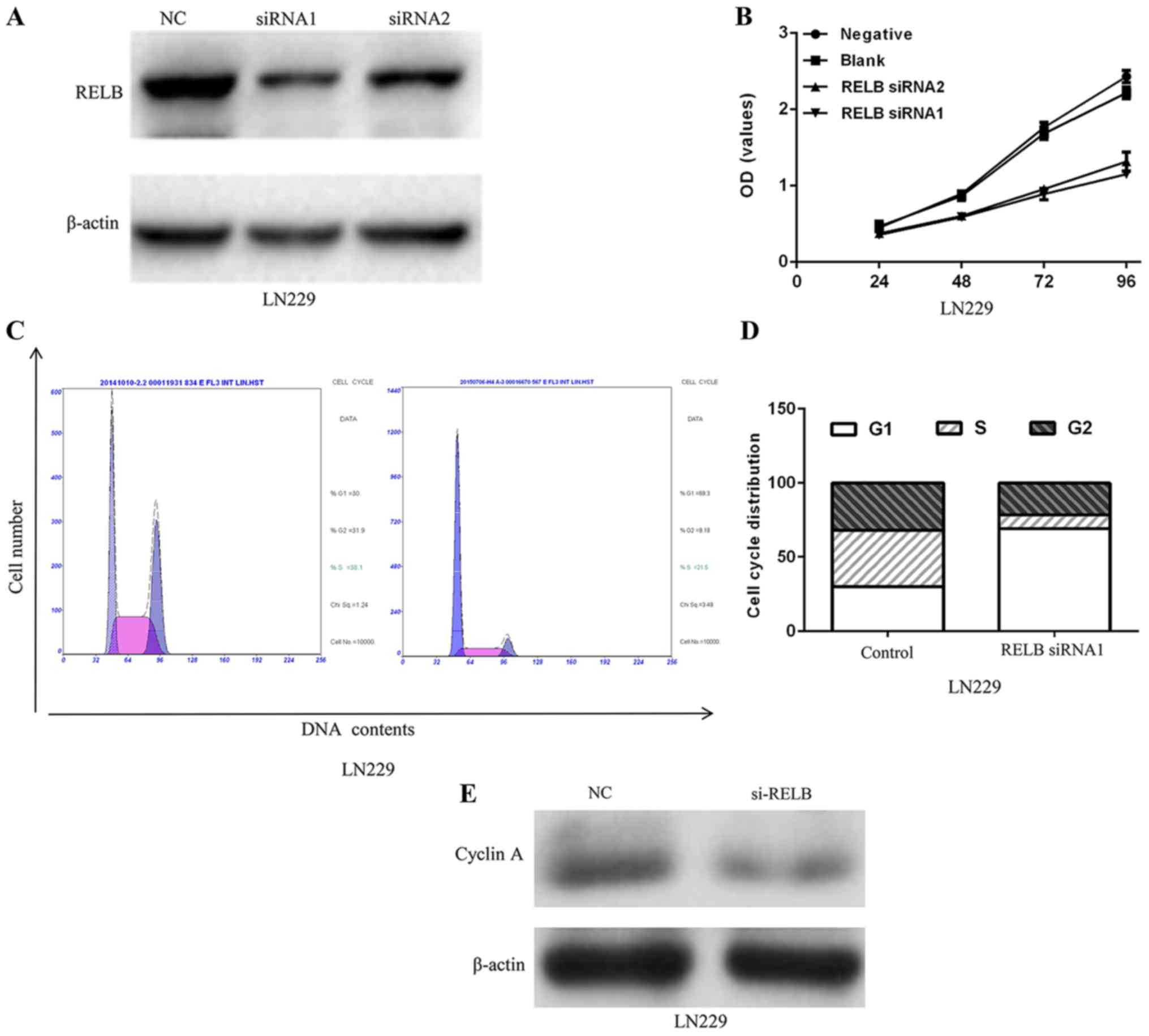
functional role in glioma. After 48 h of infection, relB protein
expression levels in LN229 were decreased (Fig. 5A). To investigate the biological
implication of relB in glioma, we performed functional assays to
determine the influence of relB on glioma cell proliferation.
Decreased relB expression in LN229 inhibited the proliferation of
cell line (Fig. 5B). Moreover, flow
cytometry analysis showed that the cell cycle was blocked in the
G0-G1 phase as a result of decreased relB (Fig. 5C and D). A significant decrease of
cyclin A protein expression level in the LN229 cells was observed
after relB knockdown compared with the control group (Fig. 5E).
The down-regulation of relB expression
inhibits glioma cell migration and invasion by regulating the MMPs
in vitro
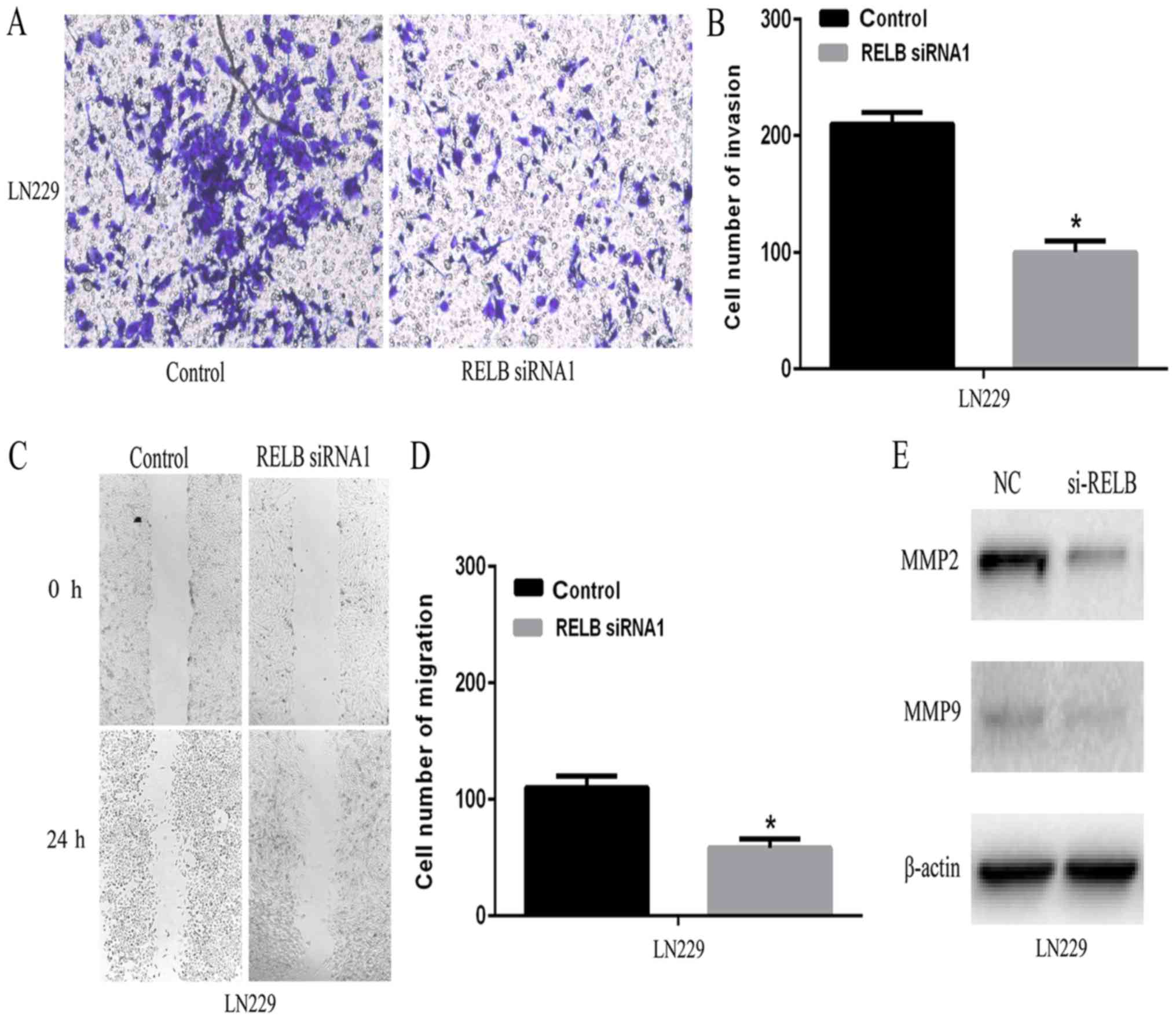
To explore the potential role of relB on the
invasiveness of glioma cells, wound healing and transwell assay
were employed in vitro. As shown in Fig. 6A and B, relB knockdown resulted in a
marked decrease in cell invasion compared to the control group.
Subsequently, the percentage of migrating cells in relB knockdown
group was significantly lower than that of the control group
(Fig. 6C and D). MMPs family genes
were critical for cancer cell migration and invasion. Our
bioinformatics analysis implied that MMP family genes were
positively correlated with relB. In particular, MMP2 and MMP9 were
known to promote cell invasion and migration in cancer cells
(16–18). Therefore, we further tested MMP2 and
MMP9 expression in relB knockdown and control cells by western
blotting. Compared with the control cells, relB knockdown cells
showed decreased MMP9 and MMP2 expression (Fig. 6E). Furthermore, transwell and wound
healing assays showed that overexpression of relB promoted glioma
cell invasion and migration whereas introduction of si-MMP9 or
si-MMP2 abrogated relB overexpression induced cellular invasion and
migration. These results suggest that relB modulates malignant
progression at least partially through MMP9 and MMP2.
Discussion
Glioma is the most common lethal intracranial tumor
in adults. Despite of tremendous efforts work on developing
multimodal treatments and mechanisms, the overall prognosis of
glioma remains poor (1-3). It is a matter of great
urgency to identified novel biomarkers for patients of particular
High-grade gliomas (HGGs).
RelB is originally found as a key transcription
factors of Nuclear factor kB (NF-kB) which regulates a wide range
of biological processes, such as cell survival, immune and
inflammatory responses (19–21). Emerging evidences have implicated that
relB plays an important role in the progression in different types
of cancers. In prostate cancer, relB promotes cancer cell growth
and decreases the radiosensitivity of cancer cells (22,23). In
breast tumor, relB enhances cellular survival and shows a highly
invasive phenotypes preference (24).
In chronic lymphocytic leukemia (CLL) cells, overexpression relB
increases the sensitivity of CLL cells to the proteasome inhibitor,
bortezomib (25). In the present
study, we demonstrate that relB expression is associated with
glioma grade and shows a mesenchymal subtype preference. Besides,
low expression of relB is a better prognostic marker in anaplastic
gliomas and glioblastomas. Therefore, our results suggest that relB
may be associated with glioma progression.
To further clarify the role of relB in the
development and progression of glioma, bioinformatics and
experiment in vitro were applied to analyze the molecular
function of relB. Through bioinformatics analysis, relB-associated
genes were mainly involved in cell-cycle and migration biological
process. To further verify the biological behavior of relB in
gliomas, we constructed lentiviral vectors expressing nonsense
control or relB siRNA, and subsequently infected the LN229 glioma
cells. In vitro assay, Repression of relB induces cell cycle
arrest and inhibits glioma cell migration and invasion of glioma
cells. The basement membrane consist of a network of extracellular
matrix (ECM) proteins which participate in tumor growth, invasion,
migration and tumor angiogenesis (26). MMPs are a large family of
Ca2+ and Zn+ dependent endopeptidases,
especially among members MMP2 and MMP9, possessing to hydrolyze
components of the basement membrane (27,28).
Furthermore, our results suggest that relB modulates malignant
progression at least partially through MMP9 and MMP2.
In summary, our results reveal that relB is
upregulated in glioma and exhibit pro-oncogenic activity partially
through MMP9 and MMP2. Importantly, relB can be used as a potential
diagnostic and prognostic marker for some HGGs, especially for
mesenchymal subtype glioblastoma.
References
|
1
|
Wang Y and Jiang T: Understanding high
grade glioma: Molecular mechanism, therapy and comprehensive
management. Cancer Lett. 331:139–146. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
van den Bent M, Chinot OL and Cairncross
JG: Recent developments in the molecular characterization and
treatment of oligodendroglial tumors. Neuro Oncol. 5:128–138. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Kalpathy-Cramer J, Gerstner ER, Emblem KE,
Andronesi OC and Rosen B: Advanced magnetic resonance imaging of
the physical processes in human glioblastoma. Cancer Res.
74:4622–4637. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Van Schaeybroeck S, Allen WL, Turkington
RC and Johnston PG: Implementing prognostic and predictive
biomarkers in CRC clinical trials. Nat Rev Clin Oncol. 8:222–232.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Reis-Filho JS and Pusztai L: Gene
expression profiling in breast cancer: Classification,
prognostication, and prediction. Lancet. 378:1812–1823. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Hayden MS and Ghosh S: NF-κB, the first
quarter-century: Remarkable progress and outstanding questions.
Genes Dev. 26:203–234. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Perkins ND: The diverse and complex roles
of NF-κB subunits in cancer. Nat Rev Cancer. 12:121–132. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
DiDonato JA, Mercurio F and Karin M: NF-κB
and the link between inflammation and cancer. Immunol Rev.
246:379–400. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Cha ST, Tan CT, Chang CC, Chu CY, Lee WJ,
Lin BZ, Lin MT and Kuo ML: Retraction: G9a/RelB regulates
self-renewal and function of colon-cancer-initiating cells by
silencing Let-7b and activating the K-RAS/β-catenin pathway. Nat
Cell Biol. 19:762016. View
Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kitamura T, Kometani K, Hashida H,
Matsunaga A, Miyoshi H, Hosogi H, Aoki M, Oshima M, Hattori M,
Takabayashi A, et al: SMAD4-deficient intestinal tumors recruit
CCR1+ myeloid cells that promote invasion. Nat Genet. 39:467–475.
2007. View
Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kenagy RD, Hart CE, Stetler-Stevenson WG
and Clowes AW: Primate smooth muscle cell migration from aortic
explants is mediated by endogenous platelet-derived growth factor
and basic fibroblast growth factor acting through matrix
metalloproteinases 2 and 9. Circulation. 96:3555–3560. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Ge QL, Liu SH, Ai ZH, Tao MF, Ma L, Wen
SY, Dai M, Liu F, Liu HS, Jiang RZ, et al: RelB/NF-κB links cell
cycle transition and apoptosis to endometrioid adenocarcinoma
tumorigenesis. Cell Death Dis. 7:e24022016. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Sau A, Lau R, Cabrita MA, Nolan E, Crooks
PA, Visvader JE and Pratt MA: Persistent activation of NF-κB in
BRCA1-deficient mammary progenitors drives aberrant proliferation
and accumulation of DNA damage. Cell Stem Cell. 19:52–65. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Gardella KA, Muro I, Fang G, Sarkar K,
Mendez O and Wright CW: Aryl hydrocarbon receptor nuclear
translocator (ARNT) isoforms control lymphoid cancer cell
proliferation through differentially regulating tumor suppressor
p53 activity. Oncotarget. 7:10710–10722. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Hänzelmann S, Castelo R and Guinney J:
GSVA: Gene set variation analysis for microarray and RNA-seq data.
BMC Bioinformatics. 14:72013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zheng H, Takahashi H, Murai Y, Cui Z,
Nomoto K, Niwa H, Tsuneyama K and Takano Y: Expressions of MMP-2,
MMP-9 and VEGF are closely linked to growth, invasion, metastasis
and angiogenesis of gastric carcinoma. Anticancer Res.
26:3579–3583. 2006.PubMed/NCBI
|
|
17
|
Deryugina EI and Quigley JP: Tumor
angiogenesis: MMP-mediated induction of intravasation- and
metastasis-sustaining neovasculature. Matrix Biol 44–46. 1–112.
2015.
|
|
18
|
Gao M, Zhang X, Li D, He P, Tian W and
Zeng B: Expression analysis and clinical significance of eIF4E,
VEGF-C, E-cadherin and MMP-2 in colorectal adenocarcinoma.
Oncotarget. 7:85502–85514. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Guo F, Tänzer S, Busslinger M and Weih F:
Lack of nuclear factor-kappa B2/p100 causes a RelB-dependent block
in early B lymphopoiesis. Blood. 112:551–559. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Bellet MM, Zocchi L and Sassone-Corsi P:
The RelB subunit of NFκB acts as a negative regulator of circadian
gene expression. Cell Cycle. 11:3304–3311. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
McMillan DH, Baglole CJ, Thatcher TH,
Maggirwar S, Sime PJ and Phipps RP: Lung-targeted overexpression of
the NF-κB member RelB inhibits cigarette smoke-induced
inflammation. Am J Pathol. 179:125–133. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Xu Y, Josson S, Fang F, Oberley TD, St
Clair DK, Wan XS, Sun Y, Bakthavatchalu V, Muthuswamy A and St
Clair WH: RelB enhances prostate cancer growth: Implications for
the role of the nuclear factor-kappaB alternative pathway in
tumorigenicity. Cancer Res. 69:3267–3271. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Zhu L, Zhu B, Yang L, Zhao X, Jiang H and
Ma F: RelB regulates Bcl-xl expression and the irradiation-induced
apoptosis of murine prostate cancer cells. Biomed Rep. 2:354–358.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Mineva ND, Wang X, Yang S, Ying H, Xiao
ZX, Holick MF and Sonenshein GE: Inhibition of RelB by
1,25-dihydroxyvitamin D3 promotes sensitivity of breast cancer
cells to radiation. J Cell Physiol. 220:593–599. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Xu J, Zhou P, Sun A and Guo F: Effect of
nuclear transcription factor RelB on the proteasome
inhibitor-sensitivity of chronic lymphocytic leukemia cells.
Zhonghua Xue Ye Xue Za Zhi. 35:524–527. 2014.(In Chinese).
PubMed/NCBI
|
|
26
|
Roskelley CD, Srebrow A and Bissell MJ: A
hierarchy of ECM-mediated signalling regulates tissue-specific gene
expression. Curr Opin Cell Biol. 7:736–747. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Park HI, Ni J, Gerkema FE, Liu D,
Belozerov VE and Sang QX: Identification and characterization of
human endometase (Matrix metalloproteinase-26) from endometrial
tumor. J Biol Chem. 275:20540–20544. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Shah FD, Shukla SN, Shah PM, Shukla HK and
Patel PS: Clinical significance of matrix metalloproteinase 2 and 9
in breast cancer. Indian J Cancer. 46:194–202. 2009. View Article : Google Scholar : PubMed/NCBI
|