Introduction
Hepatocellular carcinoma (HCC) is the sixth most
prevalent cancer worldwide, and is associated with an extremely
poor prognosis (1–3). A principal reason for the high mortality
of this disease is the failure of early diagnosis for patients with
HCC and the lack of effective therapies for patients with HCC in
advanced stages. Despite a number of advances made in therapeutic
strategies and surgery, the overall prognosis for patients with HCC
remains poor due to the high rate of metastasis and recurrence
(4–6).
Hence, the characterization of the molecular mechanisms in HCC is
urgently required to allow the development of novel treatment
methods for patients with HCC.
MicroRNAs (miRNAs/miRs) are small non-coding RNAs
(21–23 nucleotides) that are transcribed as precursors in the
nucleus and are subsequently processed into mature miRNAs in the
cytoplasm. Mature miRNAs primarily function by sequence specific
interactions with the 3′-untranslated regions of mRNAs, leading to
the translational suppression or degradation of the mRNAs (7). An increasing number of studies have
suggested that miRNAs serve an essential role as prognostic and
predictive biomarkers in various types of cancer. For example,
miR-1290 and miR-196b have been demonstrated to predict the
chemotherapeutic response of patients with lung adenocarcinoma
(8). miR-149, an anti-tumor miRNA,
has been identified as dysregulated in a variety of types of
cancer, including gastric (9), breast
(10) and colorectal cancer (11,12).
It is also reported that a number of are associated
with HCC carcinogenesis, progression and metastasis, including
miR-15b (13), miR-122 (14) and miR-29 (15). Furthermore, the low expression of
miR-124 is significantly associated with a more aggressive
phenotype and a poorer prognosis (16), whereas a high expression of miR-182
has been associated with intrahepatic metastasis and poor prognosis
in HCC (17). However, the mechanism
of these miRNAs and their regulatory networks in HCC remain
elusive, and a number of miRNAs that are associated with HCC have
yet to be considered.
miR-33a was originally demonstrated to regulate
lipid and cholesterol metabolism (18), and is an intronic miRNA located within
the sequence of the sterol regulatory element binding protein 2
(SREBP-2) gene. miR-33a has also been implicated as a tumor
suppressor miRNA; Kuo et al (19) identified that miR-33a is downregulated
in lung cancer cells and inhibits osteolytic bone metastasis by
targeting parathyroid hormone. miR-33a has also been demonstrated
to act as a tumor suppressor miRNA, and may downregulate the
expression of the oncogenic kinase Pim-1 in K562 lymphoma cells and
colon carcinoma (20,21). However, the role of miR-33a in HCC is
not yet fully characterized. In the present study, the miR-33a
expression was quantified, and its significance in the prediction
of a prognosis was assessed in patients with HCC.
Materials and methods
Patients and tissue samples
A total of 149 HCC biopsies with a median age of
65.4 years (range 45 to 81 years), 36 of which were pairs of tissue
with para-carcinoma tissues, were extracted between July 2004 and
October 2013 from Tissue Bank, China-Japan Union Hospital, Jilin
University (Jilin, China). All tissues were snap-frozen immediately
in liquid nitrogen and stored at −80°C until the reverse
transcription-quantitative polymerase chain reaction (RT-qPCR)
assay. None of the participants had undergone chemotherapy or
radiotherapy prior to surgery. Clinical features were recorded,
including each participant's characteristics (age, sex,), tumor
characteristics (foci number, diameter, tumor differentiation, and
distant metastasis) and miR-33a expression status, as included in
Table I. The HCC tissues were staged
based on 7th edition of the American Joint Committee on Cancer
Tumor-Node-Metastasis staging system for HCC (22). All patients were grouped as ≥60 or
<60 years of age, and tumors were grouped as ≥5 or <5 cm
according to the tumor diameter.
 | Table I.The association between miR-33a
expression and clinicopathological characteristics in patients with
hepatocellular carcinoma. |
Table I.
The association between miR-33a
expression and clinicopathological characteristics in patients with
hepatocellular carcinoma.
|
|
| miR-33a expression,
n (%) |
|
|---|
|
|
|
|
|
|---|
| Parameters | Cases, n (%) | Low | High | P-value |
|---|
| Age, years |
|
|
| 0.211 |
|
<60 | 66 (44.9) | 29 (19.7) | 37 (25.2) |
|
|
≥60 | 81 (55.1) | 44 (29.9) | 37 (25.2) |
|
|
Unknown | 2 |
|
|
|
| Sex |
|
|
| 0.045 |
|
Male | 117 (79.6) | 63 (42.9) | 54 (36.7) |
|
|
Female | 30 (20.4) | 10 (6.8) | 20 (13.6) |
|
|
Unknown | 2 |
|
|
|
| Number of foci |
|
|
| 0.007 |
|
Single | 97 (68.3) | 39 (27.5) | 58 (40.8) |
|
|
Multiple | 45 (31.7) | 29 (20.4) | 16 (11.3) |
|
|
Unknown | 7 |
|
|
|
| Diameter |
|
|
| 0.078 |
| <5
cm | 91 (68.3) | 40 (27.2) | 51 (34.7) |
|
| ≥5
cm | 56 (31.7) | 33 (22.4) | 23 (15.6) |
|
|
Unknown | 2 |
|
|
|
| Tumor
differentiation |
|
|
| 0.105 |
|
Poor | 10 (7.7) | 7 (5.4) | 3 (2.3) |
|
|
Moderate | 96 (73.8) | 51 (39.2) | 45 (34.6) |
|
|
Well | 24 (18.5) | 8 (6.2) | 16 (12.3) |
|
|
Unknown | 19 |
|
|
|
| Distant
metastasis |
|
|
| 0.223 |
|
Absence | 89 (91.8) | 26 (26.8) | 63 (64.9) |
|
|
Presence | 8 (8.2) | 4 (4.1) | 4 (4.1) |
|
|
Unknown | 52 |
|
|
|
A 150-month follow-up was conducted. The information
required in order to complete the follow-up was received via
outpatient visits or telephone calls, and was updated at
three-month intervals. OS (overall survival) was defined as the
time period from diagnosis to the time of mortality, irrespective
of the cause. PFS (progression free survival) was defined from the
initial date of diagnosis to the time of tumor progression, as
assessed by a computed tomography (CT) scan, or to the time of
mortality due to HCC.
Ethics statement
The present study was approved by the Ethics
Committee of Shanghai Tenth People's Hospital, Tongji University
School of Medicine (Shanghai, China; approval no.,
SHSY-IEC-pap-15-18). Each participant provided written informed
consent prior to participating in the study. All samples were
handled anonymously, according to applicable ethical and legal
standards.
RNA extraction
Total RNA was isolated from HCC and para-carcinoma
tissue specimens using TRIzol reagent (Thermo Fisher Scientific,
Inc., Waltham, MA, USA), according to the manufacturer's protocol.
The RNA concentration was determined using a Nanodrop 1000
spectrophotometer (Nanodrop; Thermo Fisher Scientific, Inc.;
Wilmington, DE, USA) and the purity was identified with 1.5%
denaturing agarose gel.
RT-qPCR
RT reactions were performed using AMV Reverse
Transcriptase (Takara Biotechnology Co., Ltd., Dalian, China) and
qPCR was performed on the Applied Biosystems 7900HT Sequence
Detection System (Thermo Fisher Scientific, Inc.). TaqMan
probe-based qPCR was carried out using TaqMan MicroRNA Reverse
Transcription kit (cat. no. 4366597) and Universal Master Mix II
(cat. no. 4440048; Applied Biosystems; Thermo Fisher Scientific,
Inc.) according to the protocol of the manufacturer (23). Specific RT primers and TaqMan probes
for hsa-miR-33a (cat. no. 000424) and U6 (cat. no. 4427975;
(Applied Biosystems; Thermo Fisher Scientific, Inc.) were used to
quantify the expression of hsa-miR-33a. The specific primers are as
follows: hsa-miR-33a, forward, 5′-GCACTTTCATGATACAAGCCG-3′ and
reverse, 5′-GACCACTCAGTTTAGAGCCA-3′; U6, forward,
5′-CTCGCTTCGGCAGCACATATACT-3′ and reverse,
5′-ACGCTTCACGAATTTGCGTGTC-3′. Thermocycling conditions were as
follows: Initial denaturation at 94°C for 10 min, followed by 35
cycles of 94°C for 30 sec, 60°C for 30 sec and 72°C for 30 sec,
with a final extension at 72°C for 10 min. Each reaction was
independently tested a minimum of three times. U6 was used as the
internal control and miR-33a levels were quantified using the
2−ΔΔCq method (24).
miR-33a level was summarized and recorded as high vs. low levels of
expression based on the median value.
Statistical analysis
Statistical analysis was conducted using SPSS 20.0
(IBM Corp., Armonk, NY, USA) and the production of figures was
performed with GraphPad 5.0 software (GraphPad Software, Inc., La
Jolla, CA, USA). An independent t-test was used to evaluate
differences between two groups, and a χ2 test was used
to examine differences between multiple groups. OS and DFS curves
were generated by GraphPad 5.0 software. Univariate and
multivariate survival analyses were performed with Cox proportional
hazard regression. P<0.05 was considered to indicate a
statistically significant difference.
Results
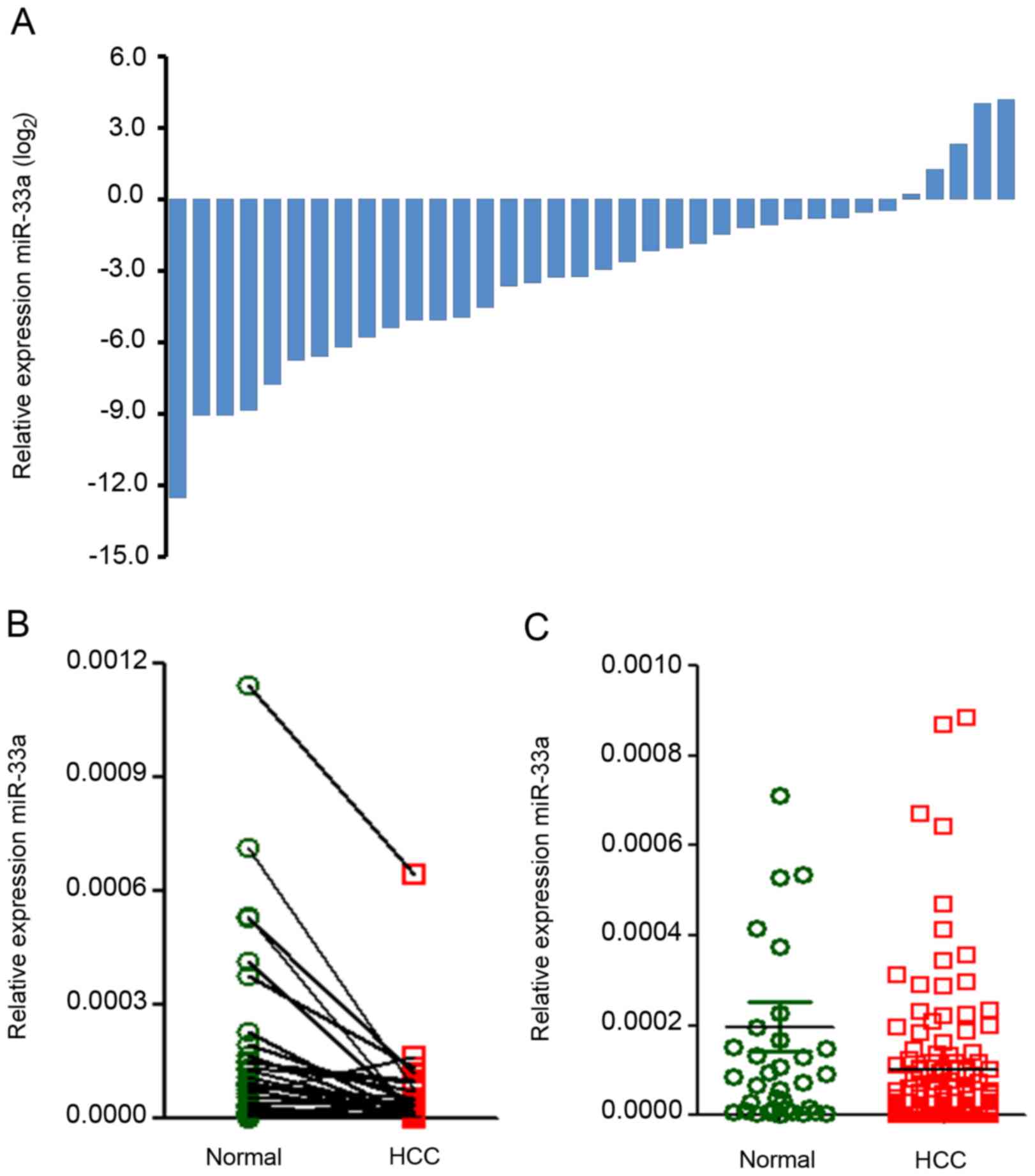
miR-33a expression in HCC and para-carcinoma tissue
samples. In order to investigate the expression and prognostic
significance of miR-33a in patients with HCC, the present study
evaluated miR-33a expression levels via RT-qPCR in 36 biopsy pairs
and 113 unpaired HCC biopsies. The miR-33a expression levels were
classified as high or low compared with the median value. miR-33a
expression levels were significantly lower in the 36 tumor biopsies
than in the paired adjacent non-tumor tissues [fold change (FC),
0.11; P=0.004; Fig. 1A and B]. It was
also identified that the miR-33a expression was significantly
downregulated in 149 HCC tissue samples relative to 36 non-tumor
tissue samples (FC, 0.51, P=0.043; Fig.
1C).
Association of miR-33a expression with
the clinical characteristics of HCC
The associations between miR-33a expression and
individual clinical characteristics were investigated, and are
listed in Table I. The results
revealed that miR-33a expression levels were significantly
correlated with sex (P=0.045) and tumor foci number (P=0.007) in
HCC samples. However, there was no association of miR-33a
expression with patient age, tumor diameter, tumor differentiation
or distant metastasis (P>0.05).
Association between clinical characteristics and
prognosis in HCC. The association between the clinical
characteristics and HCC prognosis were further investigated by
univariate survival analyses based on a Cox proportional hazard
regression model. In accord with our prior hypothesis, multiple
tumor foci [hazard ratio (HR), 1.995; 95% confidence interval (CI),
1.208–3.293; P=0.007] increased tumor size (HR, 1.945; 95% CI,
1.170–3.246; P=0.011), poorly differentiated tumors (HR, 0.471; 95%
CI, 0.262–0.842; P=0.011) and distant metastasis (HR, 3.468; 95%
CI, 1.130–10.644; P=0.032) were positively associated with a poor
prognosis (Table II).
 | Table II.Cox regression model analysis for OS
and PFS based on various clinical characteristics in patients with
hepatocellular carcinoma. |
Table II.
Cox regression model analysis for OS
and PFS based on various clinical characteristics in patients with
hepatocellular carcinoma.
|
| Univariate analysis
for OS | Multivariate
analysis for OS | Univariate analysis
for PFS | Multivariate
analysis for PFS |
|---|
|
|
|
|
|
|
|---|
| Factor | HR | 95% CI | P-value | HR | 95% CI | P-value | HR | 95% CI | P-value | HR | 95% CI | P-value |
|---|
| Age | 1.193 | 0.709–2.009 | 0.507 |
|
|
| 1.062 | 0.692–1.655 | 0.979 |
|
|
|
| Sex | 0.493 | 0.225–1.084 | 0.079 |
|
|
| 0.609 | 0.330–1.124 | 0.113 |
|
|
|
| Number of foci | 1.995 | 1.208–3.293 | 0.007 | 16.665 | 6.330–43.873 | <0.001 | 1.634 | 1.040–2.569 | 0.033 | 5.589 | 2.975–10.503 | <0.001 |
| Diameter | 1.945 | 1.170–3.246 | 0.011 |
|
|
| 1.549 | 0.991–2.419 | 0.055 |
|
|
|
| Tumor
differentiation | 0.471 | 0.262–0.842 | 0.011 |
|
|
| 0.512 | 0.308–0.851 | 0.011 |
|
|
|
| Distant
metastasis | 3.468 | 1.130–10.644 | 0.032 |
|
|
| 4.879 | 2.199–10.825 | <0.001 |
|
|
|
| miR-33a
expression | 0.072 | 0.033–0.159 | <0.001 |
|
|
| 0.194 | 0.118–0.317 | <0.001 |
|
|
|
Low expression of miR-33a is a
prognostic marker for patients with HCC
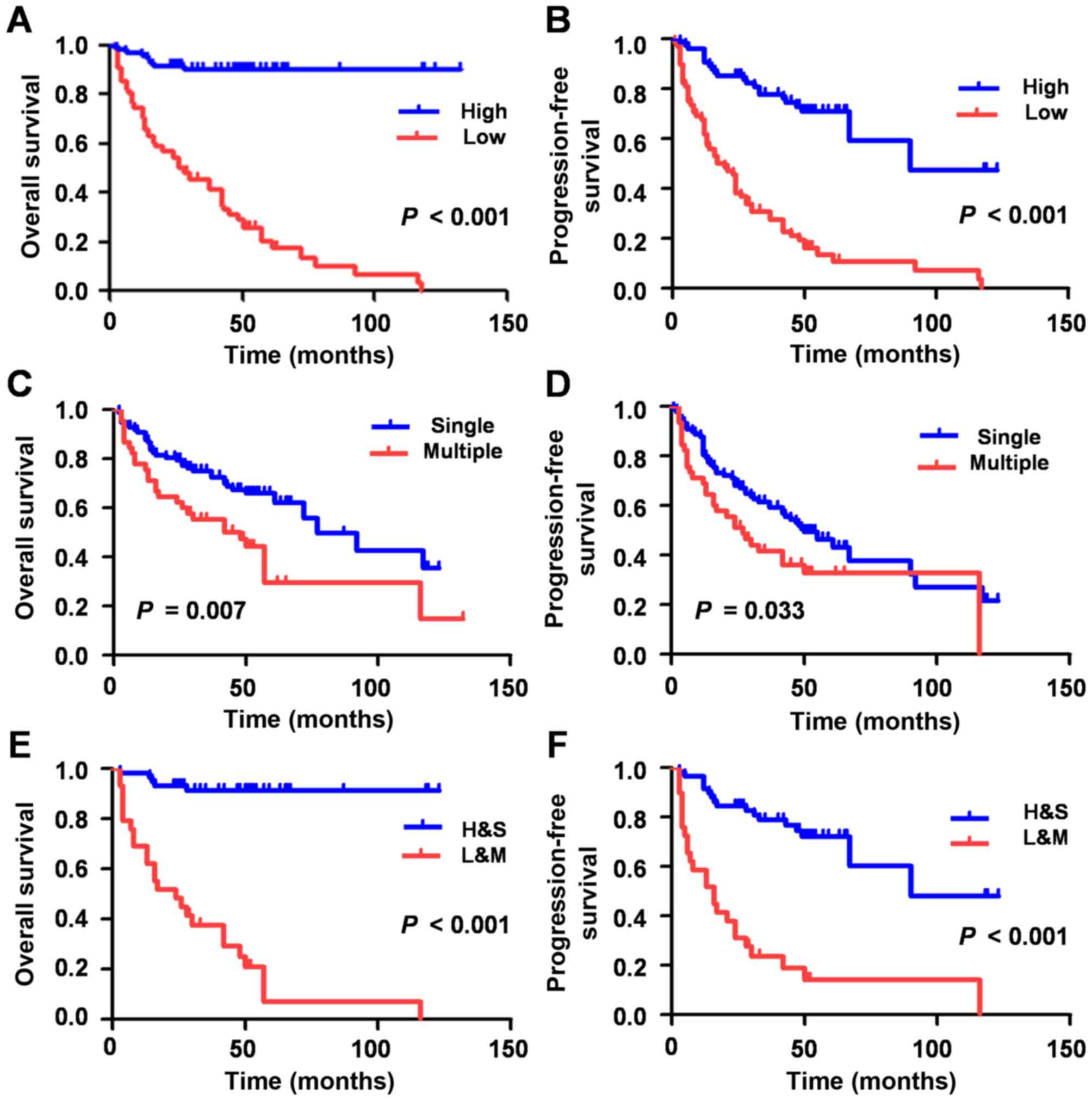
As univariate survival (Table II) and Kaplan-Meier survival analyses
(Fig. 2A-D) demonstrated, the lower
expression of miR-33a group exhibited a shorter OS (HR, 0.072; 95%
CI, 0.033–0.159; P<0.001) and PFS (HR, 0.194, 95% CI,
0.118–0.317; P<0.001), which indicated that the low expression
of miR-33a may be a negative factor for HCC prognosis.
In order to explore whether the expression of
miR-33a may unite with other clinical factors to influence HCC
survival, a multivariate Cox proportional hazards regression
analysis was performed. Associations between miR-33a expression and
parameters (including age, sex, tumor foci number, tumor diameter,
tumor differentiation and distant metastasis) that are predictive
of HCC prognosis were initially analyzed by the model. As presented
in Table I, the results demonstrated
that the patient sex (P=0.045) and tumor foci number (P=0.007) were
positively correlated with the miR-33a expression levels in the HCC
tissue samples. A forward stepwise univariate survival analysis
revealed that tumor foci number, tumor diameter, differentiation,
distant metastasis and miR-33a expression were positively
associated with HCC prognosis. The above results demonstrated that
the number of tumor foci and the miR-33a expression level may
jointly influence HCC prognosis.
The 149 HCC patients were subsequently divided into
two groups. One group was comprised of patients with low miR-33
expression levels and multiple tumor foci (L+M), whereas patients
with high miR-33 expression levels and single tumor foci, comprised
the other group (H+S). The L+M group experienced a significantly
shorter OS (HR, 16.665; 95% CI, 6.330–43.873; P<0.001) and PFS
(HR, 5589; 95% CI, 2.975–10.503; P<0.001) time compared with
H+S, as determined via multivariate Cox regression model and
Kaplan-Meier survival analyses (Table
II; Fig. 2).
In summary, the data of the present study
demonstrated that the expression of miR-33a may serve a significant
role in HCC progression, and that miR-33a may have exhibited
potential as a tumor biomarker in the determination of the
prognosis of HCC.
Discussion
Molecular biomarkers have started to serve an
important role in the selection of patient therapeutics; they may
serve as indicators of a patient's individual likelihood of a
chemotherapeutic response. The HCC mortality rate is the fastest
growing among all types of cancer and it is the third most common
cause of tumor-associated mortality (25–27).
Although there have been improvements in surgery and other
therapeutic methods, the 5-year survival rate has remained <15%
for a number of years (28–31). In order to seek a more efficient and
individualized treatment, it is essential for researchers to
comprehensively understand the molecular mechanisms of HCC
progression.
Accumulating studies have demonstrated that the
expression of miRNA is dysregulated in various types of human
cancer, and may be associated with oncogenesis. It has been
reported that miRNAs can indirectly repress the expression of a
number of cancer-associated genes, and directly work as tumor
suppressors or oncogenes (32). A
number of studies have described the role of miRNAs in cancer
treatment and diagnosis as a prognostic indicator. For example, a
seven-miRNA signature of could be a robust predictor for OS and
relapse-free survival in gastric cancer (33), or the low expression of miR-26 in the
diagnosis of HCC (34).
A number of miRNAs have a confirmed effect on the
initiation and progression of HCC. For example, the overexpression
of miR-149 suppressed the migration and invasion of HCC by
targeting protein phosphatase, Mg2+/Mn2+
dependent 1F directly (35). miR-148a
induces hepatocytic differentiation by inhibiting the inhibitor of
nuclear factor κα/NUMB/NOTCH pathway (36).
miR-33a serves a significant role in fatty acid
metabolism and cholesterol synthesis (37,38), and
inhibiting miR-33a has been considered as a method to reduce the
risk of cardiovascular disease (39).
It is suggested that miR-33 may function as a tumor-associated
molecule, as miR-33b can inhibit cell growth and induce apoptosis
through suppressing the activity of WNT/β-catenin signaling in lung
adenocarcinoma cells (40), and also
inhibit the proliferation and migration of osteosarcoma cells by
targeting hypoxia-inducible factor-1α (41). With further in-depth study of miR-33a,
it was identified that mir-33a can also affect cell proliferation
and cell cycle progression in tumors by regulating cyclin-dependent
kinase 5, cyclin D1 and Pim-1 (42,43),
inhibit bone metastasis by targeting parathyroid hormone-related
protein (19), and inhibit cancer
cell growth, invasion and metastasis by regulating the expression
of high mobility group AT-hook 2 (44) and β-catenin (45). However, to the best of our knowledge,
no report previously existed regarding the role of miR-33a in
HCC.
In the present study, HCC and para-carcinoma tissues
were examined to determine the prognostic significance of miR-33a
expression. Kaplan-Meier survival curve analysis indicated that
patients with lower miR-33a expression exhibited significantly
poorer survival. miR-33a expression was significantly associated
with the number of tumor foci, which is an important clinical
determinant of the prognosis of patients with HCC. In a univariate
Cox model, it was identified that low miR-33a expression was an
independent predictive factor for the OS time of HCC patients. In a
multivariate Cox model, it was identified that the presence of
multiple foci was associated with the low expression of miR-33a,
and the decreased OS and PFS time of patients with HCC.
In summary, the data of the present study revealed
that the miR-33a expression level was significantly associated with
the number of tumor foci, and the combination of low miR-33a
expression with multiple foci number was associated with
significantly decreased OS and PFS time. miR-33a may, therefore,
promote regional metastasis and serve as a potential prognostic
biomarker for HCC in clinical practice. However, further study with
a larger cohort is required in order to validate this view.
Acknowledgements
The present study was supported in part by grants
from the National Natural Science Foundation of China (81201535,
81302065, 81472202, 81772932, 81472209 and 81702243), Jilin
Provincial Science and Technology Department (20140414061GH),
Shanghai Natural Science Foundation (12ZR1436000 and 16ZR1428900)
and Shanghai Municipal Commission of Health and Family Planning
(201440398 and 201540228).
References
|
1
|
Ferlay J, Shin HR, Bray F, Forman D,
Mathers C and Parkin DM: Estimates of worldwide burden of cancer in
2008: GLOBOCAN 2008. Int J Cancer. 127:2893–2917. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Torre LA, Bray F, Siegel RL, Ferlay J,
Lortet-Tieulent J and Jemal A: Global cancer statistics, 2012. CA
Cancer J Clin. 65:87–108. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Thorgeirsson SS and Grisham JW: Molecular
pathogenesis of human hepatocellular carcinoma. Nat Genet.
31:339–346. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Forner A, Hessheimer AJ, Isabel Real M and
Bruix J: Treatment of hepatocellular carcinoma. Crit Rev Oncol
Hematol. 60:89–98. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Guglielmi A, Ruzzenente A, Conci S,
Valdegamberi A, Vitali M, Bertuzzo F, De Angelis M, Mantovani G and
Iacono C: Hepatocellular carcinoma: Surgical perspectives beyond
the barcelona clinic liver cancer recommendations. World J
Gastroenterol. 20:7525–7533. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ishizawa T, Hasegawa K, Aoki T, Takahashi
M, Inoue Y, Sano K, Imamura H, Sugawara Y, Kokudo N and Makuuchi M:
Neither multiple tumors nor portal hypertension are surgical
contraindications for hepatocellular carcinoma. Gastroenterology.
134:1908–1916. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Bushati N and Cohen SM: microRNA
functions. Annu Rev Cell Dev Biol. 23:175–205. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Saito M, Shiraishi K, Matsumoto K,
Schetter AJ, Ogata-Kawata H, Tsuchiya N, Kunitoh H, Nokihara H,
Watanabe S, Tsuta K, et al: A three-microRNA signature predicts
responses to platinum-based doublet chemotherapy in patients with
lung adenocarcinoma. Clin Cancer Res. 20:4784–4793. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Wang Y, Zheng X, Zhang Z, Zhou J, Zhao G,
Yang J, Xia L, Wang R, Cai X, Hu H, et al: MicroRNA-149 inhibits
proliferation and cell cycle progression through the targeting of
ZBTB2 in human gastric cancer. PLoS One. 7:e416932012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Chan SH, Huang WC, Chang JW, Chang KJ, Kuo
WH, Wang MY, Lin KY, Uen YH, Hou MF, Lin CM, et al: MicroRNA-149
targets GIT1 to suppress integrin signaling and breast cancer
metastasis. Oncogene. 33:4496–4507. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Wang F, Ma YL, Zhang P, Shen TY, Shi CZ,
Yang YZ, Moyer MP, Zhang HZ, Chen HQ, Liang Y and Qin HL: SP1
mediates the link between methylation of the tumour suppressor
miR-149 and outcome in colorectal cancer. J Pathol. 229:12–24.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Øster B, Linnet L, Christensen LL, Thorsen
K, Ongen H, Dermitzakis ET, Sandoval J, Moran S, Esteller M, Hansen
TF, et al: Non-CpG island promoter hypomethylation and miR-149
regulate the expression of SRPX2 in colorectal cancer. Int J
Cancer. 132:2303–2315. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Chung GE, Yoon JH, Myung SJ, Lee JH, Lee
SH, Lee SM, Kim SJ, Hwang SY, Lee HS and Kim CY: High expression of
microRNA-15b predicts a low risk of tumor recurrence following
curative resection of hepatocellular carcinoma. Oncol Rep.
23:113–119. 2010.PubMed/NCBI
|
|
14
|
Tsai WC, Hsu PW, Lai TC, Chau GY, Lin CW,
Chen CM, Lin CD, Liao YL, Wang JL, Chau YP, et al: MicroRNA-122, a
tumor suppressor microRNA that regulates intrahepatic metastasis of
hepatocellular carcinoma. Hepatology. 49:1571–1582. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Xiong Y, Fang JH, Yun JP, Yang J, Zhang Y,
Jia WH and Zhuang SM: Effects of microRNA-29 on apoptosis,
tumorigenicity, and prognosis of hepatocellular carcinoma.
Hepatology. 51:836–845. 2010.PubMed/NCBI
|
|
16
|
Zheng F, Liao YJ, Cai MY, Liu YH, Liu TH,
Chen SP, Bian XW, Guan XY, Lin MC, Zeng YX, et al: The putative
tumour suppressor microRNA-124 modulates hepatocellular carcinoma
cell aggressiveness by repressing ROCK2 and EZH2. Gut. 61:278–289.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wang J, Li J, Shen J, Wang C, Yang L and
Zhang X: MicroRNA-182 downregulates metastasis suppressor 1 and
contributes to metastasis of hepatocellular carcinoma. BMC Cancer.
12:2272012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Najafi-Shoushtari SH, Kristo F, Li Y,
Shioda T, Cohen DE, Gerszten RE and Näär AM: MicroRNA-33 and the
SREBP host genes cooperate to control cholesterol homeostasis.
Science. 328:1566–1569. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Kuo PL, Liao SH, Hung JY, Huang MS and Hsu
YL: MicroRNA-33a functions as a bone metastasis suppressor in lung
cancer by targeting parathyroid hormone related protein. Biochim
Biophys Acta. 1830:3756–3766. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Thomas M, Lange-Grünweller K, Weirauch U,
Gutsch D, Aigner A, Grünweller A and Hartmann RK: The
proto-oncogene Pim-1 is a target of miR-33a. Oncogene. 31:918–928.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Ibrahim AF, Weirauch U, Thomas M,
Grünweller A, Hartmann RK and Aigner A: MicroRNA replacement
therapy for miR-145 and miR-33a is efficacious in a model of colon
carcinoma. Cancer Res. 71:5214–5224. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Edge SB and Compton CC: The American joint
committee on cancer: The 7th edition of the AJCC cancer staging
manual and the future of TNM. Ann Surg Oncol. 17:1471–1474. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Schaap-Oziemlak AM, Raymakers RA,
Bergevoet SM, Gilissen C, Jansen BJ, Adema GJ, Kögler G, Le Sage C,
Agami R, van der Reijden BA and Jansen JH: MicroRNA hsa-miR-135b
regulates mineralization in osteogenic differentiation of human
unrestricted somatic stem cells. Stem Cells Dev. 19:877–885. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Ma YS, Wu TM, Lv ZW, Lu GX, Cong XL, Xie
RT, Yang HQ, Chang ZY, Sun R, Chai L, et al: High expression of
miR-105-1 positively correlates with clinical prognosis of
hepatocellular carcinoma by targeting oncogene NCOA1. Oncotarget.
8:11896–11905. 2017.PubMed/NCBI
|
|
26
|
Fang Y, Fu D, Tang WQ, Cai Y, Ma D, Wang
H, Xue R, Liu T, Huang X, Dong L, et al: Ubiquitin C-terminal
hydrolase 37, a novel predictor for hepatocellular carcinoma
recurrence, promotes cell migration and invasion via interacting
and deubiquitinating PRP19. Biochim Biophys Acta. 1833:559–572.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Wu SD, Ma YS, Fang Y, Liu LL, Fu D and
Shen XZ: Role of the microenvironment in hepatocellular carcinoma
development and progression. Cancer Treat Rev. 38:218–225. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Wang Y, Ma Y, Fang Y, Wu S, Liu L, Fu D
and Shen X: Regulatory T cell: A protection for tumor cells. J Cell
Mol Med. 16:425–436. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Fang Y, Mu J, Ma Y, Ma D, Fu D and Shen X:
The interaction between ubiquitin C-terminal hydrolase 37 and
glucose-regulated protein 78 in hepatocellular carcinoma. Mol Cell
Biochem. 359:59–66. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Liu LL, Fu D, Ma YS and Shen XZ: The power
and the promise of liver cancer stem cell markers. Stem Cells Dev.
20:2023–2030. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Fang Y, Fu D and Shen XZ: The potential
role of ubiquitin C-terminal hydrolases in oncogenesis. Biochim
Biophys Acta. 1806:1–6. 2010.PubMed/NCBI
|
|
32
|
Wang CJ, Zhou ZG, Wang L, Yang L, Zhou B,
Gu J, Chen HY and Sun XF: Clinicopathological significance of
microRNA-31, −143 and −145 expression in colorectal cancer. Dis
Markers. 26:27–34. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Li X, Zhang Y, Zhang Y, Ding J, Wu K and
Fan D: Survival prediction of gastric cancer by a seven-microRNA
signature. Gut. 59:579–585. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Ji J, Shi J, Budhu A, Yu Z, Forgues M,
Roessler S, Ambs S, Chen Y, Meltzer PS, Croce CM, et al: MicroRNA
expression, survival, and response to interferon in liver cancer. N
Engl J Med. 361:1437–1447. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Luo G, Chao YL, Tang B, Li BS, Xiao YF,
Xie R, Wang SM, Wu YY, Dong H, Liu XD and Yang SM: miR-149
represses metastasis of hepatocellular carcinoma by targeting
actin-regulatory proteins PPM1F. Oncotarget. 6:37808–37823. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Jung KH, Zhang J, Zhou C, Shen H, Gagea M,
Rodriguez-Aguayo C, Lopez-Berestein G, Sood AK and Beretta L:
Differentiation therapy for hepatocellular Carcinoma: Multifaceted
effects of miR-148a on tumor growth and phenotype and liver
fibrosis. Hepatology. 63:864–879. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Dávalos A, Goedeke L, Smibert P, Ramírez
CM, Warrier NP, Andreo U, Cirera-Salinas D, Rayner K, Suresh U,
Pastor-Pareja JC, et al: miR-33a/b contribute to the regulation of
fatty acid metabolism and insulin signaling. Proc Natl Acad Sci
USA. 108:pp. 9232–9237. 2011; View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Ramirez CM, Goedeke L, Rotllan N, Yoon JH,
Cirera-Salinas D, Mattison JA, Suárez Y, de Cabo R, Gorospe M and
Fernández-Hernando C: MicroRNA 33 regulates glucose metabolism. Mol
Cell Biol. 33:2891–2902. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Rayner KJ, Esau CC, Hussain FN, McDaniel
AL, Marshall SM, van Gils JM, Ray TD, Sheedy FJ, Goedeke L, Liu X,
et al: Inhibition of miR-33a/b in non-human primates raises plasma
HDL and lowers VLDL triglycerides. Nature. 478:404–407. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Qu J, Li M, An J, Zhao B, Zhong W, Gu Q,
Cao L, Yang H and Hu C: MicroRNA-33b inhibits lung adenocarcinoma
cell growth, invasion, and epithelial-mesenchymal transition by
suppressing Wnt/β-catenin/ZEB1 signaling. Int J Oncol.
47:2141–2152. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Zhou Y, Yang C, Wang K, Liu X and Liu Q:
MicroRNA-33b inhibits the proliferation and migration of
osteosarcoma cells via targeting hypoxia-inducible factor-1α. Oncol
Res. 25:397–405. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Cirera-Salinas D, Pauta M, Allen RM,
Salerno AG, Ramírez CM, Chamorro-Jorganes A, Wanschel AC, Lasuncion
MA, Morales-Ruiz M, Suarez Y, et al: Mir-33 regulates cell
proliferation and cell cycle progression. Cell Cycle. 11:922–933.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Wang Y, Zhou X, Shan B, Han J, Wang F, Fan
X, Lv Y, Chang L and Liu W: Downregulation of microRNA-33a promotes
cyclin-dependent kinase 6, cyclin D1 and PIM1 expression and
gastric cancer cell proliferation. Mol Med Rep. 12:6491–6500. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Rice SJ, Lai SC, Wood LW, Helsley KR,
Runkle EA, Winslow MM and Mu D: MicroRNA-33a mediates the
regulation of high mobility group AT-hook 2 gene (HMGA2) by thyroid
transcription factor 1 (TTF-1/NKX2-1). J Biol Chem.
288:16348–16360. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Zhu C, Zhao Y, Zhang Z, Ni Y, Li X and
Yong H: MicroRNA-33a inhibits lung cancer cell proliferation and
invasion by regulating the expression of β-catenin. Mol Med Rep.
11:3647–3651. 2015. View Article : Google Scholar : PubMed/NCBI
|