Introduction
Cerebroma, when it appears as a nervous system tumor
located in the cranial cavity, can have harmful effects (1). Cerebroma seriously affects health and
may even become life-threatening (2).
The onset of cerebroma is a complex process caused by multiple
factors, including virus infections and radiation. At present the
cause of the disease and its molecular mechanism has not been
completely understood (3).
Cluster of differentiation 44 (CD44) is the
forty-fourth cluster of differentiation molecule, which is widely
distributed in T cells especially memory T cells (4). Previous studies showed that CD44
molecules are associated with tumor invasion and metastasis. The
abnormal expression of CD44 may cause and accelerate the occurrence
and development of tumors (5). Golgi
protein (GP73) is a transmembrane protein located in Golgi. In a
previous study, it was demonstrated that GP73 can be used as a
serum marker of liver cancer, which plays an important role in HCC
(6). As such, GP73 may be involved in
other types of cancer.
In the present study, immunofluorescence and
immunohistochemical methods were utilized to detect the expression
intensities of CD44 and GP73 in cerebroma tissues. Reverse
transcription-polymerase chain reaction (RT-PCR) and western blot
analysis were performed to measure the expression levels of CD44
and GP73 mRNAs as well as proteins to understand the differences in
the expression levels of CD44 and GP73 in varying tissues, discuss
the changes of CD44 and GP73 expression levels in cerebroma tissues
and analyze their correlation, thus providing new insights and
directions for the genetic diagnosis and treatment of
cerebroma.
Materials and methods
Collection of cerebroma tissues
The specimens were tissues resected during surgical
procedures at Dezhou People's Hospital, fixed in 10% formaldehyde
and then embedded in paraffin. Sixteen specimens of paraffin blocks
were collected from patients with cerebroma, which was resected via
surgery and confirmed by postoperative histopathological
examination. Among the patients, 8 were men and 8 were women, aged
30–60 years. In addition, 4 specimens of normal brain tissues,
provided by the Department of Neurosurgery of Dezhou People's
Hospital (Shandong, China) were taken as the control group.
Main reagents
Reagents used in the study included: Bicinchoninic
acid (BCA) Protein Assay kit (Beyotime, Shanghai, China);
TRIzol® Total RNA extraction kit (Tiangen Biotech Co.,
Ltd., Beijing, China); RT-PCR reverse transcription kit (Tiangen
Biotech Co., Ltd.); and the immunohistochemical staining kit was
purchased from Zhongshan Goldenbridge Biotechnology Co., Ltd.
(Beijing, China). 4,6-Diamino-2-phenyl indole (DAPI), anti-β-actin
(β-actin) were used with the monoclonal antibodies for CD44 and
GP73 and immunofluorescence was used for the secondary antibody
(Cell Signaling Technology, Boston, MA, USA). For RT-PCR detection,
the tissues in each group were added into the TRIzol reagent and
ground to homogenate. The specimens were left to stand at room
temperature for 5 min until they were completely lysed. This was
followed by centrifugation at 12,000 × g for 5 min at 4°C, and the
supernatant was extracted carefully. Chloroform was added to the
supernatant and mixed thoroughly, after which the mixture was left
to stand at room temperature for 5 min. This was followed by
centrifugation at 12,000 × g for 15 min at 4°C, and the supernatant
was extracted carefully. Next, isopropyl alcohol of the same volume
was added and left to stand at room temperature for 10 min. The
mixture was then centrifuged at 12,000 × g at 4°C for 10 min, and
the precipitate was reserved. Then, 75% ethanol was added and mixed
together, which was used to wash the RNA precipitate. Finally,
RNase-free water was added to completely dissolve the precipitate.
The value of optical density (OD) 260/280 and the concentration of
RNA were measured. Amplification was performed step by step
according to the instructions. The primer sequence template shown
in Table I was used, and RT-PCR
analysis was conducted to determine the reaction products.
 | Table I.RT-PCR primer sequences for CD44 and
GP73 mRNAs. |
Table I.
RT-PCR primer sequences for CD44 and
GP73 mRNAs.
| Gene | Primer sequences |
|---|
| CD44 | U:
CAGACCTGCCCAATGCCTTTGATGGACC |
|
| D:
TCCACCTTCTTGACTCCCATGTGAGT |
| GP73 | U:
CGGGATCCATGATGGGCTTGGGAAACGGGCGTC |
|
| D:
CGGAATTCTCAGAGTGTATGATTCCGCTTTTCAC |
| β-actin | U:
GAGCCGGGAAATCGTGCGT |
|
| D:
GGAAGGAAGGCTGGAAGATG |
Western blot analysis
An appropriate amount of the four kinds of cerebroma
tissues to be tested was taken and washed with ice-cold normal
saline. According to the instructions in the total protein
extraction kit, lysis buffer for immunoprecipitation (IP)
[containing phenylmethylsulfonyl fluoride (PMSF) and protease
inhibitors] was added and then placed on the ice to adequately
grind the tissues. Next, the tissue homogenate was centrifuged at
12,000 × g for 10 min at 4°C, and the supernatant was taken to
extract new supernatant by centrifugation at 12,000 × g at 4°C for
another 20 min. After protein quantification was implemented to the
supernatant according to the instructions in the protein kit,
protein samples were loaded with an equal amount of total protein.
The supernatant was separated by sodium dodecyl
sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) at a constant
voltage of 220 V. Electrophoresis was not stopped until bromophenol
blue reached the bottom of the gel. The gel was sliced in
accordance with the molecular weight of the target protein and then
transferred to a polyvinylidene fluoride (PVDF) membrane.
Subsequently, it was placed in 5% skim milk and blocked at room
temperature for 3 h on a shaking table, prior to being incubated
overnight in mouse anti-human CD44, GP73 and β-actin primary
monoclonal antibodies (dilution, 1:1,000; cat. nos. 5640, 97537 and
3700) at 4°C. The next day, after the membrane was thoroughly
washed in Tween/Tris-buffered saline (TTBS) (3 times for 10 min),
the horse anti-mouse secondary polyclonal antibody (dilution,
1:2,000; cat. no. 7076) was added and incubated at room temperature
for 1 h. Enhanced chemiluminescence (ECL) was added for color
development after the specimen was washed in TTBS (3 times for 10
min), and images were captured.
Immunohistochemistry
The section was placed in 0.01 mol/l sodium citrate
buffer (pH 6.0) after dewaxing and hydration, and heated for
antigen retrieval. The sections were then incubated in 0.3%
hydrogen peroxide solution at 37°C for 30 min to inactivate
endogenous peroxidase. Blocking buffer was added and blocked at
room temperature for 30 min. Blocking buffer was then discarded,
and primary antibody solution was added and incubated overnight in
a wet box at 4°C. After the section was washed repeatedly,
biotinylated secondary antibody working solution and horseradish
peroxidase-conjugated streptavidin working solution were added,
respectively, and the section was placed in diaminobenzidine (DAB)
solution for color development. Finally, the section was mounted in
buffered glycerol, observed and photographed under a microscope
(Olympus Corporation, Tokyo, Japan).
Immunofluorescence
The paraffin section was dewaxed with xylene,
hydrated with graded alcohol for antigen retrieval, and washed in
0.01 M phosphate-buffered saline (PBS) (pH 7.4) 3 times for 5 min.
The section was then blocked in a wet box containing 10% bovine
serum albumin (BSA) for 30 min at 37°C. The specimen, added with
properly diluted fluorescence-labeled antibody (diluted at 1:70),
was placed in the wet box and incubated overnight at 4°C. After the
specimen was washed in PBS (pH 7.4) 3 times (5 min per time), the
fluorescent secondary antibody (diluted at 1:100) was added in the
dark and incubated in the wet box at 37°C for another 2 h. After
being washed in PBS (pH 7.4) 3 times (5 min per time), the specimen
was stained using DAPI in the dark for 15 min. Finally, the section
was mounted in buffered glycerol, observed and photographed right
under a fluorescence microscope.
Statistical analysis
Statistical analysis of the experimental data were
presented as mean ± standard deviation (mean ± SD). SPSS 17.0
software (SPSS Inc., Chicago, IL, USA) was used for statistical
analysis of the experimental results. A t-test was applied for mean
comparisons between the two groups, and one-way analysis of
variance (ANOVA) was used for mean comparisons among multiple
groups. P-test was performed for pairwise comparisons. P<0.05
was considered to indicate a statistically significant
difference.
Results
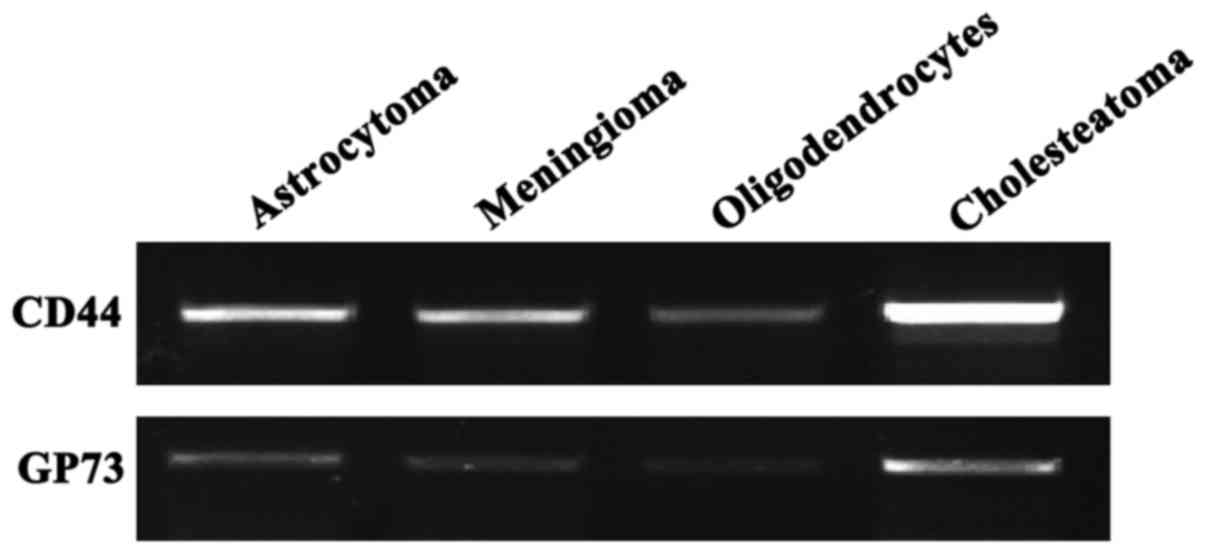
RT-PCR results
The total RNAs of astrocytoma, meningioma,
oligodendrocyte and cholesteatoma were extracted, respectively.
After the RT-PCR experiment, objective bands of CD44 and GP73 were
detected in the four groups. In particular, the expression levels
of CD44 and GP73 in cholesteatoma were very high, indicating that
CD44 and GP73 mRNAs were transcribed in the four
types of cerebroma tissues (Fig.
1).
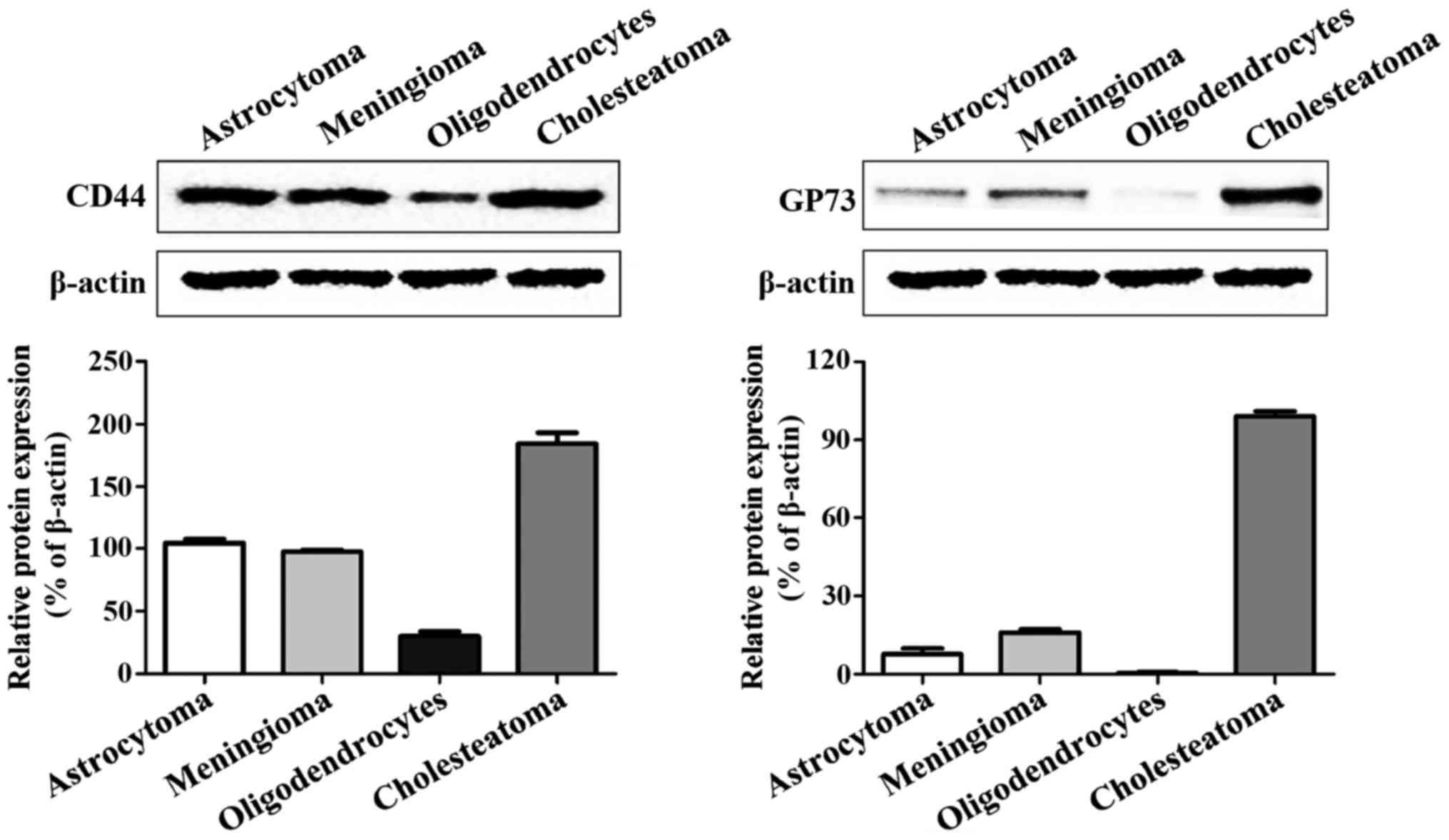
Expression levels of CD44 and GP73
proteins
The western blot analysis results revealed that CD44
and GP73 proteins were expressed in four kinds of cerebroma
tissues, of which CD44 was highly expressed in all four kinds of
cerebroma, while the expression levels of GP73 in oligodendrocytes
and cholesteatoma were higher than those in meningioma and
astrocytoma (Fig. 2).
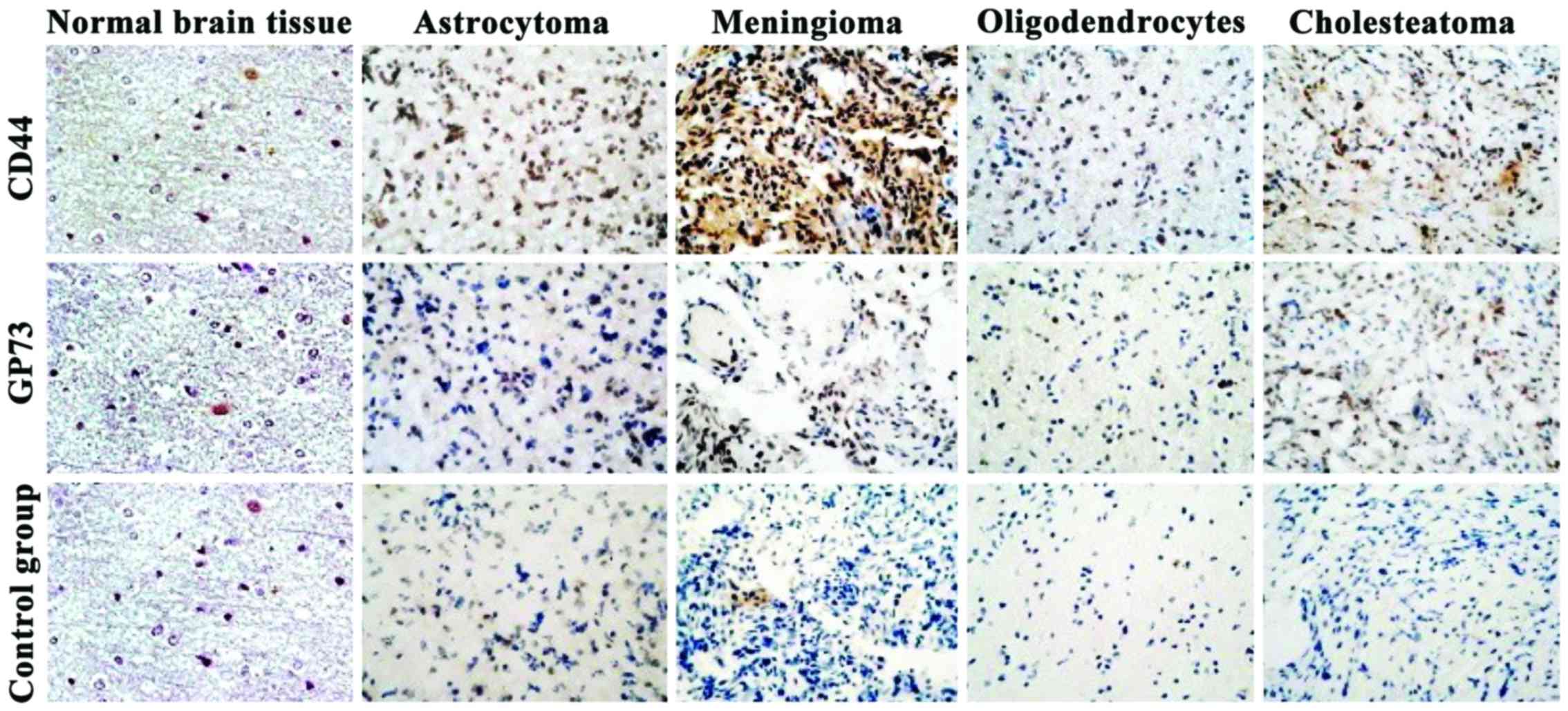
Expression levels of CD44 and GP73
detected by streptavidin-biotin complex (SABC)
The expression levels of CD44 and GP73 proteins were
mainly located in the cytoplasm (Fig.
3). By comparing the expression levels of CD44 and GP73
proteins in normal brain tissues with those in four kinds of
cerebroma tissues, the differences were statistically significant
(P<0.01) (Table II).
 | Table II.Expression levels of CD44 and
GP73 in tumor tissues and normal brain tissues. |
Table II.
Expression levels of CD44 and
GP73 in tumor tissues and normal brain tissues.
|
| Positive expression
rate |
|---|
|
|
|
|---|
| Proteins | Normal brain
tissues | Astrocytoma | Meningioma | Oligodendrocytes | Cholesteatoma |
|---|
| CD44 |
4.90±1.20 |
68.40±4.10a |
62.12±2.13a |
60.12±6.83a |
40.12±2.21a |
| GP73 |
0.02±0.04 |
2.10±0.60a |
10.03±2.68a |
7.44±2.10a |
3.08±0.47a |
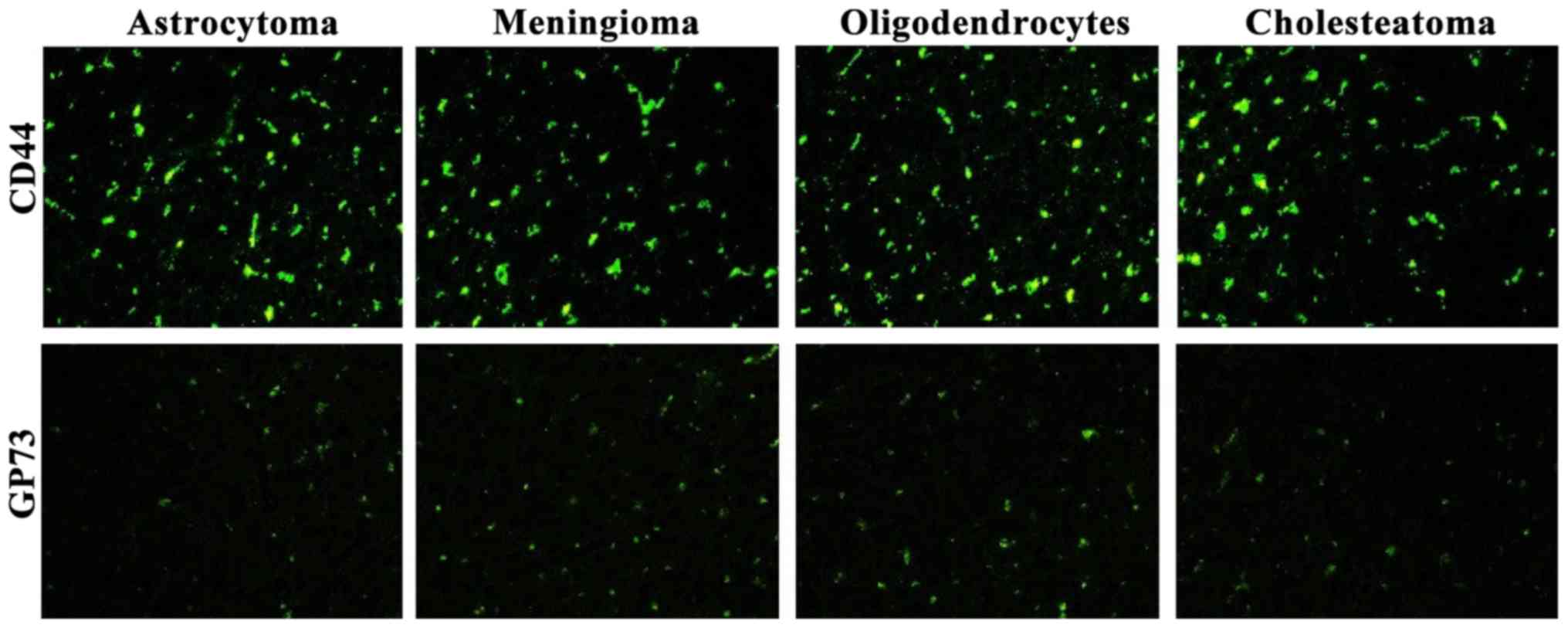
Expression levels of CD44 and GP73 in
the four kinds of tumor tissues detected by immunofluorescence
Fluorescence expression levels of CD44 and GP73 were
detected in the four kinds of cerebroma tissues. CD44 was highly
expressed in the four kinds of cerebroma tissues while the
expression levels of GP73 were slightly decreased (Fig. 4).
Discussion
Malignant cerebroma is a common tumor with extremely
high malignancy in recent years. Characterized by high incidence
rate and death rate, cerebroma is a malignant disease threatening
people's health (7–8). The majority of patients are in the
intermediate and advanced stage when they are diagnosed with
cerebroma, which greatly limits the application of radical
resection (9–10). Thus, it constitutes a global problem
for the treatment of cerebroma. Therefore, it is imperative to
identify a new, safe and effective method to succesfully treat
cerebroma. Previous studies conducted on hepatocellular carcinoma
have aimed to identify genes closely related to hepatocellular
carcinoma. Appropriate intervention in the correspondence of these
genes may become a novel direction for the treatment of
hepatocellular carcinoma (11).
In recent years, with the rapid development of
scientific research, an increasing number of genes have been found
to be closely correlated with the occurrence of tumors. Previous
findings have shown that CD44 plays an important role in
tumors. CD44 protein has several different and crucial biological
functions in organisms. The protein itself participates in organ
development, multiple immune functions and hematopoietic functions,
and also has important functions in tumors (12–14). CD44
not only has an important position in regulating growth of
cerebroma tissues, but also has a crucial effect on survival,
differentiation and migration of tumors, including promotion of
adhesion, metastasis and dissemination, of tumor tissues (15–17).
Previous findings have shown that GP73 also plays a key role in
tumors. GP73 is a kind of transmembrane protein existing in
Golgi apparatus, of which the content in different tissues (blood
and body fluids) in vivo has an obviously rising trend when
malignant cerebroma occurs (18–21). Thus,
it can be seen that GP73 is closely related to the occurrence and
development of cerebroma. Both CD44 and GP73 play an important role
in tumors, and they can be used for the genetic diagnosis and
treatment of tumors.
The results of the present study have shown that
CD44 and GP73 were highly expressed in cerebroma
tissues. RT-PCR and western blot results indicated that CD44
and GP73 were expressed in the four kinds of cerebroma
tissues. By comparing the expression levels of CD44 and GP73
proteins in tissues of the cerebroma group with those of the blank
control group, the differences were statistically significant
(P<0.05). It is believed that this research on the expression
levels of CD44 and GP73 proteins can provide a new theoretical
basis for exploring the mechanisms of tumor infiltration and
metastasis and offer a new direction for the diagnosis and
treatment of cerebroma.
Helsinki Declaration of 1975, as revised in 2008.
The study design was a multi institutional retrospective review of
medical records. Research protocols were assessed and accepted by
the institutional research boards of individual institutions.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Singh SK, Clarke ID, Terasaki M, Bonn VE,
Hawkins C, Squire J and Dirks PB: Identification of a cancer stem
cell in human brain tumors. Cancer Res. 63:5821–5828.
2003.PubMed/NCBI
|
|
2
|
Galli R, Binda E, Orfanelli U, Cipelletti
B, Gritti A, De Vitis S, Fiocco R, Foroni C, Dimeco F and Vescovi
A: Isolation and characterization of tumorigenic, stem-like neural
precursors from human glioblastoma. Cancer Res. 64:7011–7021. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Singh SK, Hawkins C, Clarke ID, Squire JA,
Bayani J, Hide T, Henkelman RM, Cusimano MD and Dirks PB:
Identification of human brain tumour initiating cells. Nature.
432:396–401. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Orian-Rousseau V and Ponta H: Perspectives
of CD44 targeting therapies. Arch Toxicol. 89:3–14. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Goodison S, Urquidi V and Tarin D: CD44
cell adhesion molecules. Mol Pathol. 52:189–196. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Bachert C, Fimmel C and Linstedt AD:
Endosomal trafficking and proprotein convertase cleavage of cis
Golgi protein GP73 produces marker for hepatocellular carcinoma.
Traffic. 8:1415–1423. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Clement V, Sanchez P, de Tribolet N,
Radovanovic I and Ruiz i Altaba A: HEDGEHOG-GLI1 signaling
regulates human glioma growth, cancer stem cell self-renewal, and
tumorigenicity. Curr Biol. 17:165–172. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Gladson CL: The extracellular matrix of
gliomas: Modulation of cell function. J Neuropathol Exp Neurol.
58:1029–1040. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Diamandis P, Wildenhain J, Clarke ID,
Sacher AG, Graham J, Bellows DS, Ling EK, Ward RJ, Jamieson LG,
Tyers M, et al: Chemical genetics reveals a complex functional
ground state of neural stem cells. Nat Chem Biol. 3:268–273. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Sonoda Y, Ozawa T, Aldape KD, Deen DF,
Berger MS and Pieper RO: Akt pathway activation converts anaplastic
astrocytoma to glioblastoma multiforme in a human astrocyte model
of glioma. Cancer Res. 61:6674–6678. 2001.PubMed/NCBI
|
|
11
|
Lagadec C, Vlashi E, Della Donna L, Meng
Y, Dekmezian C, Kim K and Pajonk F: Survival and self-renewing
capacity of breast cancer initiating cells during fractionated
radiation treatment. Breast Cancer Res. 12:R132010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Marhaba R and Zöller M: CD44 in cancer
progression: Adhesion, migration and growth regulation. J Mol
Histol. 35:211–231. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Xu Y, Stamenkovic I and Yu Q: CD44
attenuates activation of the hippo signaling pathway and is a prime
therapeutic target for glioblastoma. Cancer Res. 70:2455–2464.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Anido J, Sáez-Borderías A, Gonzàlez-Juncà
A, Rodón L, Folch G, Carmona MA, Prieto-Sánchez RM, Barba I,
Martínez-Sáez E, Prudkin L, et al: TGF-β receptor inhibitors target
the CD44(high)/Id1(high) glioma-initiating cell population in human
glioblastoma. Cancer Cell. 18:655–668. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Naor D, Sionov RV and Ish-Shalom D: CD44:
Structure, function, and association with the malignant process.
Adv Cancer Res. 71:241–319. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Iczkowski KA, Bai S and Pantazis CG:
Prostate cancer overexpresses CD44 variants 7–9 at the messenger
RNA and protein level. Anticancer Res. 23:3129–3140.
2003.PubMed/NCBI
|
|
17
|
Georgolios A, Batistatou A,
Charalabopoulos A, Manolopoulos L and Charalabopoulos K: The role
of CD44 adhesion molecule in oral cavity cancer. Exp Oncol.
28:94–98. 2006.PubMed/NCBI
|
|
18
|
Kladney RD, Cui X, Bulla GA, Brunt EM and
Fimmel CJ: Expression of GP73, a resident Golgi membrane protein,
in viral and nonviral liver disease. Hepatology. 35:1431–1440.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Kladney RD, Tollefson AE, Wold WS and
Fimmel CJ: Upregulation of the Golgi protein GP73 by adenovirus
infection requires the E1A CtBP interaction domain. Virology.
301:236–246. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Hu JS, Wu DW, Liang S and Miao XY: GP73, a
resident Golgi glycoprotein, is sensibility and specificity for
hepatocellular carcinoma of diagnosis in a hepatitis B-endemic
Asian population. Med Oncol. 27:339–345. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Sun Y, Yang H, Mao Y, Xu H, Zhang J, Li G,
Lu X, Sang X, Zhao H, Zhong S, et al: Increased Golgi protein 73
expression in hepatocellular carcinoma tissue correlates with tumor
aggression but not survival. J Gastroenterol Hepatol. 26:1207–1212.
2011. View Article : Google Scholar : PubMed/NCBI
|