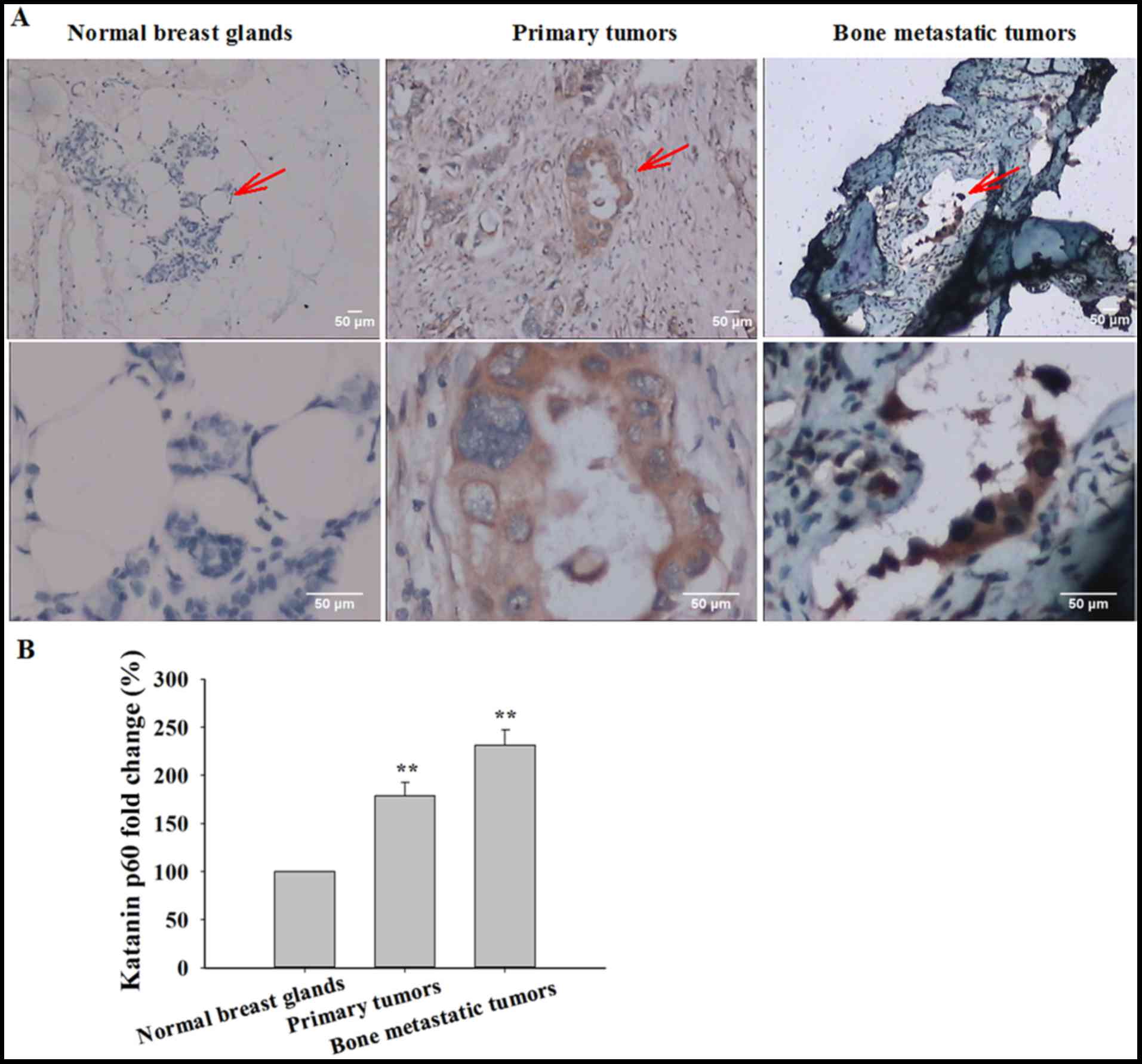
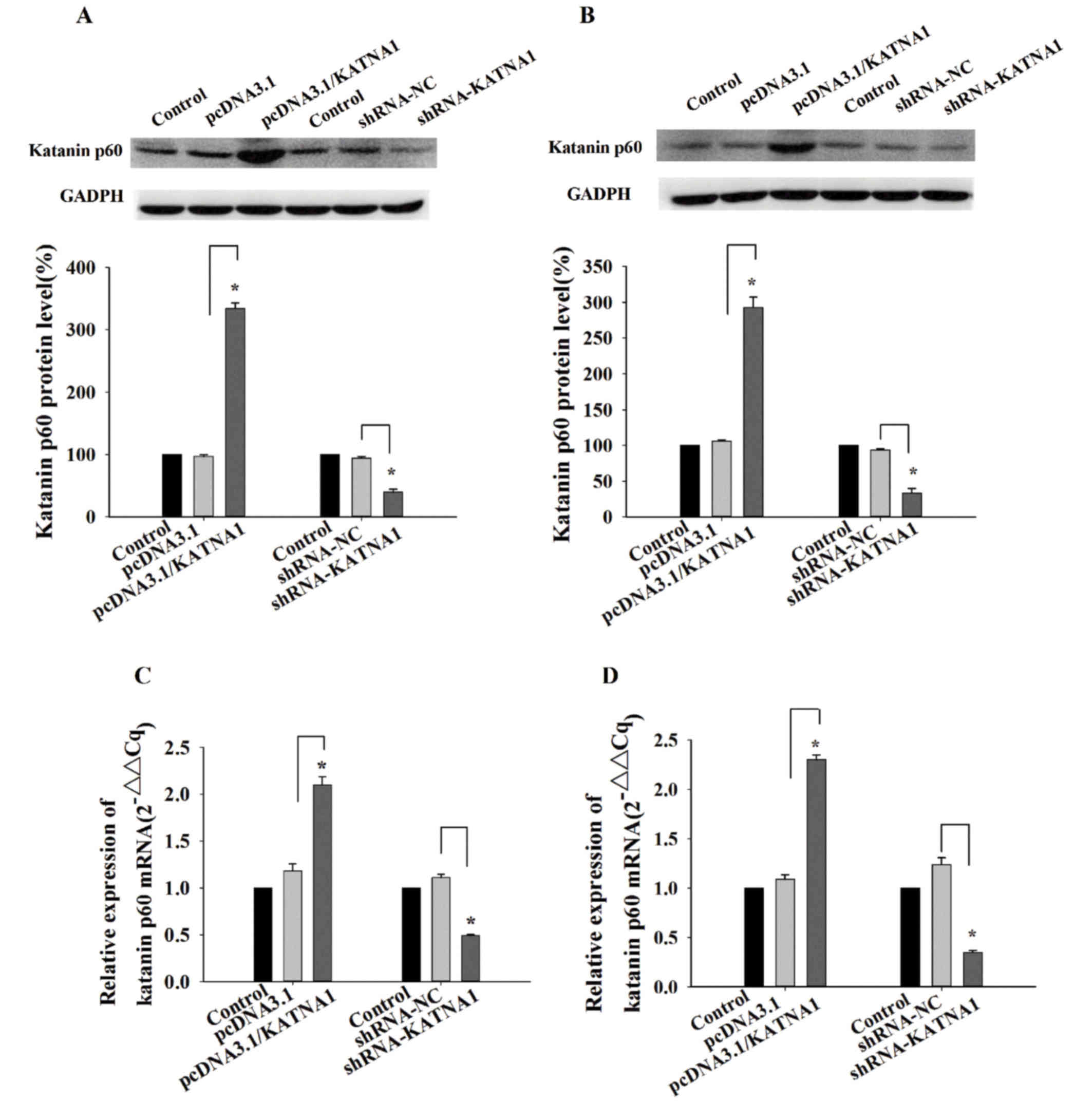
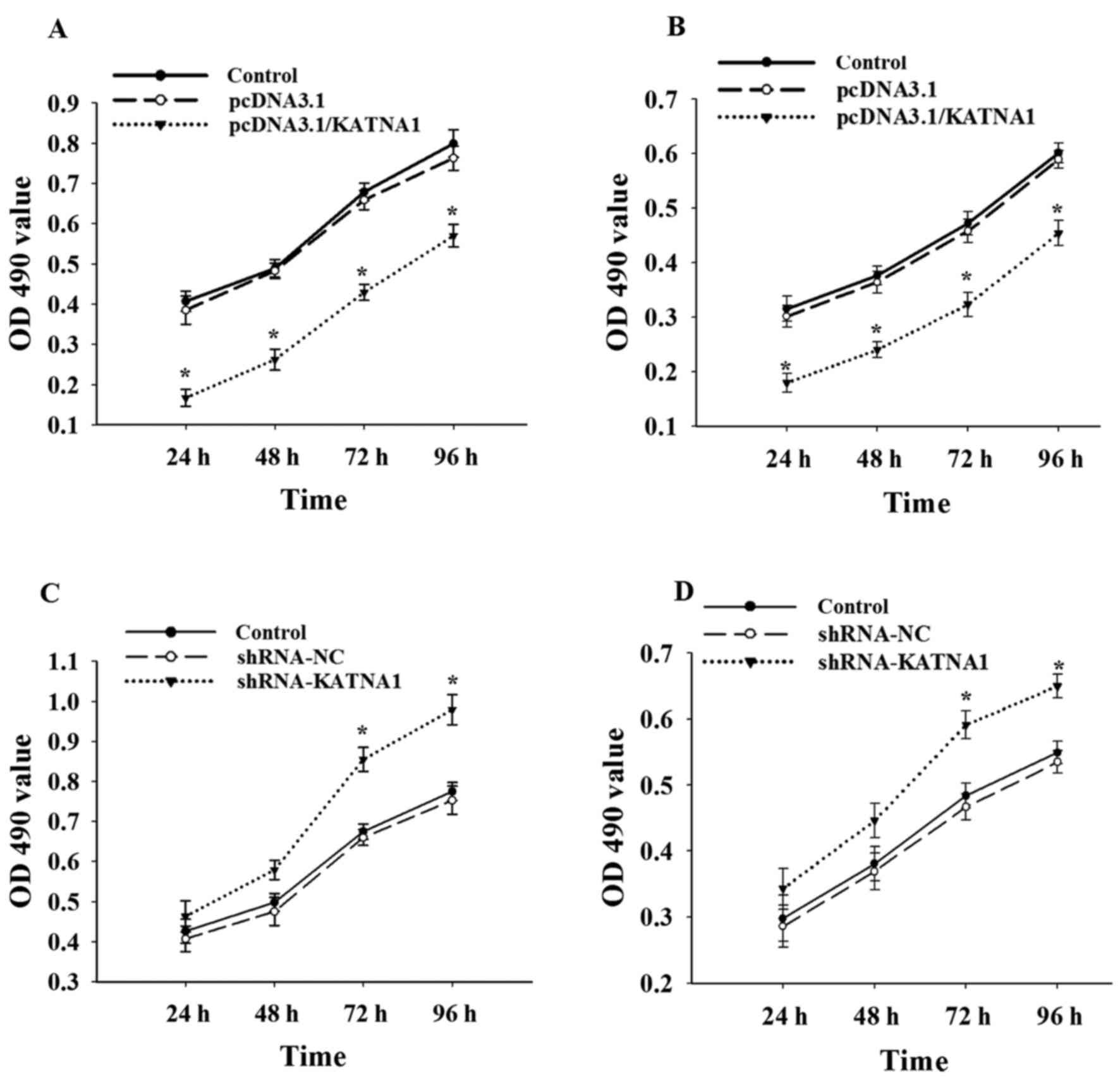
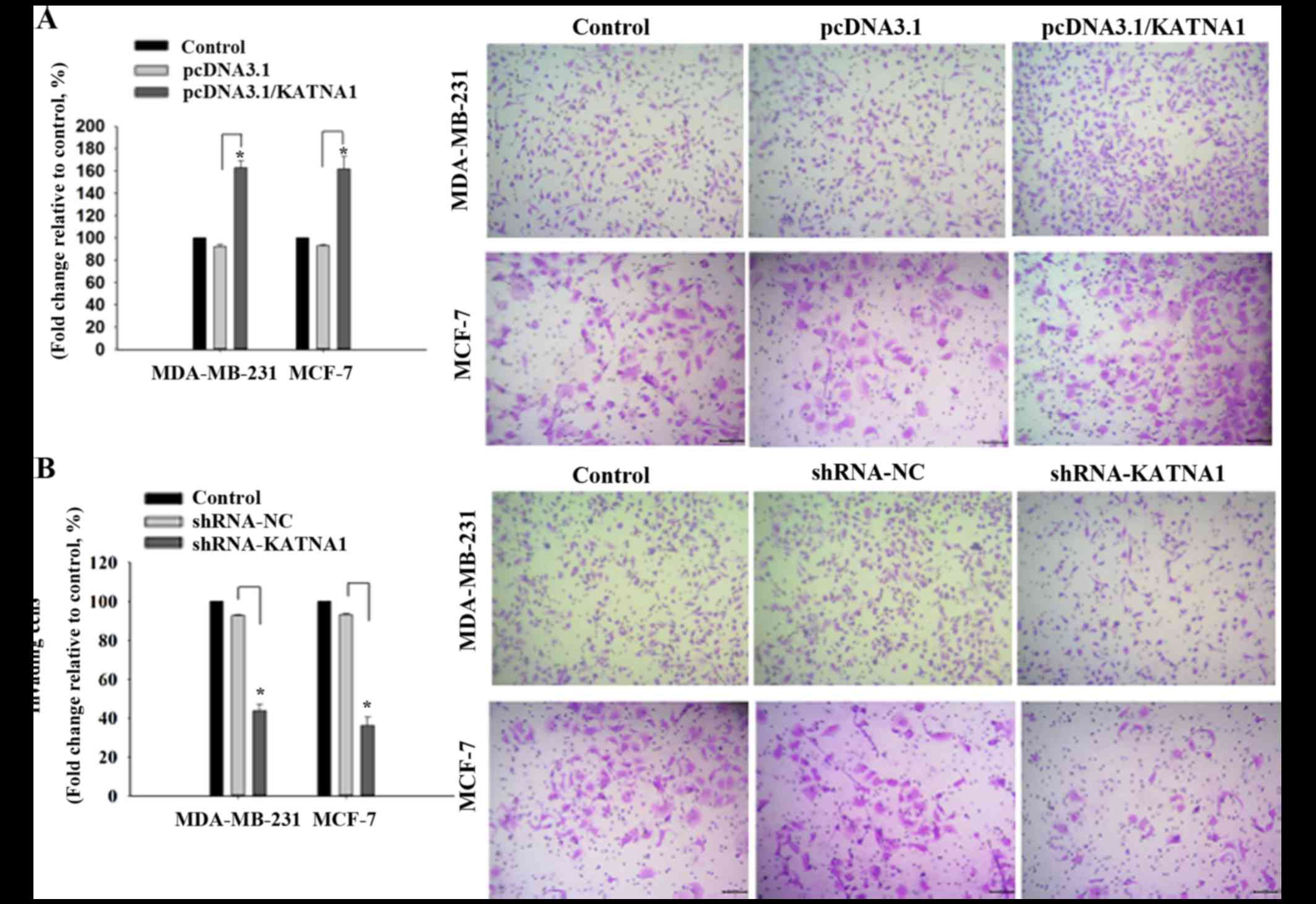
|
1
|
Kozlow W and Guise TA: Breast cancer
metastasis to bone: Mechanisms of osteolysis and implications for
therapy. J Mammary Gland Biol Neoplasia. 10:169–80. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Mundy GR: Metastasis to bone: Causes,
consequences and therapeutic opportunities. Nat Rev Cancer.
2:584–593. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
3
|
Cetin K, Christiansen CF, Sværke C,
Jacobsen JB and Sørensen HT: Survival in patients with breast
cancer with bone metastasis: A Danish population-based cohort study
on the prognostic impact of initial stage of disease at breast
cancer diagnosis and length of the bone metastasis-free interval.
BMJ Open. 5:e0077022015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Nabi IR: The polarization of the motile
cell. J Cell Sci. 112:1803–1811. 1999.PubMed/NCBI
|
|
5
|
Roll-Mecak A and McNally FJ:
Microtubule-severing enzymes. Curr Opin Cell Biol. 22:96–103. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Hartman JJ, Mahr J, McNally K, Okawa K,
Iwamatsu A, Thomas S, Cheesman S, Heuser J, Vale RD and McNally FJ:
A microtubule-severing protein, is a novel AAA ATPase that targets
to the centrosome using a WD40-containing subunit. Cell.
93:277–287. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Bershadsky AD and Vasiliev JM: Mechanisms
of regulation of pseudopodial activity by the microtubule system.
Symp Soc Exp Biol. 47:353–373. 1993.PubMed/NCBI
|
|
8
|
McNally FJ and Vale RD: Identification of
katanin, an ATPase that severs and disassembles stable
microtubules. Cell. 75:419–429. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Yu W, Solowska JM, Qiang L, Karabay A,
Baird D and Baas PW: Regulation of microtubule severing by katanin
subunits during neuronal development. J Neurosci. 25:5573–5583.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Sudo H and Maru Y: LAPSER1 is a putative
cytokinetic tumor suppressor that shows the same centrosome and
midbody subcellular localization pattern as p80 katanin. FASEB J.
21:2086–2100. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Johjima A, Noi K, Nishikori S, Ogi H,
Esaki M and Ogura T: Microtubule severing by katanin p60 AAA+
ATPase requires the C-terminal acidic tails of both alpha- and
beta-tubulins and basic amino acid residues in the AAA+ ring pore.
J Biol Chem. 290:11762–11770. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Whitehead E, Heald R and Wilbur JD:
N-terminal phosphorylation of p60 katanin directly regulates
microtubule severing. J Mol Biol. 425:214–221. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Cummings CM, Bentley CA, Perdue SA, Baas
PW and Singer JD: The Cul3/Klhdc5 E3 ligase regulates p60/katanin
and is required for normal mitosis in mammalian cells. J Biol Chem.
284:11663–11675. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Yang SW, Oh KH, Park E, Chang HM, Park JM,
Seong MW, Ka SH, Song WK, Park DE, Baas PW, et al: USP47 and C
terminus of Hsp70-interacting protein (CHIP) antagonistically
regulate katanin-p60-mediated axonal growth. J Neurosci.
33:12728–12738. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Yu W, Qiang L, Solowska JM, Karabay A,
Korulu S and Baas PW: The microtubule-severing proteins spastin and
katanin participate differently in the formation of axonal
branches. Mol Biol Cell. 19:1485–1498. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Chen K, Ye Y, Ji Z, Tan M, Li S, Zhang J,
Guo G and Lin H: Katanin p60 promotes neurite growth and collateral
formation in the hippocampus. Int J Clin Exp Med. 7:2463–2470.
2014.PubMed/NCBI
|
|
17
|
Korulu S, Yildiz-Unal A, Yuksel M and
Karabay A: Protein kinase C activation causes neurite retraction
via cyclinD1 and p60-katanin increase in rat hippocampal neurons.
Eur J Neurosci. 37:1610–1619. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Ye X, Lee YC, Choueiri M, Chu K, Huang CF,
Tsai WW, Kobayashi R, Logothetis CJ, Yu-Lee LY and Lin SH: Aberrant
expression of katanin p60 in prostate cancer bone metastasis.
Prostate. 72:291–300. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Hartman JJ and Vale RD: Microtubule
disassembly by ATP-dependent oligomerization of the AAA enzyme
katanin. Science. 286:782–785. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Rasi MQ, Parker JD, Feldman JL, Marshall
WF and Quarmby LM: Katanin knockdown supports a role for
microtubule severing in release of basal bodies before mitosis in
Chlamydomonas. Mol Biol Cell. 20:379–388. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
McNally K, Audhya A, Oegema K and McNally
FJ: Katanin controls mitotic and meiotic spindle length. J Cell
Biol. 175:881–891. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Matsuo M, Shimodaira T, Kasama T, Hata Y,
Echigo A, Okabe M, Arai K, Makino Y, Niwa S, Saya H and Kishimoto
T: Katanin p60 contributes to microtubule instability around the
midbody and facilitates cytokinesis in rat cells. PLoS One.
8:e803922013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
McNally KP, Bazirgan OA and McNally FJ:
Two domains of p80 katanin regulate microtubule severing and
spindle pole targeting by p60 katanin. J Cell Sci. 113:1623–1633.
2000.PubMed/NCBI
|
|
25
|
Shankar J, Messenberg A, Chan J, Underhill
TM, Foster LJ and Nabi IR: Pseudopodial actin dynamics control
epithelial-mesenchymal transition in metastatic cancer cells.
Cancer Res. 70:3780–3790. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Guirguis R, Margulies I, Taraboletti G,
Schiffmann E and Liotta L: Cytokine-induced pseudopodial protrusion
is coupled to tumour cell migration. Nature (Lond). 329:261–263.
1987. View
Article : Google Scholar
|
|
27
|
Zhang D, Grode KD, Stewman SF,
Diaz-Valencia JD, Liebling E, Rath U, Riera T, Currie JD, Buster
DW, Asenjo AB, et al: Drosophila Katanin is a microtubule
depolymerase that regulates cortical-microtubule plus-end
interactions and cell migration. Nat Cell Biol. 13:361–370. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Toyo-Oka K, Sasaki S, Yano Y, Mori D,
Kobayashi T, Toyoshima YY, Tokuoka SM, Ishii S, Shimizu T,
Muramatsu M, et al: Recruitment of katanin p60 by phosphorylated
NDEL1, an LIS1 interacting protein, is essential for mitotic cell
division and neuronal migration. Hum Mol Genet. 14:3113–3128. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Kuo TC, Li LW, Pan SH, Fang JM, Liu JH,
Cheng TJ, Wang CJ, Hung PF, Chen HY, Hong TM, et al: Purine-type
compounds induce microtubule fragmentation and lung cancer cell
death through interaction with Katanin. J Med Chem. 59:8521–8534.
2016. View Article : Google Scholar : PubMed/NCBI
|