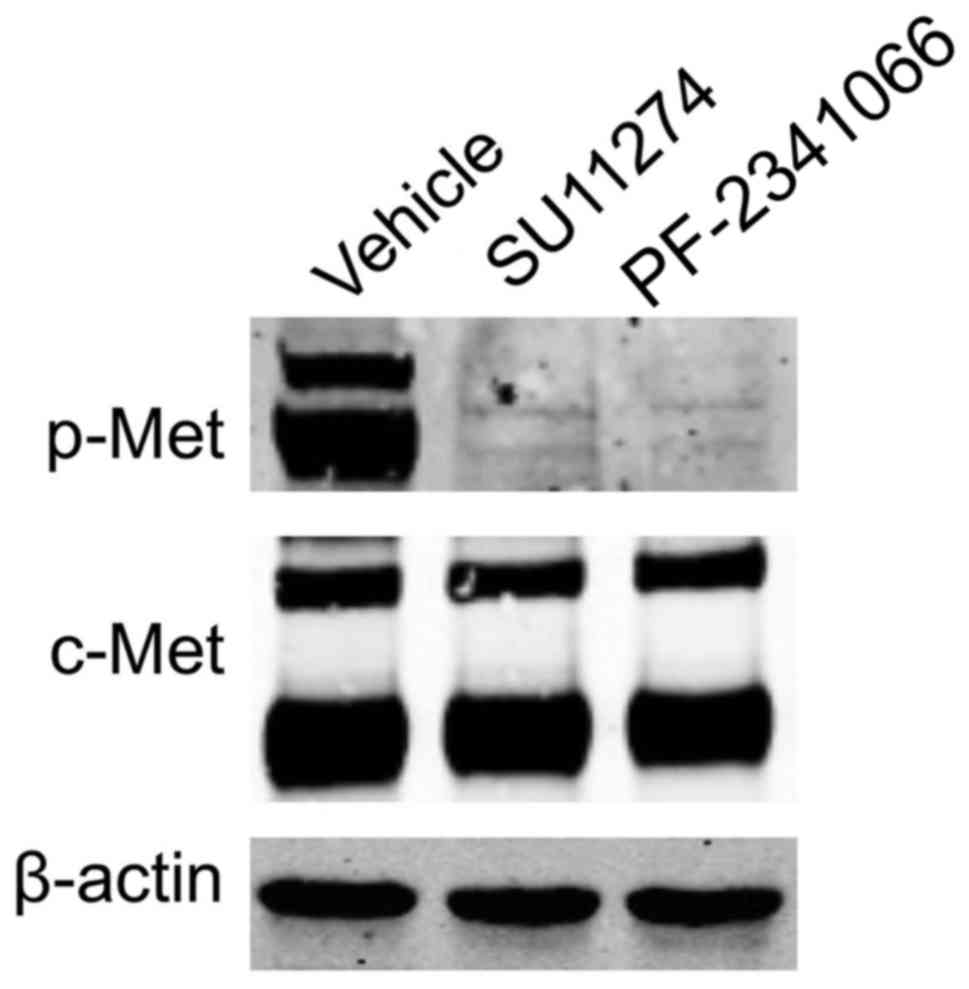
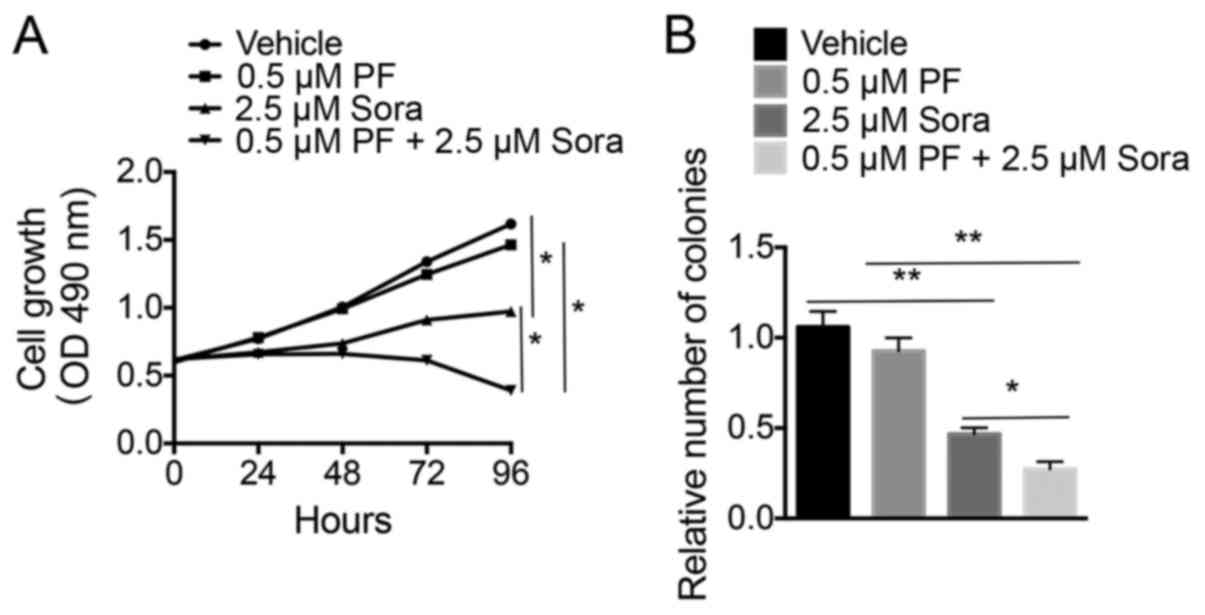
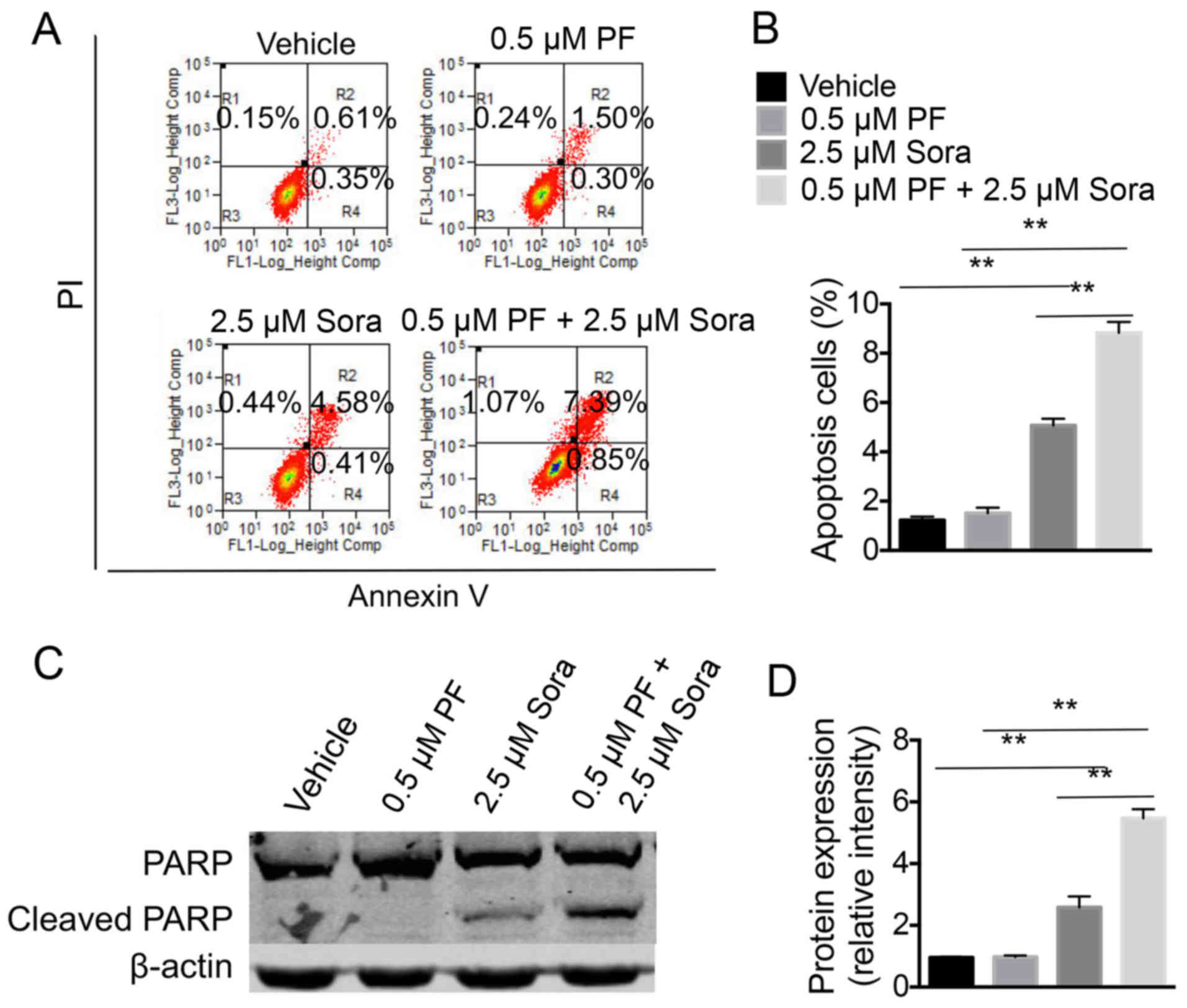
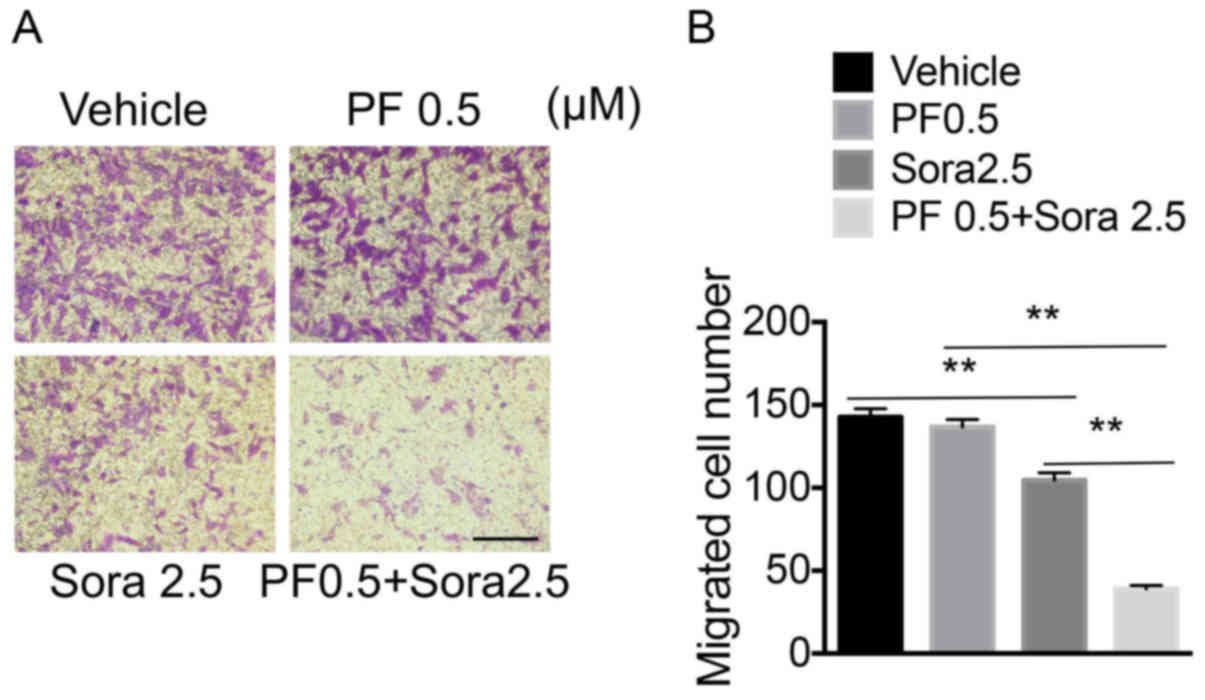
|
1
|
Torre LA, Bray F, Siegel RL, Ferlay J,
Lortet-Tieulent J and Jemal A: Global cancer statistics, 2012. CA
Cancer J Clin. 65:87–108. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Ott DE and Marcu KB: Molecular
requirements for immunoglobulin heavy chain constant region gene
switch-recombination revealed with switch-substrate retroviruses.
Int Immunol. 1:582–591. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Jemal A, Siegel R, Xu J and Ward E: Cancer
statistics, 2010. CA Cancer J Clin. 60:277–300. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ettinger DS, Wood DE, Akerley W, Bazhenova
LA, Borghaei H, Camidge DR, Cheney RT, Chirieac LR, D'Amico TA,
Demmy TL, et al: Non-small cell lung cancer, version 1.2015. J Natl
Compr Canc Netw. 12:1738–1761. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Liu CY, Wang CL, Li SH, Hsu PC, Chen CH,
Lin TY, Kuo CH, Fang YF, Ko HW, Yu CT, et al: The efficacy of 40 mg
versus dose de-escalation to less than 40 mg of afatinib (Giotrif)
as the first-line therapy for patients with primary lung
adenocarcinoma harboring favorable epidermal growth factor
mutations. Oncotarget. 8:97602–97612. 2017.PubMed/NCBI
|
|
6
|
Lategahn J, Keul M and Rauh D: Lessons to
be learned: The molecular basis of kinase-targeted therapies and
drug resistance in non-small cell lung cancer. Angew Chem Int Ed
Engl. Nov 27–2017.(Epub ahead of print).
|
|
7
|
Thabitha A, Dravid AA, Tripathi R and Lulu
SS: Database of transcription factors in lung cancer (DBTFLC): A
novel resource for exploring transcription factors associated with
lung cancer. J Cell Biochem. Dec 13–2017.(Epub ahead of print).
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kharaziha P, Chioureas D, Baltatzis G,
Fonseca P, Rodriguez P, Gogvadze V, Lennartsson L, Björklund AC,
Zhivotovsky B, Grandér D, et al: Sorafenib-induced defective
autophagy promotes cell death by necroptosis. Oncotarget.
6:37066–37082. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Bruix J, Cheng AL, Meinhardt G, Nakajima
K, De Sanctis Y and Llovet J: Prognostic factors and predictors of
sorafenib benefit in patients with hepatocellular carcinoma:
Analysis of two phase III studies. J Hepatol. 67:999–1008. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Tafreshi A, Thientosapol E, Liew MS, Guo
Y, Quaggiotto M, Boyer M and Davis ID: Efficacy of sorafenib in
advanced renal cell carcinoma independent of prior treatment,
histology or prognostic group. Asia Pac J Clin Oncol. 10:60–65.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kudo M: Immune checkpoint inhibition in
hepatocellular carcinoma: Basics and ongoing clinical trials.
Oncology. 92 Suppl 1:S50–S62. 2017. View Article : Google Scholar
|
|
12
|
Rautenberg C, Nachtkamp K, Dienst A,
Schmidt PV, Heyn C, Kondakci M, Germing U, Haas R, Kobbe G and
Schroeder T: Sorafenib and azacitidine as salvage therapy for
relapse of FLT3-ITD mutated AML after allo-SCT. Eur J Haematol.
98:348–354. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Wilhelm SM, Carter C, Tang L, Wilkie D,
McNabola A, Rong H, Chen C, Zhang X, Vincent P, McHugh M, et al:
BAY 43–9006 exhibits broad spectrum oral antitumor activity and
targets the RAF/MEK/ERK pathway and receptor tyrosine kinases
involved in tumor progression and angiogenesis. Cancer Res.
64:7099–7109. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Gridelli C, Maione P, Del Gaizo F,
Colantuoni G, Guerriero C, Ferrara C, Nicolella D, Comunale D, De
Vita A and Rossi A: Sorafenib and sunitinib in the treatment of
advanced non-small cell lung cancer. Oncologist. 12:191–200. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zhou Q, Guo X and Choksi R: Activation of
focal adhesion kinase and Src mediates acquired sorafenib
resistance in A549 human lung adenocarcinoma xenografts. J
Pharmacol Exp Ther. 363:428–443. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Degen A, Weichenthal M, Ugurel S, Trefzer
U, Kilian K, Garbe C, Egberts F, Poppe LM, Hauschild A and Gutzmer
R: Cutaneous side effects of combined therapy with sorafenib and
pegylated interferon alpha-2b in metastatic melanoma (phase II
DeCOG trial). J Dtsch Dermatol Ges. 11:846–853. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Liang H and Wang M: Mechanism of c-Met in
non-small cell lung cancer and its treatment and testing. Zhongguo
Fei Ai Za Zhi. 18:745–751. 2015.(In Chinese). PubMed/NCBI
|
|
18
|
Ma PC, Jagadeeswaran R, Jagadeesh S,
Tretiakova MS, Nallasura V, Fox EA, Hansen M, Schaefer E, Naoki K,
Lader A, et al: Functional expression and mutations of c-Met and
its therapeutic inhibition with SU11274 and small interfering RNA
in non-small cell lung cancer. Cancer Res. 65:1479–1488. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Deying W, Feng G, Shumei L, Hui Z, Ming L
and Hongqing W: CAF-derived HGF promotes cell proliferation and
drug resistance by up-regulating the c-Met/PI3K/Akt and GRP78
signalling in ovarian cancer cells. Biosci Rep. 37(pii):
BSR201604702017. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Gohda E, Tsubouchi H, Nakayama H, Hirono
S, Sakiyama O, Takahashi K, Miyazaki H, Hashimoto S and Daikuhara
Y: Purification and partial characterization of hepatocyte growth
factor from plasma of a patient with fulminant hepatic failure. J
Clin Invest. 81:414–419. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Konstorum A and Lowengrub JS: Activation
of the HGF/c-Met axis in the tumor microenvironment: A multispecies
model. J Theor Biol. 439:86–99. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Kucerova L, Demkova L, Skolekova S,
Bohovic R and Matuskova M: Tyrosine kinase inhibitor SU11274
increased tumorigenicity and enriched for melanoma-initiating cells
by bioenergetic modulation. BMC Cancer. 16:3082016. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Zou HY, Li Q, Lee JH, Arango ME, McDonnell
SR, Yamazaki S, Koudriakova TB, Alton G, Cui JJ, Kung PP, et al: An
orally available small-molecule inhibitor of c-Met, PF-2341066,
exhibits cytoreductive antitumor efficacy through antiproliferative
and antiangiogenic mechanisms. Cancer Res. 67:4408–4417. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Cascone T, Xu L, Lin HY, Liu W, Tran HT,
Liu Y, Howells K, Haddad V, Hanrahan E, Nilsson MB, et al: The
HGF/c-Met pathway is a driver and biomarker of VEGFR-inhibitor
resistance and vascular remodeling in non-small cell lung cancer.
Clin Cancer Res. 23:5489–5501. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Tarhini AA, Rafique I, Floros T, Tran P,
Gooding WE, Villaruz LC, Burns TF, Friedland DM, Petro DP, Farooqui
M, et al: PPhase 1/2 study of rilotumumab (AMG 102), a hepatocyte
growth factor inhibitor, and erlotinib in patients with advanced
non-small cell lung cancer. Cancer. 123:2936–2944. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Engelman JA, Zejnullahu K, Mitsudomi T,
Song Y, Hyland C, Park JO, Lindeman N, Gale CM, Zhao X, Christensen
J, et al: MET amplification leads to gefitinib resistance in lung
cancer by activating ERBB3 signaling. Science. 316:1039–1043. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Bignold LP, Ferrante A and Haynes DR:
Studies of chemotactic, chemotactic movement-inhibiting and random
movement-inhibiting effects of interleukin-1 alpha and beta, tumour
necrosis factor alpha and beta and interferon gamma on human
neutrophils in assays using ‘sparse-pore’ polycarbonate (Nuclepore)
membranes in the Boyden chamber. Int Arch Allergy Appl Immunol.
91:1–7. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Yamaoka T, Ohmori T, Ohba M, Arata S,
Murata Y, Kusumoto S, Ando K, Ishida H, Ohnishi T and Sasaki Y:
Distinct afatinib resistance mechanisms identified in lung
adenocarcinoma harboring an EGFR mutation. Mol Cancer Res.
15:915–928. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Zhao J, Fang L, Zhang X, Liang Y and Gou
S: Synthesis and biological evaluation of new
[1,2,4]triazolo[4,3-a]pyridine derivatives as potential c-Met
inhibitors. Bioorg Med Chem. 24:3483–3493. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Li D, Yang H, Li R, Wang Y, Wang W, Li D,
Ma S and Zhang X: Antitumor activity of gambogic acid on NCI-H1993
xenografts via MET signaling pathway downregulation. Oncol Lett.
10:2802–2806. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Zhao L, Zhu Z, Yao C, Huang Y, Zhi E, Chen
H, Tian R, Li P, Yuan Q, Xue Y, et al: VEGFC/VEGFR3 signaling
regulates mouse spermatogonial cell proliferation via the
activation of AKT/MAPK and cyclin D1 pathway and mediates the
apoptosis by affecting caspase 3/9 and Bcl-2. Cell Cycle. 1–50.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Katayama R: Therapeutic strategies and
mechanisms of drug resistance in anaplastic lymphoma kinase
(ALK)-rearranged lung cancer. Pharmacol Ther. 177:1–8. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Zhu YJ, Zheng B, Wang HY and Chen L: New
knowledge of the mechanisms of sorafenib resistance in liver
cancer. Acta Pharmacol Sin. 38:614–622. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Murray M, Gillani TB, Ghassabian S,
Edwards RJ and Rawling T: Differential effects of hepatic cirrhosis
on the intrinsic clearances of sorafenib and imatinib by CYPs in
human liver. Eur J Pharm Sci. 114:55–63. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Bahrami A, Hassanian SM, ShahidSales S,
Farjami Z, Hasanzadeh M, Anvari K, Aledavood A, Maftouh M, Ferns
GA, Khazaei M and Avan A: Targeting RAS signaling pathway as a
potential therapeutic target in the treatment of colorectal cancer.
J Cell Physiol. 233:2058–2066. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Liu H, Zhang Q, Li K, Gong Z, Liu Z, Xu Y,
Swaney MH, Xiao K and Chen Y: Prognostic significance of USP33 in
advanced colorectal cancer patients: New insights into
β-arrestin-dependent ERK signaling. Oncotarget. 7:81223–4020. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Li XL, Chen XQ, Zhang MN, Chen N, Nie L,
Xu M, Gong J, Shen PF, Su ZZ, Weng X, et al: SOX9 was involved in
TKIs resistance in renal cell carcinoma via Raf/MEK/ERK signaling
pathway. Int J Clin Exp Pathol. 8:3871–3881. 2015.PubMed/NCBI
|
|
38
|
Ranieri G, Gadaleta-Caldarola G, Goffredo
V, Patruno R, Mangia A, Rizzo A, Sciorsci RL and Gadaleta CD:
Sorafenib (BAY 43–9006) in hepatocellular carcinoma patients: From
discovery to clinical development. Curr Med Chem. 19:938–944. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Sharma SV, Bell DW, Settleman J and Haber
DA: Epidermal growth factor receptor mutations in lung cancer. Nat
Rev Cancer. 7:169–181. 2007. View
Article : Google Scholar : PubMed/NCBI
|
|
40
|
Bonanno L, Jirillo A and Favaretto A:
Mechanisms of acquired resistance to epidermal growth factor
receptor tyrosine kinase inhibitors and new therapeutic
perspectives in non small cell lung cancer. Curr Drug Targets.
12:922–933. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Polverino A, Coxon A, Starnes C, Diaz Z,
DeMelfi T, Wang L, Bready J, Estrada J, Cattley R, Kaufman S, et
al: AMG 706, an oral, multikinase inhibitor that selectively
targets vascular endothelial growth factor, platelet-derived growth
factor, and kit receptors, potently inhibits angiogenesis and
induces regression in tumor xenografts. Cancer Res. 66:8715–8721.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Stabile LP, Rothstein ME, Keohavong P,
Lenzner D, Land SR, Gaither-Davis AL, Kim KJ, Kaminski N and
Siegfried JM: Targeting of both the c-Met and EGFR pathways results
in additive inhibition of lung tumorigenesis in transgenic mice.
Cancers (Basel). 2:2153–2170. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Wu YL, Soo RA, Locatelli G, Stammberger U,
Scagliotti G and Park K: Does c-Met remain a rational target for
therapy in patients with EGFR TKI-resistant non-small cell lung
cancer? Cancer Treat Rev. 61:70–81. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Martinez-Marti A, Felip E, Matito J, Mereu
E, Navarro A, Cedrés S, Pardo N, Martinez de Castro A, Remon J,
Miquel JM, et al: Dual MET and ERBB inhibition overcomes intratumor
plasticity in osimertinib-resistant-advanced non-small-cell lung
cancer (NSCLC). Ann Oncol. 28:2451–2457. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Chen JC, Chuang HY, Hsu FT, Chen YC, Chien
YC and Hwang JJ: SSorafenib pretreatment enhances radiotherapy
through targeting MEK/ERK/NF-κB pathway in human hepatocellular
carcinoma-bearing mouse model. Oncotarget. 7:85450–85463. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Pisanu ME, Noto A, De Vitis C, Morrone S,
Scognamiglio G, Botti G, Venuta F, Diso D, Jakopin Z, Padula F, et
al: Blockade of Stearoyl-CoA-desaturase 1 activity reverts
resistance to cisplatin in lung cancer stem cells. Cancer Lett.
406:93–104. 2017. View Article : Google Scholar : PubMed/NCBI
|