Introduction
The constitutive photomorphogenesis 9 (COP9)
signalosome complex (CSN) is composed of 8 subunits (CSN1-CSN8) and
is involved in the development of eukaryotic organisms (1,2). CSN is a
highly conserved protein complex that is involved in the regulation
of cullin-RING family of ubiquitin ligases (CRLs) by mediating CRL
deneddylation (3). The catalytic
activity of CSN is performed by the CSN5 subunit. The Mpr1-Pad1-N
(MPN) domain of CSN5 harbors a JAB1-MPN-MOV34 (JAMM) metalloenzyme
motif (also known as the MPN+ motif), which is responsible for CRL
deneddylation (4). Among the 8
subunits, CSN5 is unique, as it possesses the catalytic center of
CSN isopeptidase activity and is also able to stably exist
independently of the CSN (5).
Although CSN5 was initially considered as a c-Jun coactivator, it
is now known to be an integral subunit of the CSN. CSN5 is also
referred to as c-Jun activation domain binding-protein-1 (Jab1)
(6,7).
It has been identified previously that the free form of Jab1 is
cytoplasmic and nuclear, whereas CSN-associated Jab1 is mainly
nuclear (8). Nevertheless, further
studies are required to validate the function of Jab1, as a monomer
or as a part of the CSN holocomplex, in the formation and
progression of tumors.
Jab1 serves an essential function in cellular
proliferation by directly interacting with and functionally
regulating the activity and stability of several key intracellular
regulatory proteins, including p53, p27, mothers against
decapentaplegic homolog 4/7, macrophage migration inhibitory factor
and hypoxia-inducible factor-1α (9–13).
Although Jab1 participates in a number of regulatory processes, its
function in tumorigenesis remains largely elusive. Indeed, Jab1 has
an emerging function in cancer. Jab1 overexpression has been
identified in various human malignancies and is inversely
associated with a poor prognosis of patients with cancer (14). For example, the increased expression
of Jab1 is detected in hepatocellular (15) and thyroid (16) carcinoma, and is associated with a
lower survival rate.
Among the various types of head and neck cancer
known, laryngeal squamous cell carcinoma (LSCC) remains a common
type of cancer. There are >500,000 novel cases of LSCC diagnosed
annually and the incidence of LSCC has increased in the last decade
(17,18). It is frustrating that the mortality
rate of patients with LSCC has not markedly decreased, regardless
of whether the treatment strategy for LSCC has improved (19–21).
Therefore, novel strategies and biomarkers are necessary for the
treatment and tumor staging of LSCC.
In the present study, the expression levels of Jab1
in laryngeal cancer cells were examined. Furthermore, small
interfering RNA (siRNA) was employed to analyze the function of
Jab1 in regulating apoptosis and proliferation of laryngeal cancer
cells in vitro. Finally, in order to investigate the
underlying molecular mechanisms by which Jab1 is involved in LSCC,
the expression levels of protein kinase B (Akt), phosphorylated
(p)-Akt, p53 and cleaved caspase-3 (c-caspase-3) were examined in
Jab1-knockdown cancer cells. The results of the present study
suggest that Jab1 serves a crucial function in the progression of
LSCC and may be a promising therapeutic target in combating
LSCC.
Materials and methods
Cell culture conditions and siRNA
transfection
AMC-HN-8 and OME cells were sourced from the Cell
Bank, China Academy of Sciences (Shanghai, China) and maintained in
Dulbecco's modified Eagle's medium (Gibco; Thermo Fisher
Scientific, Inc., Waltham, MA, USA) supplemented with 10% fetal
bovine serum (FBS; Invitrogen; Thermo Fisher Scientific, Inc.) and
incubated in the Thermo forma incubator (Thermo Fisher Scientific,
Inc.) at 37°C with 5% CO2. The cells were grown to 50%
confluence. Cells were then transfected with three sets of
Jab1-specific siRNAs (Shanghai GenePharma Co., Ltd., Shanghai,
China) or negative control siRNA (siCtrl; Shanghai GenePharma Co.,
Ltd.), using siLentFect Lipid reagent (Bio-Rad Laboratories, Inc.,
Hercules, CA, USA). The quantity of the siRNAs used for
transfection was 200 pmol for each 35 mm culture dish. The target
sequences of the Jab1-specific siRNAs used were as follows:
Jab1-siRNA1 (si1-Jab1) sense, 5′-CCAGACUAUUCCACUUAAUTT-3′ and
antisense, 5′-AUUAAGUGGAAUAGUCUGGTT-3′; Jab1-siRNA2 (si2-Jab1)
sense, 5′-GGUGAAACCAUGAUCAUUTT-3′ and antisense,
5′-UAAUGAUCAUGGUUUCACCTT-3′; Jab1-siRNA3 (si3-Jab1) sense,
5′-GGACUAAGGAUCACCAUUATT-3′ and antisense,
5′-UAAUGGUGAUCCUUAGUCCTT-3′.
At 4–6 h after transfection, the culture medium
(DMEM) containing the transfection reagent was removed, and fresh
medium containing FBS was added. After 48 h of culture, cells were
lysed and used for subsequent experiments.
Western blot analysis
At 48 h after transfection with each si-Jab1, 1 ml
of lysis buffer (Beyotime Institute of Biotechnology, Haimen,
China) was added to each dish for 30 min at 4°C, with occasional
shaking. Then, cell lysates were collected into a 1.5 ml tube
followed with centrifugation at 16,000 × g for 15 min at 4°C. The
protein concentration was determined using a bicinchoninic acid
assay kit (Pierce; Thermo Fisher Scientific, Inc.). Total proteins
were boiled with loading buffer (Vicmed Biotech Co., Ltd., Xuzhou,
China) and then loaded (100 µg/lane) and separated by SDS-PAGE
(12.5% gel) and transferred onto a nitrocellulose membrane. The
membranes were then blocked for 2 h at room temperature in 5%
non-fat milk (BD Biosciences, Franklin Lakes, NJ, USA) suspended in
tris-buffered saline with Tween-20 (TBST; 150 mM NaCl, 20 mM
Tris-HCl, pH 8.0, 0.05% Tween-20). Following blocking, the
membranes were incubated with anti-cleaved caspase-3 (Cell
Signaling Technology, Inc., Danvers, MA, USA), anti-Jab1, anti-p53,
anti-Akt, anti-p-Akt and anti-β-actin (Santa Cruz Biotechnology,
Inc., Dallas, TX, USA) antibodies at 4°C overnight. Membranes were
then washed using TBST and incubated with the horseradish
peroxidase-conjugated mouse anti-rabbit IgG secondary antibodies
(cat no. A2074; 1:20,000; Sigma; Merck KGaA, Darmstadt, Germany) at
room temperature for 2 h. The protein bands were visualized with
Enhanced Chemiluminescence reagent (Tanon Science and Technology
Co., Ltd., Shanghai, China). The densitometric analysis for the
quantification of the bands was performed using ImageJ software
(version 1.46; National Institutes of Health, Bethesda, MD,
USA).
Cell proliferation assay
Cell proliferation was evaluated using a Cell
Counting Kit-8 (CCK-8; Beyotime Institute of Biotechnology). In
brief, at 48 h post-transfection, 5,000 cells were seeded in
96-well plates with medium containing 10% FBS and incubated for 1,
2, 3 and 4 days according to the manufacturer's protocol. Then, 10
µl CCK-8 solution and 100 µl serum-free culture medium (DMEM) were
mixed and added to each well, followed by incubation at 37°C for 2
h. The absorbance was read at 450 nm using a spectrophotometer
(BioTek Instruments, Inc., Winooski, VT, USA). The experiments were
performed in triplicate.
Confocal analysis of Annexin V
binding
AMC-HN-8 cells were plated in 6-well plates and
transfected with si1-Jab1. si1-Jab1 was selected for this and
subsequent experiments as it yielded the most marked decrease in
Jab1 expression of all three Jab1-specific siRNAs. Following
transfection, treated cells were collected in a 1.5 ml tube and
washed twice with phosphate buffered saline. Following washing,
cells were labeled with 200 µl binding buffer (BD Biosciences)
containing 5 µl Annexin V-fluorescein isothiocyanate (FITC) and
added to 300 µl binding buffer containing 5 µl propidium iodide
(PI) at room temperature in darkness for 5 min. Cells were
visualized under a fluorescence microscope (Olympus Corporation,
Tokyo, Japan). The software used for analysis was Olympus cellSens
Dimension (version 1.0; Olympus Corporation). A total of 3
independent experiments were conducted and 5 horizons were randomly
selected from each group, at a magnification of ×100.
Flow cytometric analysis of
apoptosis
AMC-HN-8 cells were plated in 6-well plates and
transfected with si1-Jab1 for 48 h. The cells were collected and
labeled using an Annexin V-FITC/PI Apoptosis Detection kit (BD
Biosciences), according to the manufacturer's protocol. The
apoptotic cell fraction was detected using a FACScan flow cytometer
(BD Biosciences). Data were analyzed using ModFit LT 3.0 software
(Verity Software House, Inc., Topsham, ME, USA). Living cells
(Annexin V−FITC−/PI−), early
apoptotic cells (Annexin
V−FITC+/PI−), late apoptotic cells
(Annexin V−FITC+/PI+) and necrotic
cells (Annexin V−FITC−/PI+) were
enumerated.
Statistical analysis
Data were analyzed using SPSS software (version
16.0; SPSS, Inc., Chicago, IL, USA). The relevant data are
expressed as the mean ± standard deviation (SD). Results were
analyzed using Student's t-test when only 2 groups were compared,
or one-way analysis of variance when >2 groups were compared.
The post hoc test used was the Student-Newman-Keuls method.
P<0.05 was considered to indicate a statistically significant
difference.
Results
Jab1 expression is increased in cancer
cells
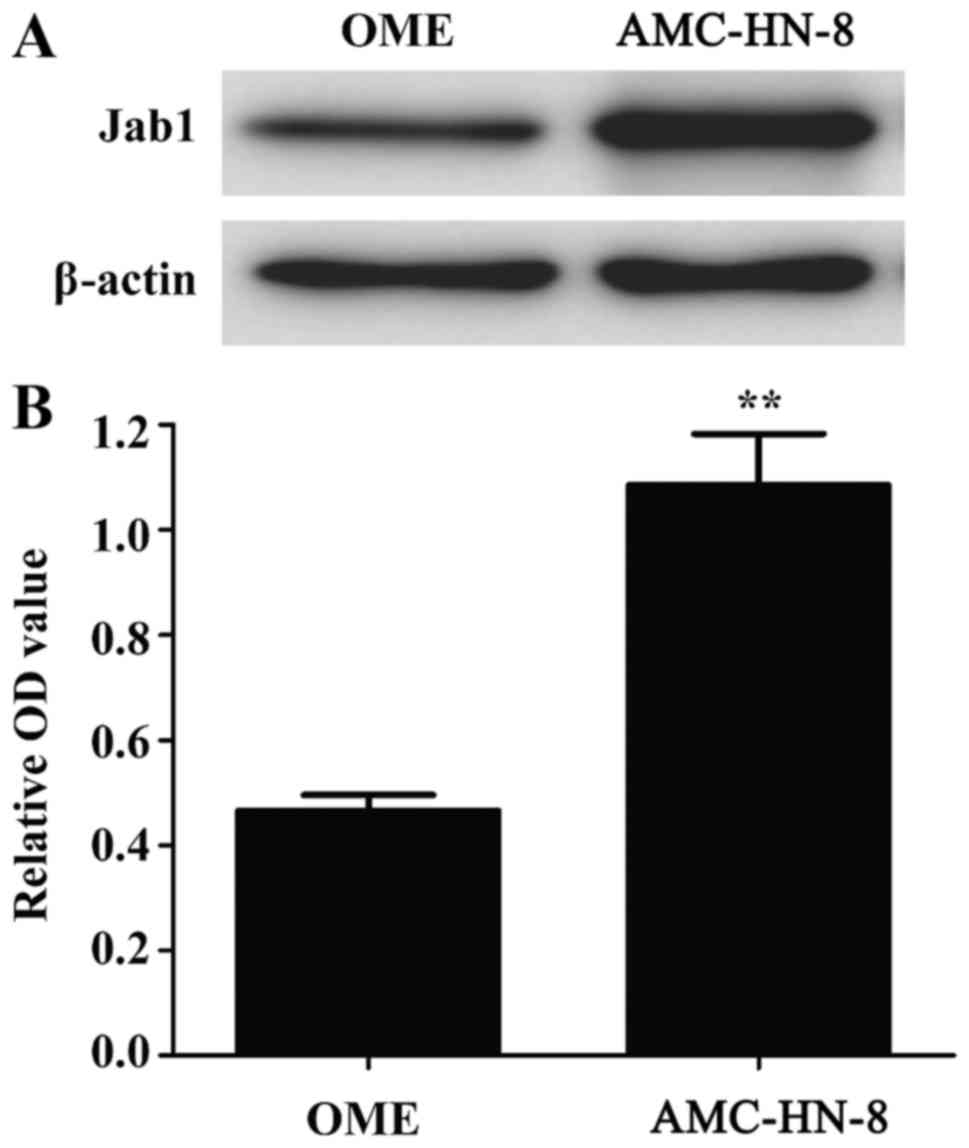
Western blot analysis was used to detect the
expression of Jab1 in normal human oral mucosal epithelial (OME)
and AMC-HN-8 cells. As presented in Fig.
1, the expression level of Jab1 protein was increased in
AMC-HN-8 cells compared with OME cells, suggesting that, although
Jab1 can be expressed in normal cells, its levels are increased in
laryngeal cancer cells.
Knockdown of Jab1 inhibits cell
proliferation and promotes apoptosis
Jab1 expression was knocked down in AMC-HN-8 cells
using three different sets of siRNAs targeting Jab1 (si1-Jab1,
si2-Jab1 and si3-Jab1). Jab1 protein expression was evaluated using
western blot analysis and it was identified that Jab1 was
significantly downregulated in si1-Jab1 and si2-Jab1 groups
compared with the siCtrl group (Fig.
2). However, si1-Jab1 exhibited the most marked effect in
decreasing the expression levels of Jab1 and was therefore used in
subsequent experiments.
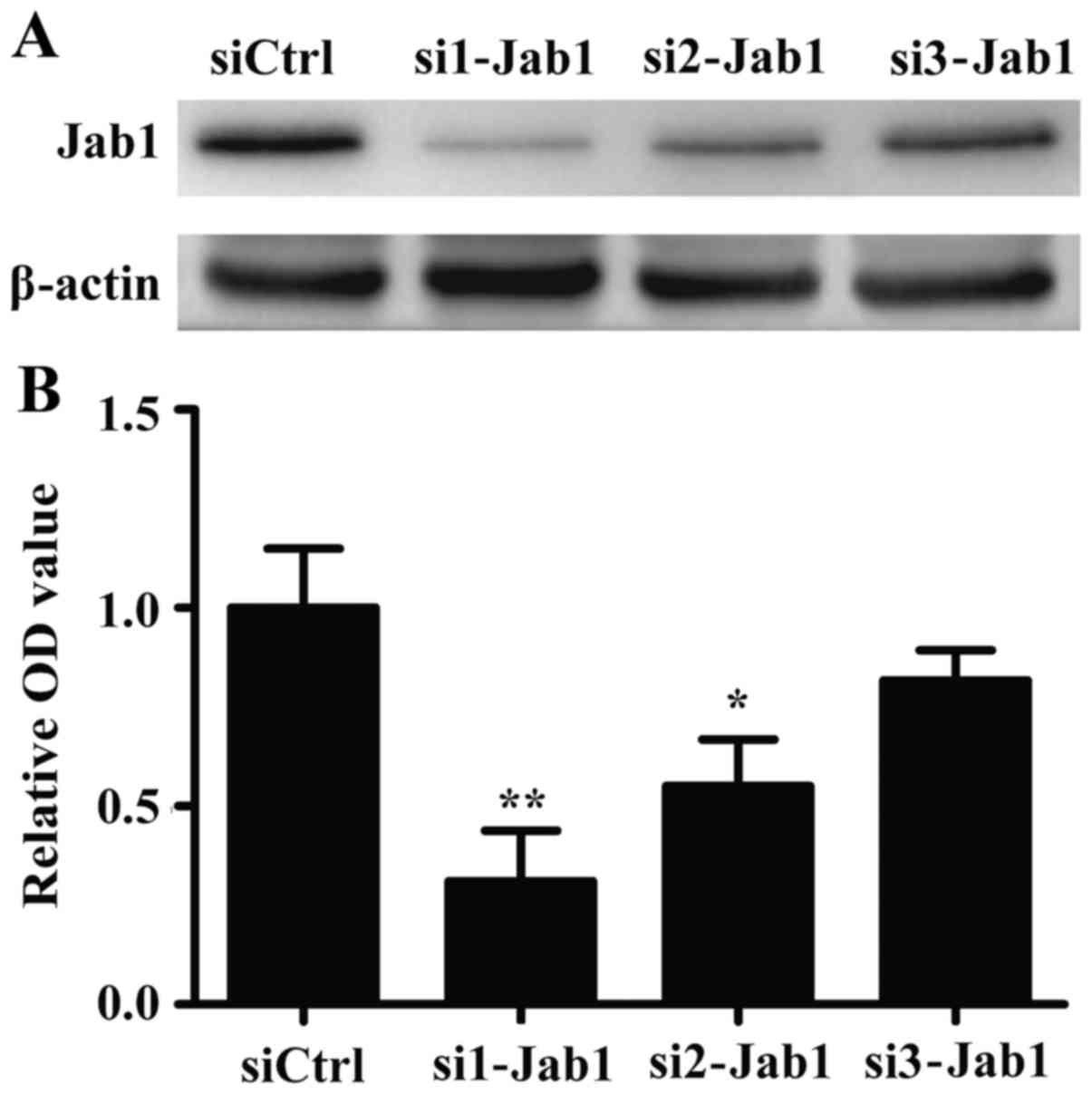 | Figure 2.Effect of Jab1 siRNA on the expression
levels of Jab1. (A) AMC-HN-8 cells were transfected with siCtrl or
with three different sets of Jab1 siRNAs: si1-Jab1, si2-Jab1 or
si3-Jab1. Following transfection, Jab1 expression was analyzed
among the different treatment groups by western blotting. si3-Jab1
group was not significant in the present study, and si1-Jabl was
selected for further experiments. (B) Relative intensity obtained
after densitometric analysis of Jab1 protein in siCtrl, si1-Jab1,
si2-Jab1 and si3-Jab1 treatment groups. β-actin was used as the
loading control. Results are expressed as the mean ± standard
deviation for triplicate experiments. *P<0.05; **P<0.01 vs.
siCtrl. Jab1, c-Jun activation domain-binding protein-1; OD,
optical density; si-Jab1, Jab1-siRNA; siCtrl, control siRNA; siRNA,
small interfering RNA. |
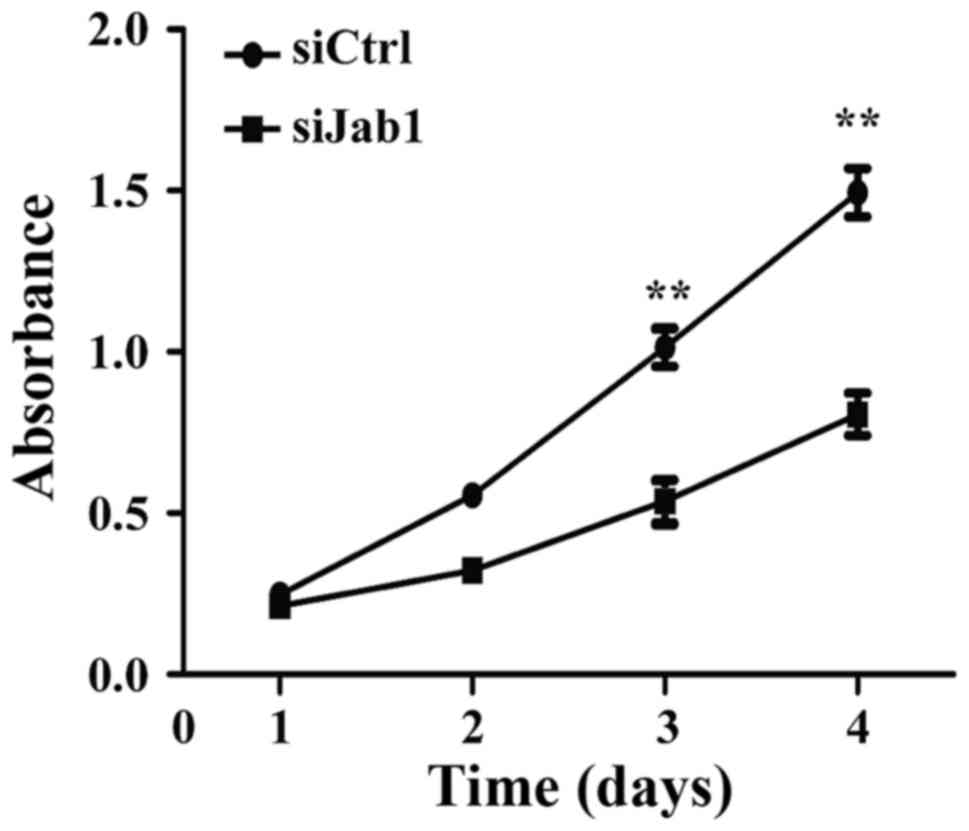
The effect of Jab1 on carcinoma cell proliferation
was investigated. Following transfection of AMC-HN-8 cells with
Jab1 siRNA, a CCK-8 assay was performed and a significant decrease
in cellular proliferation was identified on days 3 and 4 (Fig. 3). This suggests that loss of Jab1
expression has an inhibitory effect on the proliferation of
laryngeal cancer cells.
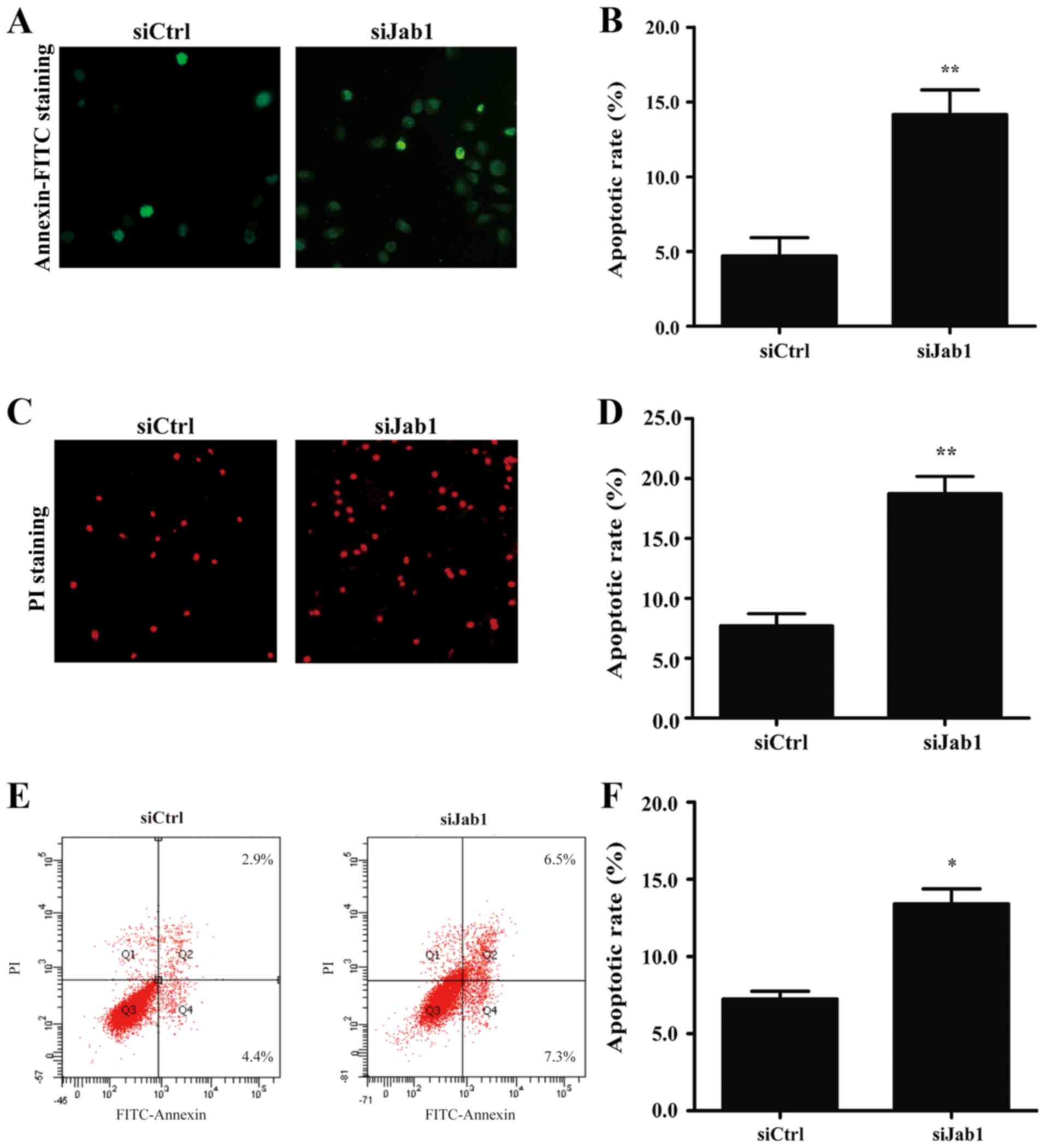
On the basis of the cell proliferation results, it
was investigated whether the loss of Jab1 expression may also have
an effect on the apoptosis of laryngeal cancer cells. An Annexin
V-FITC binding assay and fluorescence microscopy were used to
analyze changes in the total apoptotic cells including early
apoptotic cells (Annexin
V−FITC+/PI−) and late apoptotic or
necrotic cells (Annexin
V−FITC+/PI+). It was identified
that the si1-Jab1-treated cells exhibited enhanced apoptosis at
prophase (Fig. 4A and B) and anaphase
(Fig. 4C and D) compared with the
control group. Apoptosis was additionally analyzed between the two
groups by flow cytometry. According to the results, it was observed
that si1-Jab1 treatment increased the apoptotic cell fraction in
AMC-HN-8 cells (Fig. 4E and F). In
particular, the Annexin
V−FITC+/PI− early apoptotic cell
fraction accounted for 4.4% of the cells in the control and 7.3% of
the cells in the si1-Jab1-treated group. Annexin
V−FITC+/PI+ late apoptotic cells
accounted for 2.9% of the cells in the control and 6.5% of the
cells in the si1-Jab1 group, 48 h post-transfection. These results
suggest that decreasing the expression of Jab1 protein facilitates
the apoptosis of laryngeal cancer cells.
Effects of Jab1 downregulation on the
expression of Akt and p53 protein
To understand the mechanisms involved in
Jab1-mediated apoptosis and proliferation in carcinoma cells, the
effect of Jab1 deficiency on certain key factors involved in
anti-apoptotic pathways and the apoptotic cascade, including Akt,
p-Akt, c-caspase-3 and p53, were examined. Western blot analysis
revealed that the level of p-Akt expression was downregulated,
whereas c-caspase-3 and p53 were upregulated in the Jab1-knockdown
group in comparison with the control group (Fig. 5). These results indicate that these
proteins may be involved in Jab1-mediated proliferation and
apoptosis in AMC-HN-8 cells.
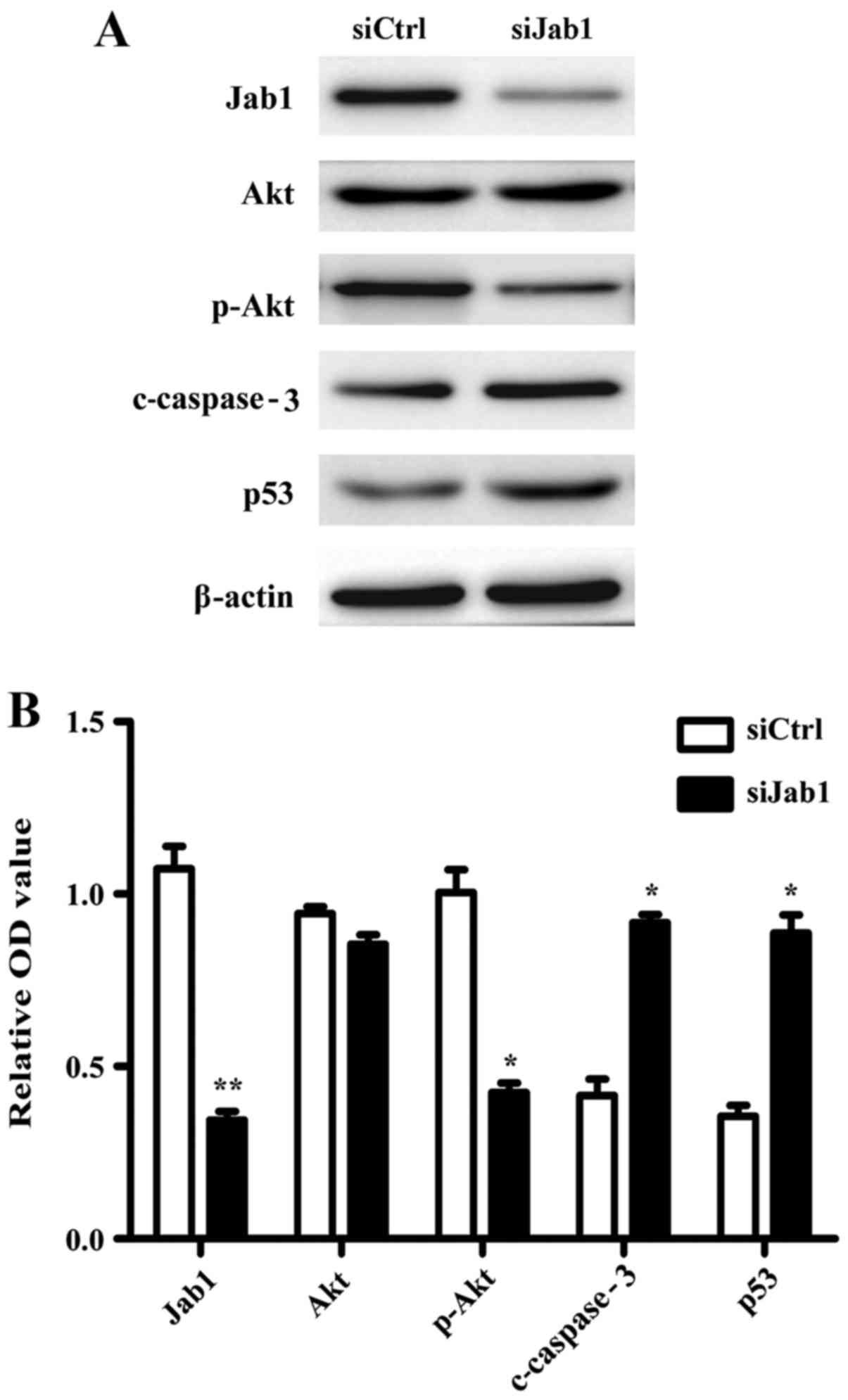 | Figure 5.Effects of Jab1 deficiency on the
expression of Akt and p53 proteins. (A) Western blot analysis of
Akt, p-Akt, c-caspase-3 and p53 expression in AMC-HN-8 cells which
were transfected with siCtrl or siJab1. (B) Relative intensity
following densitometric analysis of Jab1, Akt, p-Akt, c-caspase-3
and p53. β-actin was used as the loading control. Results are
expressed as the mean ± standard deviation for triplicate
experiments. *P<0.05; **P<0.01 vs. siCtrl. Akt, protein
kinase B; c-caspase-3, cleaved caspase-3; Jab1, c-Jun activation
domain-binding protein-1; p-Akt, phosphorylated Akt; OD, optical
density; siJab1, Jab1-siRNA1; siCtrl, control siRNA; siRNA, small
interfering RNA. |
Discussion
A number of studies have demonstrated that Jab1
expression is increased in a number of human malignant tumors
(9). Jab1 serves an intriguing
function in the tumorigenic process and increased levels of Jab1
are associated with lymph node metastasis and poor prognosis of
several types of human cancer, including LSCC. Jab1 may represent a
prognostic indicator of malignant transformation and assists in
explaining the biological behavior of a number of cancer cells,
including human colorectal cancer cell lines (22) and liver cancer cells (23). It has been reported that Jab1 acts as
a negative regulatory factor of p27 protein and has an association
with cell proliferation in LSCC (24). However, the underlying molecular
mechanism of Jab1 in LSCC is not known. In the present study, it
was identified that AMC-HN-8 cells exhibit increased Jab1
expression levels when compared with OME cells. Additionally, it
was demonstrated that downregulation of Jab1 inhibits proliferation
and increases the apoptosis of laryngeal carcinoma cells. These
results suggest that Jab1 may regulate the biological behavior of
the LSCC cells and contribute to the progression of laryngeal
carcinoma.
Free-form (non-CSN-associated) Jab1 is located in
the cytoplasm and the nucleus, whereas CSN-associated Jab1 is
primarily located in the nucleus (25). Jab1 protein is mainly localized in the
nucleus in AMC-HN-8 cells (24). The
Jab1 PMN domain contains the JAMM motif, which is essential for CSN
deneddylation activity. The functional effects of Jab1 depend on
the whole CSN assembly and deletion of any CSN subunit may lead to
the inactivation of the CSN complex (26).
In the present study the function of Jab1 on
apoptosis, a complex biological process (27), during LSCC progression was evaluated.
For this purpose, the expression levels of caspase-3 and p53 were
determined in laryngeal cancer cells, with downregulated Jab1
expression. Caspase-3 is an effector caspase involved in the
apoptotic cascade (28). According to
the results of the present study, c-caspase-3 exhibited a
significant increase following Jab1 downregulation in AMC-HN-8
cells. Additionally, p53 was identified to be upregulated following
si1-Jab1 transfection. p53 is a tumor-inhibiting factor that is
able to limit cell proliferation by inducing apoptosis, and a
number of apoptosis-related genes are transcriptionally regulated
by p53 (29,30). Although c-caspase-3 and p53 are
involved in Jab1-mediated apoptosis, it remains to be determined
whether there is a connection between p53 and c-caspase-3.
According to the results presented in Fig. 5A, p53 and c-caspase-3 were identified
to be upregulated following si1-Jab1 transfection. Therefore, it
was hypothesized that there may exist a positive association
between p53 and c-caspase-3. Finally, it was identified that the
expression level of p-Akt was decreased following si1-Jab1
transfection. On the basis of the significant function of Akt in
cellular proliferation (31), it is
likely that Jab1 siRNA inhibits cell proliferation via the
phosphoinositide 3-kinase/Akt signaling pathway.
In summary, the results of the present study
demonstrate that Jab1 serves a crucial function in LSCC
tumorigenesis, particularly in cellular proliferation and
apoptosis. Jab1 is critical for the regulation of Akt, c-caspase-3
and p53. Further experiments remain to be performed to elucidate
the underlying molecular mechanisms by which Jab1 regulates
proliferation and apoptosis during tumorigenesis. Jab1 is likely to
be a key marker and target for the treatment of LSCC.
Acknowledgements
The present study was supported by the National
Natural Science Foundation of China (grant no. 81572349), Jiangsu
Provincial Medical Talent, and the Science and Technology
Department of Jiangsu Province (grant nos. BK20130231 and
BK20141149).
References
|
1
|
Kapelari B, Bech-Otschir D, Hegerl R,
Schade R, Dumdey R and Dubiel W: Electron microscopy and
subunit-subunit interaction studies reveal a first architecture of
COP9 signalosome. J Mol Biol. 300:1169–1178. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Wei N, Serino G and Deng XW: The COP9
signalosome: More than a protease. Trends Biochem Sci. 33:592–600.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Schwechheimer C and Deng XW: COP9
signalosome revisited: A novel mediator of protein degradation.
Trends Cell Biol. 11:420–426. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Cope GA, Suh GS, Aravind L, Schwarz SE,
Zipursky SL, Koonin EV and Deshaies RJ: Role of predicted
metalloprotease motif of Jab1/Csn5 in cleavage of Nedd8 from Cul1.
Science. 298:608–611. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Wei N and Deng XW: The COP9 signalosome.
Annu Rev Cell Dev Biol. 19:261–286. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Claret FX, Hibi M, Dhut S, Toda T and
Karin M: A new group of conserved coactivators that increase the
specificity of AP-1 transcription factors. Nature. 383:453–457.
1996. View
Article : Google Scholar : PubMed/NCBI
|
|
7
|
Chamovitz DA and Segal D: JAB1/CSN5 and
the COP9 signalosome. A complex situation. EMBO Rep. 2:96–101.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kwok SF, Solano R, Tsuge T, Chamovitz DA,
Ecker JR, Matsui M and Deng XW: Arabidopsis homologs of a c-Jun
coactivator are present both in monomeric form and in the COP9
complex, and their abundance is differentially affected by the
pleiotropic cop/det/fus mutations. Plant Cell. 10:1779–1790. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Shackleford TJ and Claret FX: JAB1/CSN5: A
new player in cell cycle control and cancer. Cell Div. 5:262010.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Winner M, Koong AC, Rendon BE, Zundel W
and Mitchell RA: Amplification of tumor hypoxic responses by
macrophage migration inhibitory factor-dependent hypoxia-inducible
factor stabilization. Cancer Res. 67:186–193. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Oh W, Lee EW, Sung YH, Yang MR, Ghim J,
Lee HW and Song J: Jab1 induces the cytoplasmic localization and
degradation of p53 in coordination with Hdm2. J Biol Chem.
281:17457–17465. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Wan M and Cao X, Wu Y, Bai S, Wu L, Shi X,
Wang N and Cao X: Jab1 antagonizes TGF-beta signaling by inducing
Smad4 degradation. EMBO Rep. 3:171–176. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Tomoda K, Kubota Y, Arata Y, Mori S, Maeda
M, Tanaka T, Yoshida M, Yoneda-Kato N and Kato JY: The cytoplasmic
shuttling and subsequent degradation of p27Kip1 mediated by
Jab1/CSN5 and the COP9 signalosome complex. J Biol Chem.
277:2302–2310. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kato JY and Yoneda-Kato N: Mammalian COP9
signalosome. Genes Cells. 14:1209–1225. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Hsu MC, Huang CC, Chang HC, Hu TH and Hung
WC: Overexpression of Jab1 in hepatocellular carcinoma and its
inhibition by peroxisome proliferator-activated receptor{gamma}
ligands in vitro and in vivo. Clin Cancer Res. 14:4045–4052. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Ahn J, Hong SA, Lee SE, Kim J, Oh YS, Park
SJ and Chung YJ: Cytoplasmic localization of Jab1 and p27 Kip1
might be associated with invasiveness of papillary thyroid
carcinoma. Endocr J. 56:707–713. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Jemal A, Siegel R, Ward E, Hao Y, Xu J and
Thun MJ: Cancer statistics, 2009. CA Cancer J Clin. 59:225–249.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Liu XK, Li Q, Xu LH, Hu LJ, Liao WG, Zhang
XR, Liu ZM, Wu D and Zeng MS: Expression and clinical significance
of SIAH in laryngeal squamous cell carcinoma. Med Oncol.
30:4852013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Boyle P and Ferlay J: Cancer incidence and
mortality in Europe, 2004. Ann Oncol. 16:481–488. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Ferlay J, Parkin DM and Steliarova-Foucher
E: Estimates of cancer incidence and mortality in Europe in 2008.
Eur J Cancer. 46:765–781. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Li JJ, Yang XM, Wang SH and Tang QL:
Prognostic role of epidermal growth factor-like domain 7 protein
expression in laryngeal squamous cell carcinoma. J Laryngol Otol.
125:1152–1157. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Schütz AK, Hennes T, Jumpertz S, Fuchs S
and Bernhagen J: Role of CSN5/JAB1 in Wnt/β-catenin activation in
colorectal cancer cells. FEBS Lett. 586:1645–1651. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Lee YH, Judge AD, Seo D, Kitade M,
Gómez-Quiroz LE, Ishikawa T, Andersen JB, Kim BK, Marquardt JU,
Raggi C, et al: Molecular targeting of CSN5 in human hepatocellular
carcinoma: A mechanism of therapeutic response. Oncogene.
30:4175–4184. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Dong Y, Sui L, Watanabe Y, Yamaguchi F,
Hatano N and Tokuda M: Prognostic significance of Jab1 expression
in laryngeal squamous cell carcinomas. Clin Cancer Res. 11:259–266.
2005.PubMed/NCBI
|
|
25
|
Tomoda K, Kubota Y and Kato J: Degradation
of the cyclin-dependent-kinase inhibitor p27Kip1 is instigated by
Jab1. Nature. 398:160–165. 1999. View
Article : Google Scholar : PubMed/NCBI
|
|
26
|
Adler AS, Littlepage LE, Lin M, Kawahara
TL, Wong DJ, Werb Z and Chang HY: CSN5 isopeptidase activity links
COP9 signalosome activation to breast cancer progression. Cancer
Res. 68:506–515. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Thompson CB: Apoptosis in the pathogenesis
and treatment of disease. Science. 267:1456–1462. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Porter AG and Jänicke RU: Emerging roles
of caspase-3 in apoptosis. Cell Death Differ. 6:99–104. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Shen Y and White E: p53-dependent
apoptosis pathways. Adv Cancer Res. 82:55–84. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Abu-Qare AW and Abou-Donia MB: Biomarkers
of apoptosis: Release of cytochrome c, activation of caspase-3,
induction of 8-hydroxy-2′-deoxyguanosine, increased
3-nitrotyrosine, and alteration of p53 gene. J Toxicol Environ
Health B Crit Rev. 4:313–332. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Cicenas J: The potential role of Akt
phosphorylation in human cancers. Int J Biol Markers. 23:1–9. 2008.
View Article : Google Scholar : PubMed/NCBI
|