Introduction
With high rates of recurrence, invasion and
metastasis, bladder cancer is one of the common urinary neoplasms
and seriously affects human health worldwide. In China, bladder
cancer is the seventh most common tumor in males and it has the
highest incidence among genitourinary tumors (1). Similarly, in the US, the incidence of
bladder cancer is second only to prostate cancer (2). In addition, the incidence of bladder
cancer in females is lower than in males worldwide. Although
bladder cancer is common, its molecular mechanisms of occurrence
and progression are not yet clear. Surgery is the gold-standard
treatment for bladder cancer, yet the rate of recurrence is high
and the overall 5-year survival is low. In particular, therapeutic
strategies for advanced bladder cancer are very limited (3). Therefore, clarifying the mechanism of
proliferation, invasion and metastasis in bladder cancer, and
finding new targets for therapy, has become a key focus of
research.
It is well known that the biological behavior of
malignant tumors is closely related to blood supply. Traditional
neovascularization is common in malignant tumors, which ensures a
sufficient nutrient supply over time. In recent years, some
vascular targeting drugs, such as bevacizumab and Tyrosine Kinase
Inhibitor, have been used in clinical therapy, but these had little
effect in many malignant tumors. Some scholars have proposed that
novel tumor microcirculation patterns, different from traditional
angiogenesis, may exist in these malignant tumors. Vasculogenic
mimicry (VM), originally discovered in melanoma (4), is a novel tumor microcirculation system
that does not rely on vascular endothelial cells. It is a blood
vessel-like structure, composed of a group of tumor cells, which
can deliver erythrocytes and other nutrients by connecting with
normal blood vessels directly. The phenomenon has been observed in
many tumor types, including hepatocellular carcinoma, breast,
colorectal and prostate cancer (5–8). Numerous
studies (8–10) have shown that the existence of VM is
closely related to the invasion, metastasis and poor prognosis of
malignant tumors, which is significant in the clinic. At present,
the literature about VM in bladder cancer, particularly its
molecular mechanism, is very limited.
Zinc finger E-box binding homeobox 1 (ZEB1), which
belongs to the ZEB family of transcription factors, consists of two
zinc finger clusters, responsible for DNA binding, and a
centrally-located homeodomain. ZEB1 not only promotes tumor
invasion and metastasis by inducing epithelial-mesenchymal
transition (EMT), but it can also regulate therapeutic resistance
via different mechanisms (11). ZEB1
is overexpressed in several types of cancer, such as gastric
cancer, lung cancer and prostate cancer, and it plays an important
role in inducing EMT in tumor cells (12–14). EMT,
which describes the process by which tumor cells escape from one
site to invade the adjacent matrix and transfer to a distant site,
is a key event in the progression of malignant tumors. EMT is
regulated by many transcription factors, including the traditional
factor ZEB1. The role of EMT in the development of VM has recently
attracted attention (9,15,16). Liu
et al (8) confirmed that ZEB1
can promote VM formation by inducing EMT in colorectal cancer.
Similarly, ZEB2, a homologous protein of ZEB1, was found to have
the same function in inducing EMT, promoting VM formation in
hepatocellular carcinoma (17).
Although there is no consensus about the correlation between ZEB1
expression and tumor grade, stage, invasion and metastasis in
bladder cancer, numerous studies have verified that ZEB1 is
significantly overexpressed in bladder cancer tissues in comparison
with healthy adjacent tissues (18–20).
However, it has not been reported whether ZEB1 plays a critical
role in VM formation in bladder cancer. Therefore, it is necessary
to study the relationship between VM, ZEB1 expression and clinical
parameters in bladder cancer. More importantly, the mechanism
involving ZEB1 and VM in bladder cancer must be investigated.
In the present study, we demonstrated that ZEB1 was
significantly overexpressed in bladder cancer compared with normal
tissue, and was positively correlated with VM. In an in
vitro assay, knockdown of ZEB1 was indicated to suppress the
formation of VM in bladder cancer. Furthermore, the mechanism by
which ZEB1 promotes VM in bladder cancer requires further
investigation.
Materials and methods
Clinical tissue samples and
immunohistochemistry (IHC) staining
The current study consisted of 147 formalin-fixed
and paraffin-embedded samples, of which 135 specimens (116 males
and 19 females; mean age, 61.5 years; age range, 18 to 85 years)
were from patients with bladder cancer and a further 12 specimens
were from normal tissue adjacent to bladder cancer tissue. The
samples were obtained from the First Affiliated Hospital, Sun
Yat-sen University, between November 2015 and March 2017. All
diagnoses were confirmed by pathology. Further clinical parameters
are presented in Table I. The present
study was approved by the Medical Ethics Committee of Sun Yat-sen
University (Guangzhou, Guangdong, China) and written informed
consent was obtained from each patient. The IHC staining assays and
evaluation methods were performed as previously described (18,21). The
antibody used was rabbit polyclonal ZEB1 antibody (1:200; cat. no.
ab87280; Abcam, Cambridge, UK). ZEB1 expression was evaluated
according to the staining intensity and extent. In brief, the
staining intensity was scored as 0 (none), 1 (weak), 2 (medium) or
3 (strong), and the staining extent was scored as 0 (0–5%), 1
(6–25%), 2 (26–75%) or 3 (75–100%). Then the two scores were summed
to obtain a final score. Final scores ≤3 or >3 were considered
to indicate low or high expression, respectively. All samples were
evaluated by two independent observers.
 | Table I.Associations between vasculogenic
mimicry, zinc finger E-box binding homeobox 1 expression and the
clinicopathological parameters in bladder cancer. |
Table I.
Associations between vasculogenic
mimicry, zinc finger E-box binding homeobox 1 expression and the
clinicopathological parameters in bladder cancer.
|
|
| VM |
| ZEB1 expression |
|
|---|
|
|
|
|
|
|
|
|---|
| Variables | Total | Positive | Negative | P-value | Low expression
(scores ≤3) | High expression
(scores >3) | P-value |
|---|
| Age (years) |
|
|
|
|
|
|
|
|
<60 | 52 | 18 | 34 | 0.486 | 18 | 34 | 0.312 |
| ≥60 | 83 | 24 | 59 |
| 36 | 47 |
|
| Sex |
|
|
|
|
|
|
|
| Male | 116 | 37 | 79 | 0.626 | 48 | 68 | 0.419 |
|
Female | 19 | 5 | 14 |
| 6 | 13 |
|
| TNM stage |
|
|
|
|
|
|
|
|
Ta-T1 | 95 | 32 | 63 | 0.320 | 46 | 49 | 0.002 |
|
T2–4 | 40 | 10 | 30 |
| 8 | 32 |
|
| Tumor grade |
|
|
|
|
|
|
|
|
Low | 63 | 19 | 44 | 0.823 | 33 | 30 | 0.006 |
|
High | 72 | 23 | 49 |
| 21 | 51 |
|
| Recurrence |
|
|
|
|
|
|
|
|
Absent | 105 | 32 | 73 | 0.766 | 41 | 64 | 0.673 |
|
Present | 30 | 10 | 20 |
| 13 | 17 |
|
CD34/periodic acid Schiff (PAS) double
staining
CD34/PAS double staining was performed in order to
detect VM formation in paraffin-embedded sections. First, IHC
staining for CD34, using a mouse monoclonal antibody (1:50; cat.
no. ZM-0046; Zhongshan Goldenbridge, Beijing, China), was performed
to detect endothelial cells. Then, the sections were washed in
running water for 1 min and incubated with PAS for 30 min to detect
the basement membrane of tubular structures. The typical
characteristic of VM is a tubular structure containing red blood
cells, indicated by PAS staining of the basement membrane,
surrounded by tumor cells with negative CD34 staining. The number
of red blood cells in the tubular structure is ≥1. All the steps
were performed as previously described (22).
Cell culture and three-dimensional
(3-D) culture
The immortalized human bladder epithelium cell line
SV-HUC-1 and the bladder transitional cancer cell line J82 were
purchased from the American Type Culture Collection (ATCC;
Manassas, VA, USA). The human bladder transitional cancer cell line
UM-UC-3 was donated by Professor Chunxiao Liu (Urology Department,
Zhujiang Hospital of Southern Medical University). The base media
for SV-HUC-1, J82 and UM-UC-3 were F-12K, EMEN and 1640 medium
(Gibco; Thermo Fisher Scientific, Inc., Waltham, MA, USA),
respectively. All the base media were supplemented with a final
concentration of 1% penicillin/streptomycin (Gibco; Thermo Fisher
Scientific, Inc.) and 10% fetal bovine serum (Gibco; Thermo Fisher
Scientific, Inc.). The cells were cultured at 37°C and 5%
CO2.
3-D culture was used for the detection of VM
formation in vitro. Firstly, 96-well culture plates were
coated with 50 µl/well growth factor-reduced Matrigel (BD
Biosciences, Franklin Lakes, NJ, USA). Then, the plates were
incubated at 37°C for 2 h. Subsequently, the cells, suspended in
complete medium at 3×105 cells/ml, were plated onto the
surface of the Matrigel at 100 µl/well and incubated at 37°C for 4
h. The number of tube-like structures was measured in 3 random
fields. The average number was calculated and statistical analysis
was performed.
Small interfering RNA (siRNA)
transfection
The siRNA was purchased from RiboBio Biology
(Guangzhou, China). The target sequences for ZEB1 were as follows:
si-ZEB1#1, GCATACACCTACTCAACTA; si-ZEB1#2, CGGACGAGAGAGAGAGTTT. A
non-silencing siRNA was used as the negative control. Transfection
was performed using Lipofectamine 2000 Transfection Reagent
(Invitrogen; Thermo Fisher Scientific, Inc.). Then, 5 µl of 20
nmol/µl siRNA was added to each well of 6-well plates, which had
been seeded with cells according to the manufacturer's protocol.
After incubating the cells at 37°C for 48 h, we tested the
efficiency of gene knockdown by reverse transcription-quantitative
polymerase chain reaction (RT-qPCR) assay and western blot
analysis.
RNA purification and RT-qPCR
assay
Total RNA from different cells (SV-HUC-1, UM-UC-3,
J82) was extracted using an E.Z.N.A® HP Total RNA Kit
(Omega Bio-Tek, Norcross, GA, USA). Total RNA (1 µg) was used to
synthesize cDNA with the Revert Aid First Strand cDNA Synthesis Kit
(Thermo Fisher Scientific, Inc.). The fluorescent dye used for the
qPCR assay was SYBR® Premix Ex Taq™ (Takara, Tokyo,
Japan). All of the above experiments were conducted according to
the protocols provided by the kit manufacturers. The primers used
were as follows: ZEB1 forward, 5′-GCACCTGAAGAGGACCAGAG-3′ and ZEB1
reverse, 5′-GTGTAACTGCACAGGGAGCA-3′; GAPDH forward,
5′-GAGTCAACGGATTTGGTCGT-3′ and GAPDH reverse,
5′-TTGATTTTGGAGGGATCTCG-3′. All primers were synthesized by Thermo
Fisher Scientific, Inc. The PCR conditions were 95°C for 30 sec,
then a total of 40 cycles of 95°C for 5 sec, 60°C for 34 sec, then
a final extension at 95°C (15 sec), 60°C (1 min) and 95°C (15 sec).
The relative expression levels were calculated using the 2-Δ∆Cq
method according to the following formula: ΔCq (target gene) = Cq
(target gene) - Cq (control gene).
Western blot analysis
Cell lysates were collected using a total protein
extract kit (KeyGen Biotech, Inc., Nanjing, China) and protein
concentrations were quantified with a Pierce BCA Protein Assay Kit
(Thermo Fisher Scientific, Inc.). Proteins (30 µg/lane) were
resolved by SDS-PAGE (upper gel: 5%, lower gel: 10%) (KeyGen
Biotech, Inc.) and transferred to polyvinylidene difluoride (PVDF)
membranes (Pierce; Thermo Fisher Scientific, Inc.). Then, the
membranes were incubated overnight with primary antibodies (rabbit
antibody to ZEB1, 1:250; cat. no. sc-25388; Santa Cruz
Biotechnology, Inc., Dallas, TX, USA; GAPDH, 1:5,000; cat. no.
AC027; ABclonal Biotech Co., Ltd., Woburn, MA, USA) at 4°C.
Subsequently, the goat anti-rabbit IgG-HRP (1:5,000, cat. no.
BL003A; Biosharp, Anhui, China) was incubated at room temperature
to detect protein bands in the membranes.
Statistical analysis
All data in the present study were evaluated using
SPSS 20.0 software (IBM Corp., Armonk, NY, USA). Each experiment
was performed at least 3 times. The relationships between VM, ZEB1
expression and clinicopathological parameters were analyzed by the
Chi-square (χ2) test or Fisher's exact test. The correlation
between VM and ZEB1 expression was assessed by association
analysis. Student's t-test was performed to compare differences
between groups in cell assays. P<0.05 was considered to indicate
a statistically significant difference for all analyses.
Results
Evaluation of VM and
clinicopathological characteristics in bladder cancer
According to the aforementioned criteria, we
detected VM in 135 bladder cancer cases. As shown in Table I, 42 samples from 135 cases (31.1%) in
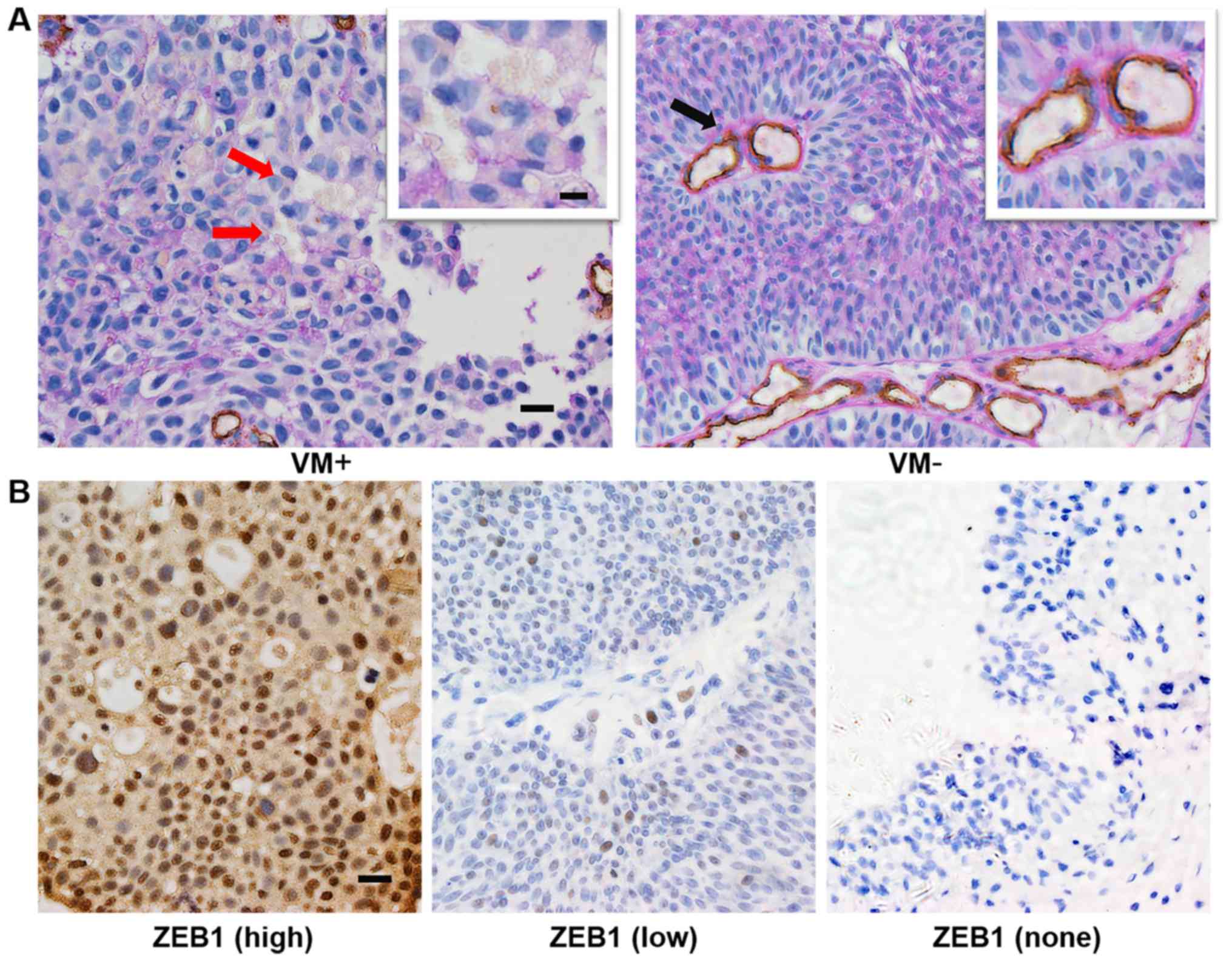
bladder cancer were VM positive. A typical VM structure, which is
positive for PAS in its membrane and negative for the endothelial
cell marker CD34, contains one or more red blood cells (Fig. 1A). In addition, we recorded the
clinicopathological parameters of all patients, including age, sex,
tumor grade, tumor stage and progression. We compared the rate of
VM in different subgroups. However, the data in our study showed no
significant correlations between VM and the clinicopathological
parameters (Table I).
VM is associated with ZEB1
overexpression in bladder cancer
In order to explore the role of ZEB1 in bladder
cancer, we evaluated ZEB1 expression in all samples from patients
(Fig. 1B). As shown in Table II, high expression of ZEB1 (score
>3) was exhibited in 60.0% (81/135) of cases, and 31 of these
cases were VM-positive (38.3%, 31/81). However, for cases with low
expression of ZEB1 (score ≤3), the rate of VM was 20.4% (11/54),
which was lower compared with the high-expression ZEB1 group. The
difference was statistically significant (χ2=4.844,
P<0.05) and VM was positively correlated with overexpression of
ZEB1 (r=0.189, P<0.05). These results indicate that there is a
strong correlation between VM and ZEB1 expression.
 | Table II.Associations between vasculogenic
mimicry and zinc finger E-box binding homeobox 1 expression in
bladder cancer. |
Table II.
Associations between vasculogenic
mimicry and zinc finger E-box binding homeobox 1 expression in
bladder cancer.
|
| ZEB1
expression |
|
|
|
|
|---|
|
|
|
|
|
|
|
|---|
| VM | High (n) | Low (n) | Total (n) | r | χ2 | P-value |
|---|
| Positive | 31 | 11 | 42 | 0.189 | 4.844 | 0.028 |
| Negative | 50 | 43 | 93 |
|
|
|
Aberrant expression of ZEB1 is related
to the stage and grade of bladder cancer
Based on its biological behavior, bladder cancer is
divided into non-muscle-invasive bladder cancer (NMIBC) and
muscle-invasive bladder cancer (MIBC). According to TNM
classification, Ta-T1 tumors are classified
as NMIBC and T2-4 tumors are classified as MIBC. In our
study, we found that MIBC sections showed higher ZEB1 expression
compared with NMIBC sections (80.0%, 32/40 vs. 51.2%, 49/95;
P<0.05) (Table I). In addition,
compared with the low-grade urothelial carcinoma group, ZEB1
expression was higher in those with high-grade urothelial carcinoma
(70.8%, 51/72 vs. 47.6%, 30/63; P<0.05) (Table I). Notably, ZEB1 expression was absent
in all 12 specimens from normal adjacent tissues (Fig. 1B). Overall, these results indicated
that ZEB1 may play an important role in invasive and aggressive
bladder cancer.
VM formation and ZEB1 expression in
bladder transitional cancer cell lines
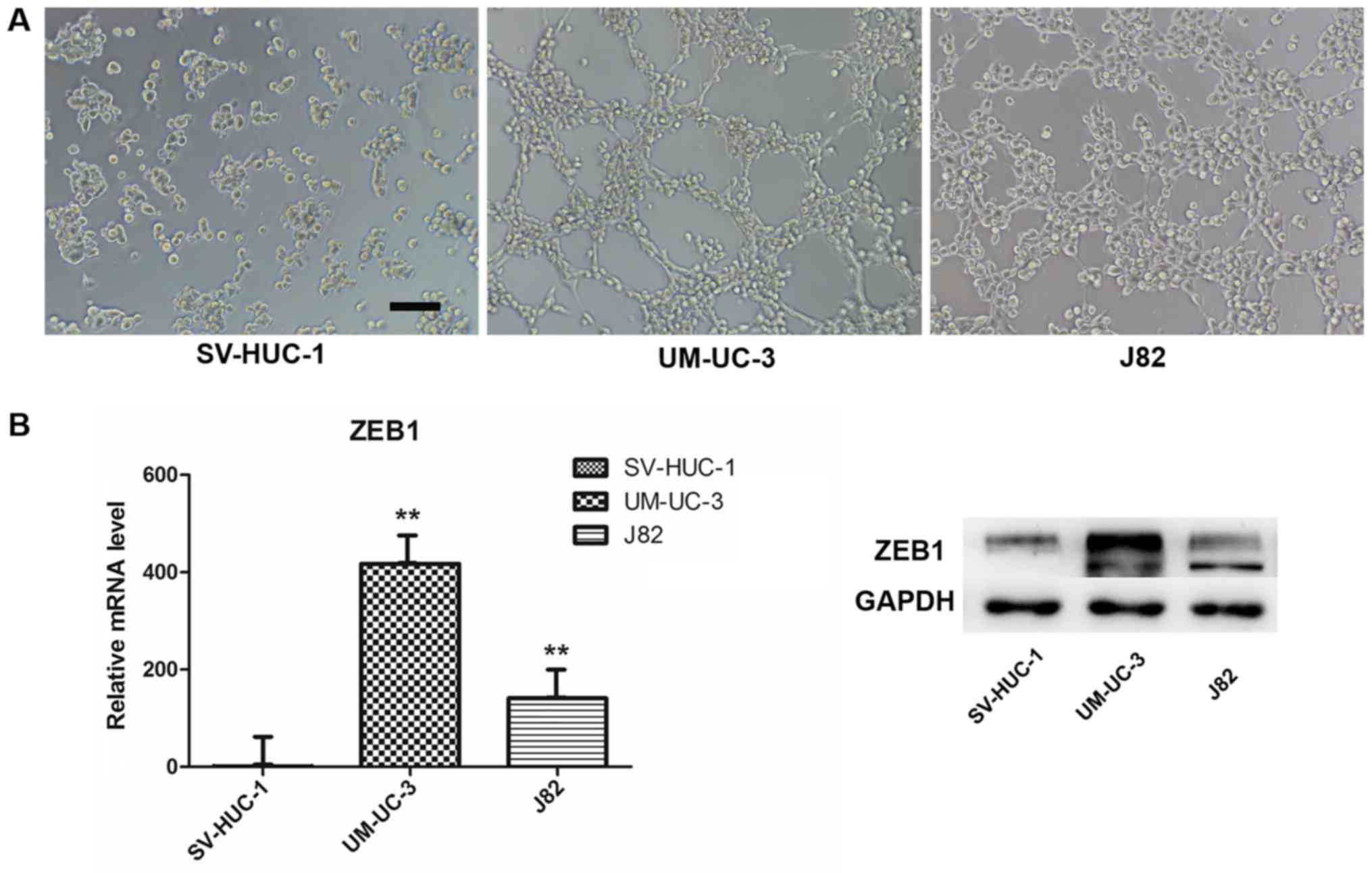
We used a well-established 3-D model to investigate
VM formation in vitro. We chose the SV-HUC-1 (normal
uroepithelium) and UM-UC-3 and J82 (transitional cell carcinoma)
cell lines and evaluated their ability to form vessel-like tubes.
The results indicated that both UM-UC-3 and J82 formed vessel-like
tubes after we cultured the cells on Matrigel for 4 h (Fig. 2A). By contrast, the normal
uroepithelium cells did not form these structures under the same
conditions, or at higher cell density or with longer culture time.
In addition, we compared ZEB1 expression in the three cell lines by
RT-qPCR and western blot assays. Interestingly, both mRNA and
protein expression of ZEB1 in SV-HUC-1 were lower compared with
UM-UC-3 and J82 (Fig. 2B), which
revealed that ZEB1 potentially promotes VM formation in bladder
cancer.
Knockdown of ZEB1 impaired VM
formation in UM-UC-3 and J82 cell lines
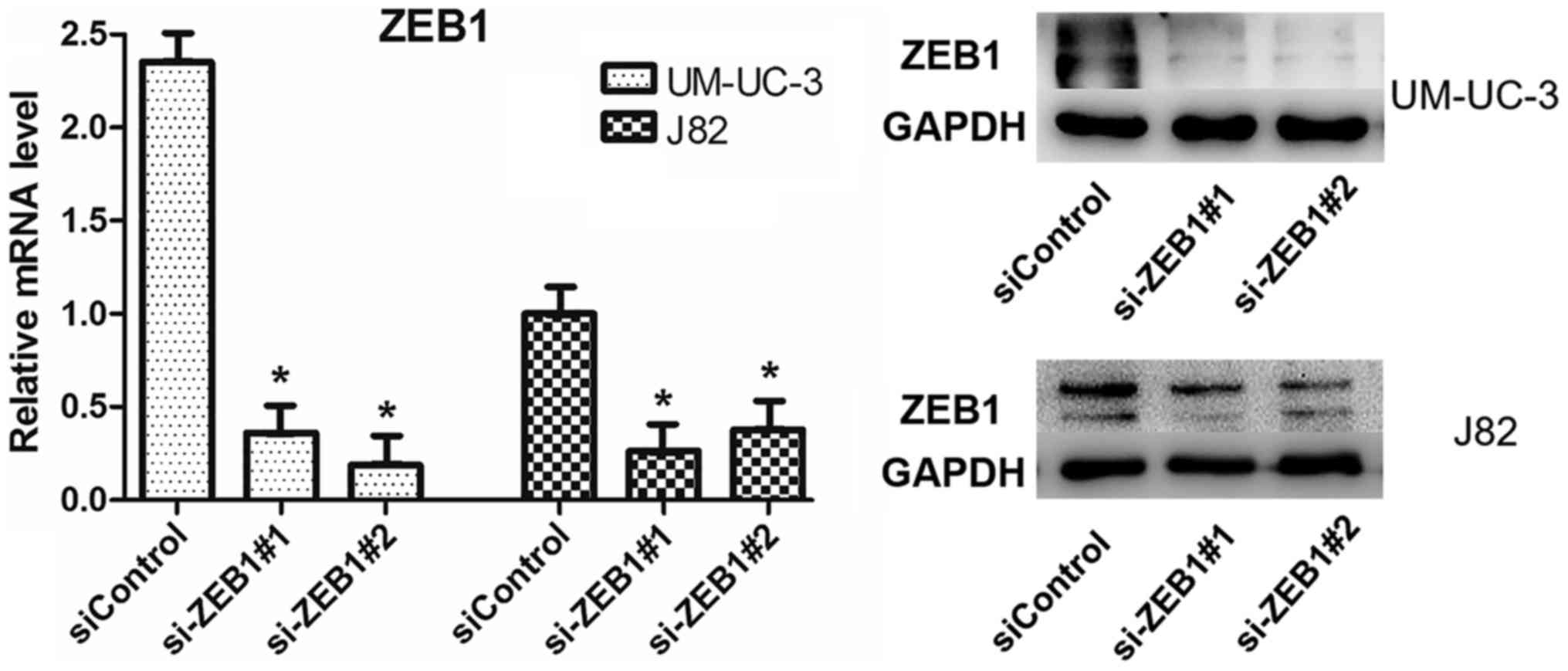
To confirm the potential role of ZEB1 in the
formation of vascular networks in bladder cancer in vitro,
we downregulated ZEB1 expression in UM-UC-3 and J82 cells by
transfecting them with specific siRNA targeting ZEB1. We
investigated the efficiency after knockdown of ZEB1 by RT-qPCR and
western blot assays and ensured that the transfection method had
been effective (Fig. 3). The results
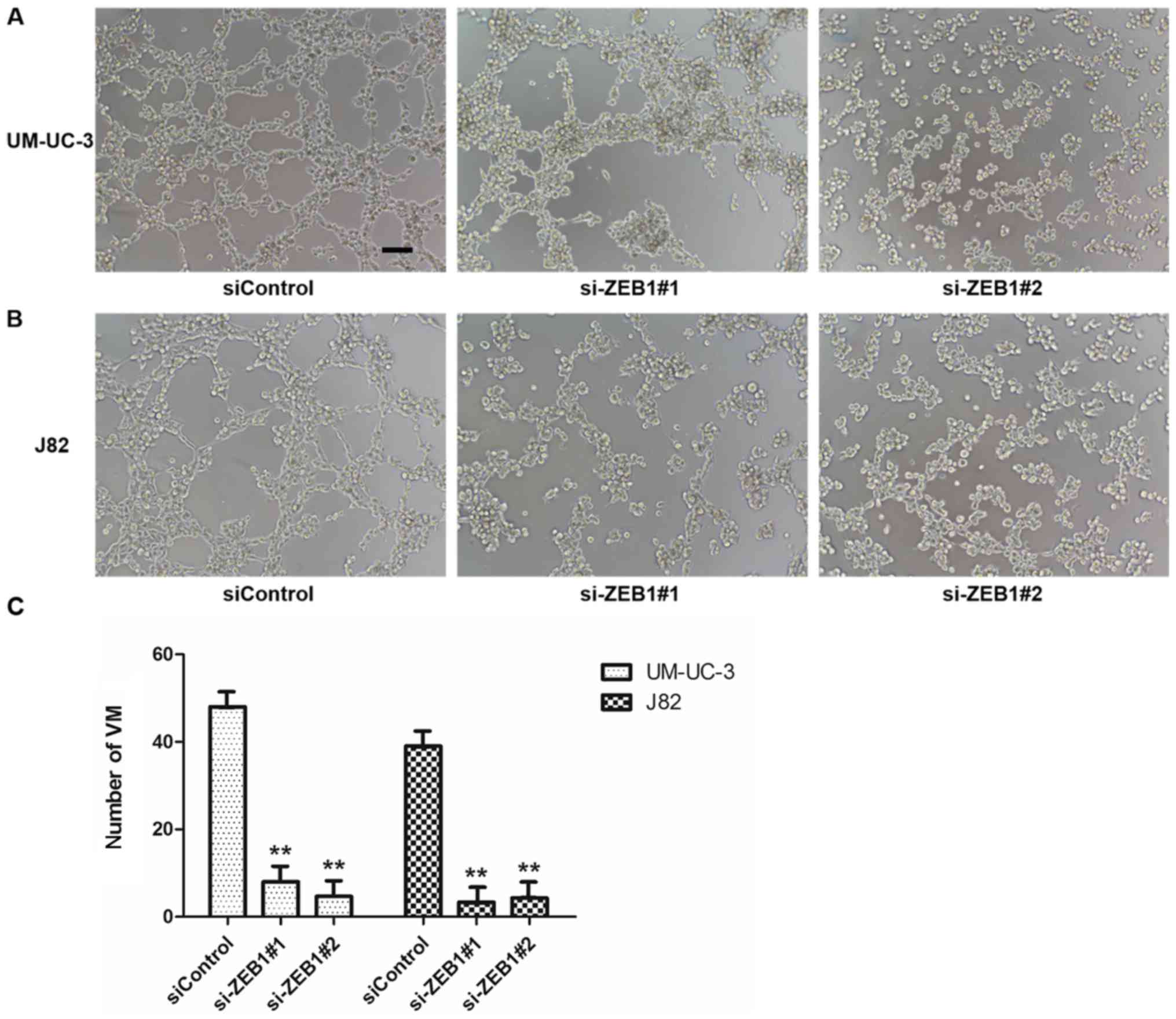
of 3-D culture in treated cells demonstrated that downregulation of
ZEB1 in UM-UC-3 and J82 cell lines inhibited the formation of
tubular structures (Fig. 4). However,
the control groups exhibited lots of tubular structures. These
results demonstrated that ZEB1 is an important regulatory factor
for VM formation in bladder cancer.
Discussion
VM is a microcirculation pattern different from the
traditional blood supply, which plays an important role in the
auxiliary functions of transferring blood and other nutrients. In
addition, VM is found in many solid tumors, including but not
limited to the cancer mentioned above: Melanoma, hepatocellular
carcinoma, colorectal and prostate cancer (4,6,8,10).
Furthermore, there is a strong correlation between VM and malignant
features of cancer, such as advanced stage or grade, poor
differentiation and short overall survival. In our study, VM was
detected in 135 specimens of bladder cancer and its positive rate
was 31.1%, which is similar to a previous study (23). Despite the presence of VM in our
specimens, we did not observe a significant correlation between VM
and clinical parameters such as TNM stage, pathological grade and
recurrence in our study. However, Zhou et al (24) reported that VM was not only closely
associated with pathological grade, stage and recurrence, but also
stimulated metastasis of bladder cancer, which the tumor cells may
transfer to distant locations through VM. The discrepancies between
these studies may be related to differences in the study
populations. For instance, the patients included in the study by
Zhou et al (24) were treated
with radical cystectomy, but our study consisted of a large number
of specimens resected from patients under transurethral resection
of bladder tumor (TURBT). Factors affecting the selection of
operation methods may have led to bias in the two study groups.
Nevertheless, the present study confirms that VM exists in bladder
cancer. Furthermore, we want to explore the molecular mechanism of
VM in bladder cancer since little research has been conducted in
this area.
In a previous study, VM was detected in
paraffin-embedded samples of bladder cancer, but further in
vitro research into its mechanism was not performed. Wang et
al (25) found that human bladder
transitional cancer cell lines J82 and T24 generated VM formation,
and this was inhibited by downregulation of UHRF1 via miR-124.
Likewise, in the current study, we confirmed that bladder
transitional cancer cell lines UM-UC-3 and J82 can generate VM
structures in a 3-D Matrigel culture, but the immortalized human
bladder epithelium cell line SV-HUC-1 did not exhibit this ability,
even when the seeding concentration was higher or the observation
time was longer. Notably, both of the VM-forming cell lines,
UM-UC-3 and J82, showed higher ZEB1 expression compared with
SV-HUC-1 and the phenomenon was verified in IHC staining of
paraffin-embedded samples. In 135 specimens resected from patients
with bladder cancer, the rate of high expression of ZEB1 was 60.0%,
yet a further 12 specimens from normal adjacent tissues were
ZEB1-negative (P<0.05). Our results also found that MIBC
(T2-4) tissue sections showed higher ZEB1 expression
compared with NMIBC (Ta-T1) sections. In
terms of pathological grade, ZEB1 was expressed at a higher level
in the high-grade group compared with the low-grade group. These
findings suggested that ZEB1 may contribute significantly to the
progression of bladder cancer. Furthermore, we demonstrated that VM
presentation in bladder cancer tissues was closely correlated with
ZEB1 overexpression, in accordance with a previous study in
colorectal carcinoma (8). However, it
is unclear whether ZEB1 can regulate VM formation in bladder
transitional cancer cell lines, which arouses us strong
interest.
Recently, many studies have proposed that EMT is
vital for VM formation and tumor progression (9). Some regulatory factors, such as Twist,
Runx2 and ZEB2, play important roles in VM formation by promoting
EMT (6,16,17). As a
crucial EMT-inducer, ZEB1 was increased in colorectal carcinoma
samples and its expression concomitantly occurred with EMT features
in vivo and in vitro. Furthermore, knockdown of ZEB1
inhibited VM formation in HCT116 cells, accompanied by upregulated
epithelial marker E-cadherin and downregulated mesenchymal marker
vimentin expression (8). Similarly,
VM was inhibited in the breast cancer cell line, Mda-MB-231, by
knockdown of ZEB1 (26). To further
clarify the relationship between VM and ZEB1 in bladder cancer, a
3-D culture assay was performed after transfection with a specific
siRNA to decrease ZEB1 expression in bladder transitional cancer
cell lines. Notably, VM formation was inhibited in both UM-UC-3 and
J82 cell lines after reduction of ZEB1. However, we did not observe
the phenomenon by which epithelial and mesenchymal markers in
bladder transitional cancer cell lines go into reverse (data not
shown), which was inconsistent with a previous study (8). We propose that ZEB1 may be an
intermediate step in the VM formation process in bladder cancer,
regulated by some unknown upstream molecules or affecting an
unknown downstream gene, and it may not be directly associated with
epithelial phenotype. Therefore, we could not observe changes in
EMT markers after we inhibited ZEB1 expression in bladder cancer.
In summary, ZEB1 is at least a key factor in VM formation in
bladder cancer, but its detailed mechanism is unclear and worthy of
further exploration.
In conclusion, the present study confirms that ZEB1
is associated with VM in bladder cancer. Moreover, ZEB1 is vital in
the process of VM formation. However, our study has certain
limitations. For instance, it was a retrospective study in a single
center and the patients admitted were only from the last two years,
meaning that there is a lack of long-term survival data. The value
of VM in bladder cancer remains to be elucidated. Furthermore, we
verified that ZEB1 is important for VM formation, but the mechanism
of it has not been investigated thoroughly. Hence, in the future, a
multicenter and prospective study must be undertaken to validate
the relationship between VM and clinical features. In addition, we
will explore the detailed mechanism of VM in bladder cancer and
find downstream genes of ZEB1 to clarify the exact ZEB1-regulation
pathway in VM formation.
Acknowledgements
The present study was supported by grants from the
National Natural Science Foundation of China (no. 81001146) and the
Science and Technology Planning Project of Guangdong Province,
China (nos. 2012B031800033 and 2017A020215168).
References
|
1
|
Chen W, Zheng R, Baade PD, Zhang S, Zeng
H, Bray F, Jemal A, Yu XQ and He J: Cancer statistics in China,
2015. CA Cancer J Clin. 66:115–132. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2018. CA Cancer J Clin. 68:7–30. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Mayr R, Fritsche HM and Pycha A and Pycha
A: Radical cystectomy and the implications of comorbidity. Expert
Rev Anticancer Ther. 14:289–295. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Maniotis AJ, Folberg R, Hess A, Seftor EA,
Gardner LM, Pe'er J, Trent JM, Meltzer PS and Hendrix MJ: Vascular
channel formation by human melanoma cells in vivo and in vitro:
Vasculogenic mimicry. Am J Pathol. 155:739–752. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Ahmadi SA, Moinfar M, Gohari Moghaddam K
and Bahadori M: Practical application of angiogenesis and
vasculogenic mimicry in prostatic adenocarcinoma. Arch Iran Med.
13:498–503. 2010.PubMed/NCBI
|
|
6
|
Ma JL, Han SX, Zhu Q, Zhao J, Zhang D,
Wang L and Lv Y: Role of twist in vasculogenic mimicry formation in
hypoxic hepatocellular carcinoma cells in vitro. Biochem Biophys
Res Commun. 408:686–691. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Karroum A, Mirshahi P, Faussat AM,
Therwath A, Mirshahi M and Hatmi M: Tubular network formation by
adriamycin-resistant MCF-7 breast cancer cells is closely linked to
MMP-9 and VEGFR-2/VEGFR-3 over-expressions. Eur J Pharmacol.
685:1–7. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Liu Z, Sun B, Qi L, Li H, Gao J and Leng
X: Zinc finger E-box binding homeobox 1 promotes vasculogenic
mimicry in colorectal cancer through induction of
epithelial-to-mesenchymal transition. Cancer Sci. 103:813–820.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Liu Q, Qiao L, Liang N, Xie J and Zhang J,
Deng G, Luo H and Zhang J: The relationship between vasculogenic
mimicry and epithelial-mesenchymal transitions. J Cell Mol Med.
20:1761–1769. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Wang H, Lin H, Pan J, Mo C, Zhang F, Huang
B, Wang Z, Chen X, Zhuang J, Wang D and Qiu S: Vasculogenic mimicry
in prostate cancer: The roles of EphA2 and PI3K. J Cancer.
7:1114–1124. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zhang P, Sun Y and Ma L: ZEB1: At the
crossroads of epithelial-mesenchymal transition, metastasis and
therapy resistance. Cell Cycle. 14:481–487. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Chen H, Lu W, Huang C, Ding K, Xia D, Wu Y
and Cai M: Prognostic significance of ZEB1 and ZEB2 in digestive
cancers: A cohort-based analysis and secondary analysis.
Oncotarget. 8:31435–31448. 2017.PubMed/NCBI
|
|
13
|
Hanrahan K, O'Neill A, Prencipe M, Bugler
J, Murphy L, Fabre A, Puhr M, Culig Z, Murphy K and Watson RW: The
role of epithelial-mesenchymal transition drivers ZEB1 and ZEB2 in
mediating docetaxel-resistant prostate cancer. Mol Oncol.
11:251–265. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Larsen JE, Nathan V, Osborne JK, Farrow
RK, Deb D, Sullivan JP, Dospoy PD, Augustyn A, Hight SK, Sato M, et
al: ZEB1 drives epithelial-to-mesenchymal transition in lung
cancer. J Clin Invest. 126:3219–3235. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Wang W, Lin P, Sun B, Zhang S, Cai W, Han
C, Li L, Lu H and Zhao X: Epithelial-mesenchymal transition
regulated by EphA2 contributes to vasculogenic mimicry formation of
head and neck squamous cell carcinoma. Biomed Res Int.
2014:8039142014.PubMed/NCBI
|
|
16
|
Cao Z, Sun B, Zhao X, Zhang Y, Gu Q, Liang
X, Dong X and Zhao N: The expression and functional significance of
Runx2 in hepatocellular carcinoma: Its role in vasculogenic mimicry
and epithelial-mesenchymal transition. Int J Mol Sci. 18:pii:
E5002017. View Article : Google Scholar
|
|
17
|
Yang Z, Sun B, Li Y, Zhao X, Zhao X, Gu Q,
An J, Dong X, Liu F and Wang Y: ZEB2 promotes vasculogenic mimicry
by TGF-β1 induced epithelial-to-mesenchymal transition in
hepatocellular carcinoma. Exp Mol Pathol. 98:352–359. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wu K, Fan J, Zhang L, Ning Z, Zeng J, Zhou
J, Li L, Chen Y, Zhang T, Wang X, et al: PI3K/Akt to
GSK3β/β-catenin signaling cascade coordinates cell colonization for
bladder cancer bone metastasis through regulating ZEB1
transcription. Cell Signal. 24:2273–2282. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Mahdavinezhad A, Yadegarazari R,
Mousavi-Bahar SH, Poorolajal J, Jafari M, Amirzargar MA, Effatpanah
H and Saidijam M: Evaluation of zinc finger E-box binding homeobox
1 and transforming growth factor-beta2 expression in bladder cancer
tissue in comparison with healthy adjacent tissue. Investig Clin
Urol. 58:140–145. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kenney PA, Wszolek MF, Rieger-Christ KM,
Neto BS, Gould JJ, Harty NJ, Mosquera JM, Zeheb R, Loda M, Darling
DS, et al: Novel ZEB1 expression in bladder tumorigenesis. BJU Int.
107:656–663. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Liu B, Miyake H, Nishikawa M and Fujisawa
M: Expression profile of epithelial-mesenchymal transition markers
in non-muscle-invasive urothelial carcinoma of the bladder:
Correlation with intravesical recurrence following transurethral
resection. Urol Oncol. 33:110.e11–8. 2015. View Article : Google Scholar
|
|
22
|
Xu Y, Li Q, Li XY, Yang QY, Xu WW and Liu
GL: Short-term anti-vascular endothelial growth factor treatment
elicits vasculogenic mimicry formation of tumors to accelerate
metastasis. J Exp Clin Cancer Res. 31:162012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Yu L, Wu S, Zhou L, Song W and Wang D:
Expressions of CD133 and CD82/KAI1 in bladder urothelial carcinoma
and their correlation with vasculogenic mimicry. Nan Fang Yi Ke Da
Xue Xue Bao. 33:1336–1340. 2013.(In Chinese). PubMed/NCBI
|
|
24
|
Zhou L, Chang Y, Xu L, Hoang ST, Liu Z, Fu
Q, Lin Z and Xu J: Prognostic value of vascular mimicry in patients
with urothelial carcinoma of the bladder after radical cystectomy.
Oncotarget. 7:76214–76223. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Wang X, Wu Q, Xu B, Wang P, Fan W, Cai Y,
Gu X and Meng F: MiR-124 exerts tumor suppressive functions on the
cell proliferation, motility and angiogenesis of bladder cancer by
fine-tuning UHRF1. FEBS J. 282:4376–4388. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
LI H, Song S, Xu Y, Zhao J and Liu H:
Knockdown of ZEB1 suppresses the formation of vasculogenic mimicry
in breast cancer cell line MDA-MB-231 through downregulation of
Flk-1. Minerva Med. 108:191–193. 2017.PubMed/NCBI
|