Introduction
Lung cancer is one of the leading causes of
cancer-associated mortality, with a 5-year-survival rate of 17%
worldwide in 2011 (1). Two major
types of lung cancer have been identified: Small-cell lung cancer
(~15%) and non-small-cell lung cancer (~85%), the latter of which
also contains three major histological subtypes: Adenocarcinoma,
squamous cell carcinoma and large cell carcinoma (2). Despite the differences between the
subtypes of lung cancer, the low survival rate of patients
suffering from lung cancer is primarily a result of delayed
diagnosis and late detection, resulting in limited treatment
options in the late stages of disease (3,4).
Therefore, it may be beneficial to identify novel biomarkers to
allow for the diagnosis of lung cancer at an early stage.
Long non-coding RNAs (lncRNAs) are a class of RNA
molecules that are >200 nucleotides in length, do not possess an
open reading frame and do not encode proteins (5). The majority of well-characterized
lncRNAs are RNA polymerase II-transcribed, capped and
polyadenylated, containing exon-exon splice junctions similar to
mRNAs (6,7). It has been demonstrated that lncRNAs
serve functions in gene regulation in various biological conditions
with distinct underlying molecular mechanisms including the
recruitment of transcriptional factors and direct interaction with
DNAs or other RNAs (8,9). It has also been reported that lncRNAs
have functions in a variety of tumors, including lung cancer. For
example, LINC00313 may be used as a diagnostic biomarker of early
stage lung adenocarcinoma (10), and
AK126698, a newly discovered lncRNA, was demonstrated to confer
cisplatin resistance by targeting the Wnt pathway (11). Therefore, the objective of the present
study was to identify novel lncRNAs that serve functions in the
tumorigenesis of lung cancer.
Zinc finger E-box-binding homeobox 1 (ZEB1) is a
transcriptional factor that serves important functions in the
process of epithelial-mesenchymal-transition, which is associated
with tumorigenesis. It was demonstrated that aberrant expression of
ZEB1 was associated with aggressive disease, low differentiation,
metastases and poor prognosis in clinical patients with multiple
types of cancer (12,13). Zinc finger E-box-binding homeobox 2
antisense RNA 1 (ZEB2-AS1) is a non-coding oncogene identified in
human hepatocellular carcinoma (HCC) (14). Li et al (15) demonstrated that the relative
transcript level of ZEB2-AS1 was upregulated in HCC in vivo
and in vitro and functioned as a prognostic factor for HCC
pathogenesis.
The present study aimed to investigate the
expression of ZEB2-AS1 in human lung cancer in clinical patients
and in cultured lung cancer cells. The detailed function of
ZEB2-AS1 in cell proliferation and cell apoptosis was also
investigated. The results of the present study indicated that
ZEB2-AS1 may function as a prognostic biomarker for lung cancer and
may aid the diagnosis and treatment of patients with lung
cancer.
Materials and methods
Human tissues
The present study was approved by an Institutional
Review Board at the General Department Beijing Chest Hospital,
Capital Medical University (Beijing, China). A total of 100 lung
cancer tissues and their adjacent non-cancerous tissues were
collected from patients (67 males and 33 females; mean age, 62; age
range, 48–79) who underwent surgical resection at Department of
General Surgery. The tissues were snap-frozen in liquid nitrogen
once dissected from the patients and used for subsequent RT-qPCR
analysis. The patients selected had not received any chemotherapies
or radiotherapies prior to surgical resection. All patients
provided informed consent.
Cell culture and transfection
The normal human lung cell line MRC-5 was purchased
from American Type Culture Collection (ATCC; Manassas, VA, USA). A
total of 5 lung cancer cell lines; H-125, A549, 95D, NCI-H292 and
H1975 were obtained from Shanghai Cell Bank of the Chinese Academy
of Sciences (Shanghai, China). All cells were cultured in
Dulbecco's modified Eagle's medium (DMEM) supplemented with 10%
fetal bovine serum (both from Gibco; Thermo Fisher Scientific,
Inc., Waltham, MA, USA). Cells were maintained in an incubator
containing 5% CO2 at 37°C. The ZEB2-AS1 expression
plasmid was constructed with the pcDNA 3.0 vector (Addgene, Inc.,
Cambridge, MA, USA). The specific small interfering RNA (siRNA)
against ZEB2-AS1 was designed and synthesized by Invitrogen; Thermo
Fisher Scientific, Inc., with the sequence,
5′-CAAAGGACACCTTTGGTTACCTGAA-3′. When A549, NCI-H929, H-125 and
H1975 cells grew to a confluence of 80%, the transfection was
conducted using Lipofectamine® 2000 (Invitrogen; Thermo
Fisher Scientific, Inc.) according to the manufacturer's
instructions. Following 6 h of transfection, cell medium was
replaced and 48 h later, cells were harvested.
RNA extraction and reverse
transcription-quantitative polymerase chain reaction (RT-qPCR)
Total RNAs were extracted from fresh frozen samples
and cells with TRIzol Reagent (Thermo Fisher Scientific, Inc.) as
per the manufacturer's instructions. A total of 1 µg RNA was
reverse transcribed into cDNA with First Strand cDNA Synthesis kit
(Takara Biotechnology Co., Ltd., Dalian, China) with the following
protocol: 37°C for 15 min and 85°C for 5 sec. The relative
expression of lncRNA ZEB2-AS1 to GAPDH control transcripts was
determined using qPCR as per the ABI 7900 Fast Real-Time PCR system
(Thermo Fisher Scientific, Inc.). The PCR conditions included: An
initial denaturation step of 94°C for 2 min, followed by 30 cycles
of 95°C for 30 sec, 59°C for 30 sec, 72°C for 2 min and a final
elongation step at 72°C for 10 min. The PCR reaction was normalized
to the GAPDH reference gene. The RT-qPCR amplification was
performed in triplicate. The relative level of ZEB2-AS1 transcript
was determined using the 2−ΔΔCq method (16). The primer sequences were as follows:
ZEB2-AS1 forward, 5′-ATGAAGAAGCCGCGAAGTGT-3′ and reverse,
5′-CACACCCTAATACACATGCCCT-3′; GAPDH forward,
5′-ACCACAGTCCATGCCATCAC-3′ and reverse,
5′-TCCACCCTGTTGCTGTA-3′.
Colony formation assay
A total of A549 (5×105/ml), NCI-H292
(5×105/ml), H-125 and 95D cells (5×105/ml) in
6-well plates were treated with siRNA targeted at ZEB2-AS1
(siZEB2-AS1) or ZEB2-AS1 expressing plasmid and 24 h after
treatment, were seeded into 12-well plates (100 cells/well) in
triplicate. Following incubation for 10 days at 37°C, the colonies
were fixed with pre-iced methanol and stained with crystal violet
(1%) at room temperature for 10 min. Colonies were counted using
light microscopy (magnification, ×200) and colonies that contained
>50 cells were designated as survivors. The following formula
was used to calculate the rate of colony formation: Colony
formation rate = (number of colonies/number of seeded cells) ×
100.
Cell proliferation assay
An MTT cell growth kit (Promega Corporation,
Madison, WI, USA) was used to measure cell proliferative abilities
according to the manufacturer's protocol. Briefly, A549, NCI-H292,
H-125 and H1975 cells were seeded into 96-well plates at an initial
concentration of 5×103 cells/well in DMEM supplemented
with 10% FBS. Each experimental group of cells was then spread in
sextuplicate and the culture medium was replaced every other day.
Cell proliferation rate was assessed for 5 consecutive days. At
each time-point (1, 2, 3, 4 and 5 days' post-transfection),
formazan crystals were dissolved in dimethyl sulfoxide, and the
cell proliferation rate was detected using a microplate reader at a
wavelength of 490 nm. The absorbance of control cells at day 1 was
designated as 1. Other absorbance values were normalized to the
control cells at day 1.
Western blot analysis
Cells were seeded into a six-well plate 24 h prior
to transfection. A549 and NCI-H292 cells were treated with specific
siZEB2-AS1. H-125 and H1975 cells were stimulated with ZEB2-AS1
expression plasmid. Total proteins were collected using NP40 lysis
buffer and quantified using a bicinchonic acid assay. An equal
quantity of protein from each sample (50 µg/lane) was subjected to
12% SDS-PAGE and electroblotted onto polyvinylidene fluoride
membranes. Subsequently, the membrane was blocked with TBS/0.1%
Tween-20, supplemented with 5% skimmed milk for 1 h at room
temperature and then incubated with primary antibodies at 4°C
overnight. Secondary antibodies horseradish peroxidase-conjugated
goat anti-mouse IgG (1:5,000; cat. no. ab6717; Abcam, Cambridge,
UK) were incubated with the membrane for 1 h at room temperature.
Next, proteins were detected using an enhanced chemiluminescence
method (EMD Millipore, Billerica, MA, USA). The immunoreactive
bands were quantified by the densitometry with ImageJ software
(v2.0; National Institutes of Health, Bethesda, MD, USA) when
necessary. Primary antibodies against caspase-9 (sc-7885; 1:1,000),
caspase-3 (sc271759; 1:1,000), B-cell lymphoma-2 (Bcl-2; sc-578;
1:1,000), GAPDH (sc-47724; 1:1,000) and secondary antibodies
(sc-2004; sc-2005; 1:2,000) were all purchased from Santa Cruz
Biotechnology, Inc. (Dallas, TX, USA). Primary antibody against
cytoplasmic Bcl-associated X protein (Bax; ab32503; 1:1,000) was
purchased from Abcam.
Relative activities of caspases
The activities of caspase-3, −8 and −9 were
determined using caspase-3 activity kits, caspase-8 activity kits,
caspase-9 activity kits, respectively (Beyotime Institute of
Biotechnology, Haimen, China), according to the manufacturers'
instructions. Briefly, A549, NCI-H292, H-125 and H1975 cells were
transfected with ZEB2-AS1 plasmid or siRNA 48 h prior to the
experiment. Subsequently, cell lysates were collected from each
group of cells. An equal amount of 10 µl (50 µg) proteins from cell
lysates were added into 96-well plates and mixed with an aliquot of
80 µl reaction buffer supplemented with caspase substrate (2 mM).
Following a 4-h incubation at 37°C, caspase activities were
determined using a microplate reader at an absorbance of 450
nm.
Statistical analysis
All data are presented as the mean ± standard
deviation. Each experiment was repeated in triplicate. GraphPad
Prism version 5.0 (GraphPad Software, Inc., La Jolla, CA, USA)
software was used for statistical analysis. Statistical evaluation
was performed using Student's t-test or one-way analysis of
variance followed by the Student-Newman-Keuls post hoc test.
P<0.05 was considered to indicate a statistically
significant difference.
Results
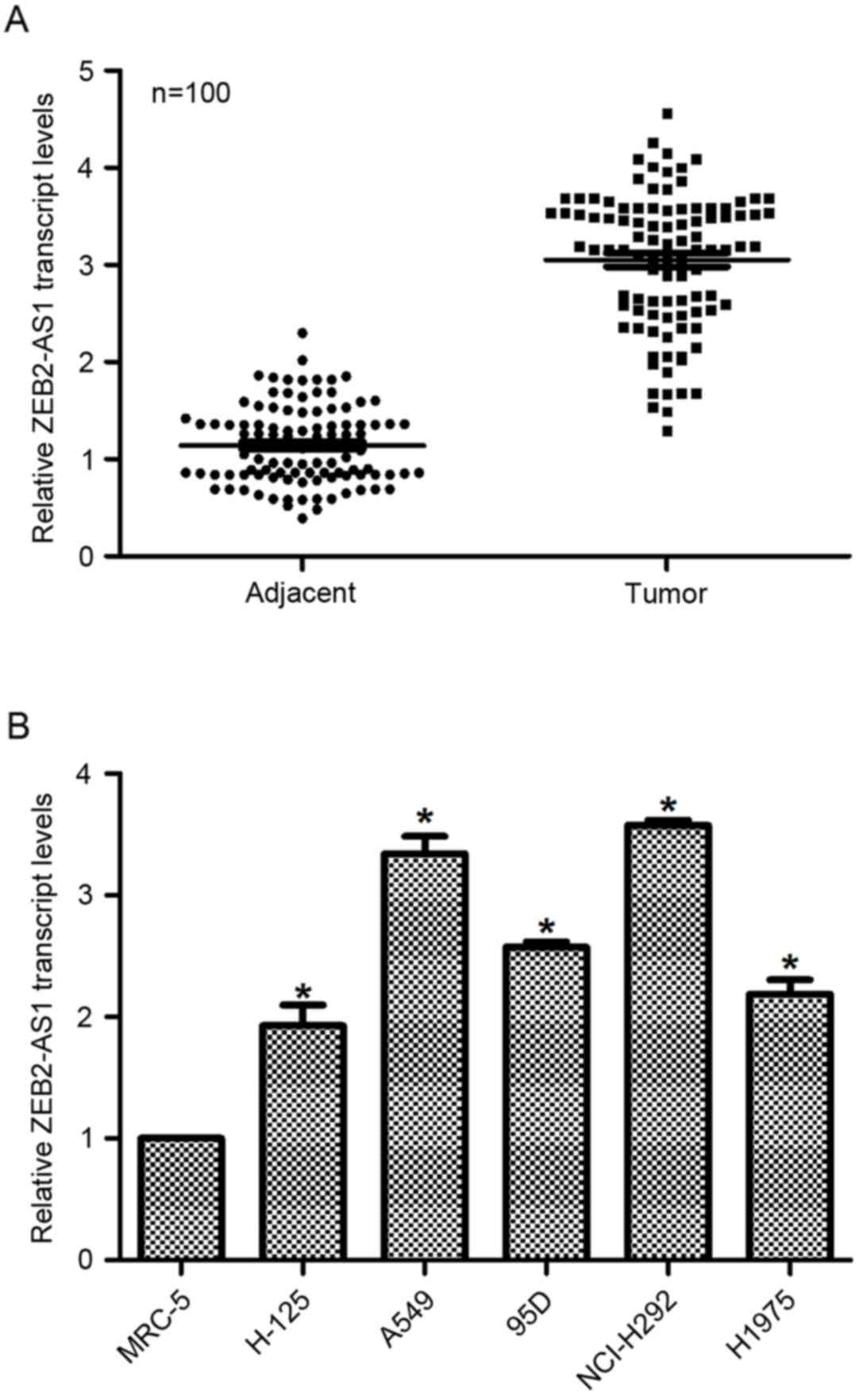
Expression of lncRNA ZEB2-AS1 is
upregulated in human lung cancer in vivo and in vitro
First, the relative transcript level of lncRNA
ZEB2-AS1 in human lung cancer was examined in vivo and in
vitro. To this end, a total of 100 patients with lung cancer
were included in the present study, and their tumor tissues as well
as their adjacent non-cancerous tissues were dissected and
collected for the subsequent RT-qPCR analysis. As presented in
Fig. 1A, the relative transcript
level of ZEB2-AS1 in tumor tissues was significantly increased
(3-fold) compared with their adjacent non-cancerous counterparts. A
total of 5 lung cancer cell lines and a normal human lung cell line
tissue were also assessed by RT-qPCR to detect the expression of
ZEB2-AS1. As presented in Fig. 1B,
the relative transcript levels of ZEB2-AS1 in lung cancer cells
were significantly upregulated, with A549 and NCI-H292 exhibiting
the highest ZEB2-AS1 expression (up to 3.4-fold and 3.5-fold,
respectively), whereas the expression levels of ZEB2-AS1 in H-125,
95D and H1975 were lower than those in A549 and NCI-H292 cells;
however, these levels were significantly increased compared with
non-tumor tissue. Thus, A549 and NCI-H292 cells were selected for
knockdown assays and H-125 and H1975 cells were selected for
overexpression analysis. These results identified that the level of
ZEB2-AS1 expression was upregulated in human lung cancer.
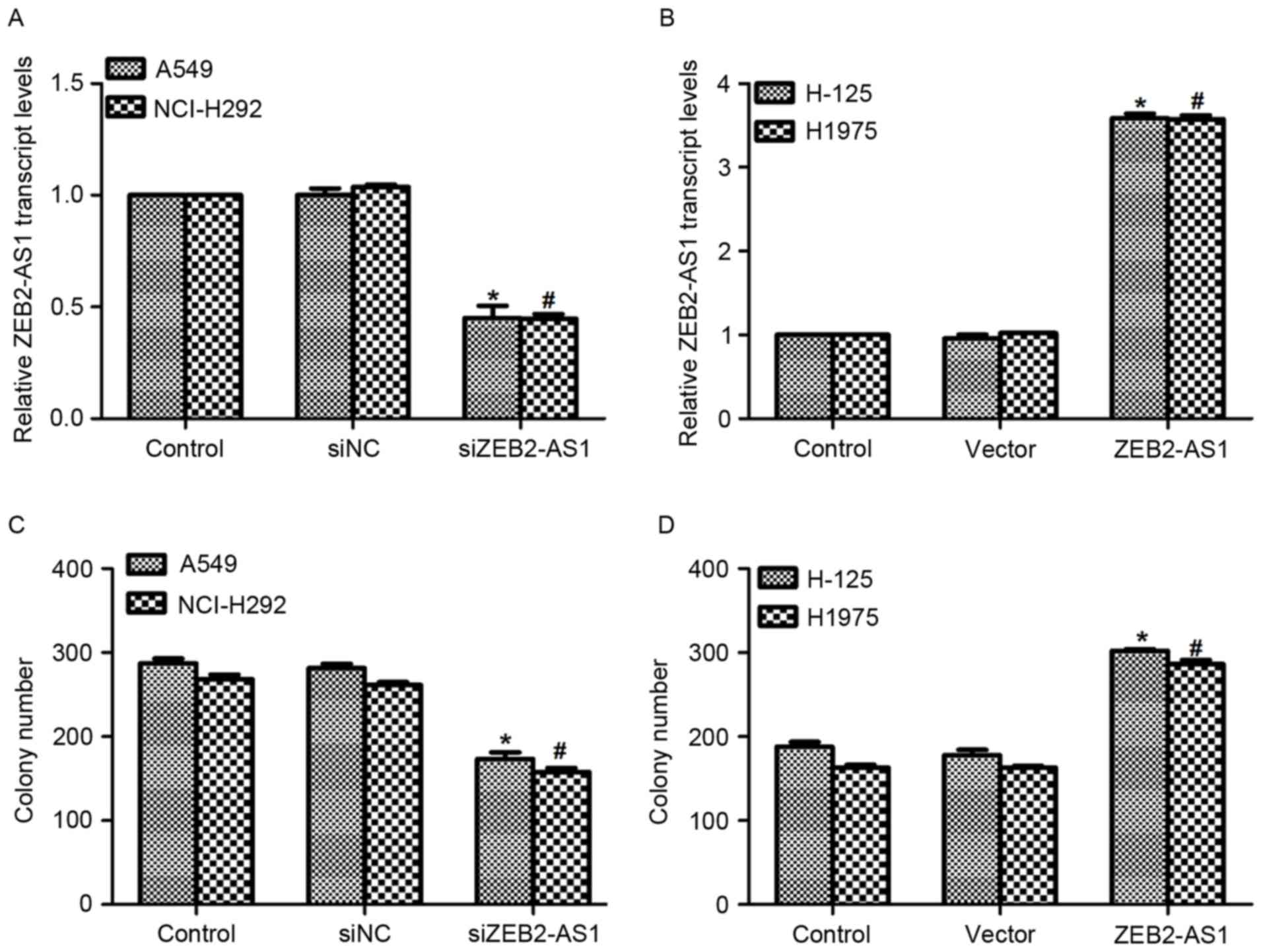
Expression of lncRNA ZEB2-AS1 is
associated with cell proliferation in human lung cancer
The functions of ZEB2-AS1 in human lung cancer were
further evaluated. A specific siRNA against ZEB2-AS1 and an
expression plasmid containing ZEB2-AS1 were constructed and
transfected into the corresponding cells. As presented in Fig. 2A, when cells were transfected with
siZEB2-AS1 for 48 h, the level of ZEB2-AS1 expression was decreased
by 55% in A549 cells and 56% in NCI-H292 cells, respectively.
Treatment with ZEB2-AS1 expressing plasmid upregulated the
transcript level of ZEB2-AS1 3.45-fold inH-125 cells and 3.40-fold
in H1975 cells (Fig. 2B).
Subsequently, a colony formation assay was performed in all four
cell lines. Transfection of A549 cells with siZEB2-AS1 inhibited
the colony formation ability of the cells, as evidenced by the
significantly decreased colony number compared with controls
(Fig. 2C). Approximately 250 colonies
were observed in NCI-H292 cells; however, only 152 colonies were
counted when siZEB2-AS1 was transfected into NCI-H292 cells
(Fig. 2C). Conversely, transfection
with the ZEB2-AS1-expressing plasmid promoted colony formation in
H-125 and H1975 cells (Fig. 2D).
These results demonstrate that overexpression of ZEB2-AS1 in human
lung cancer cells increased their colony formation ability.
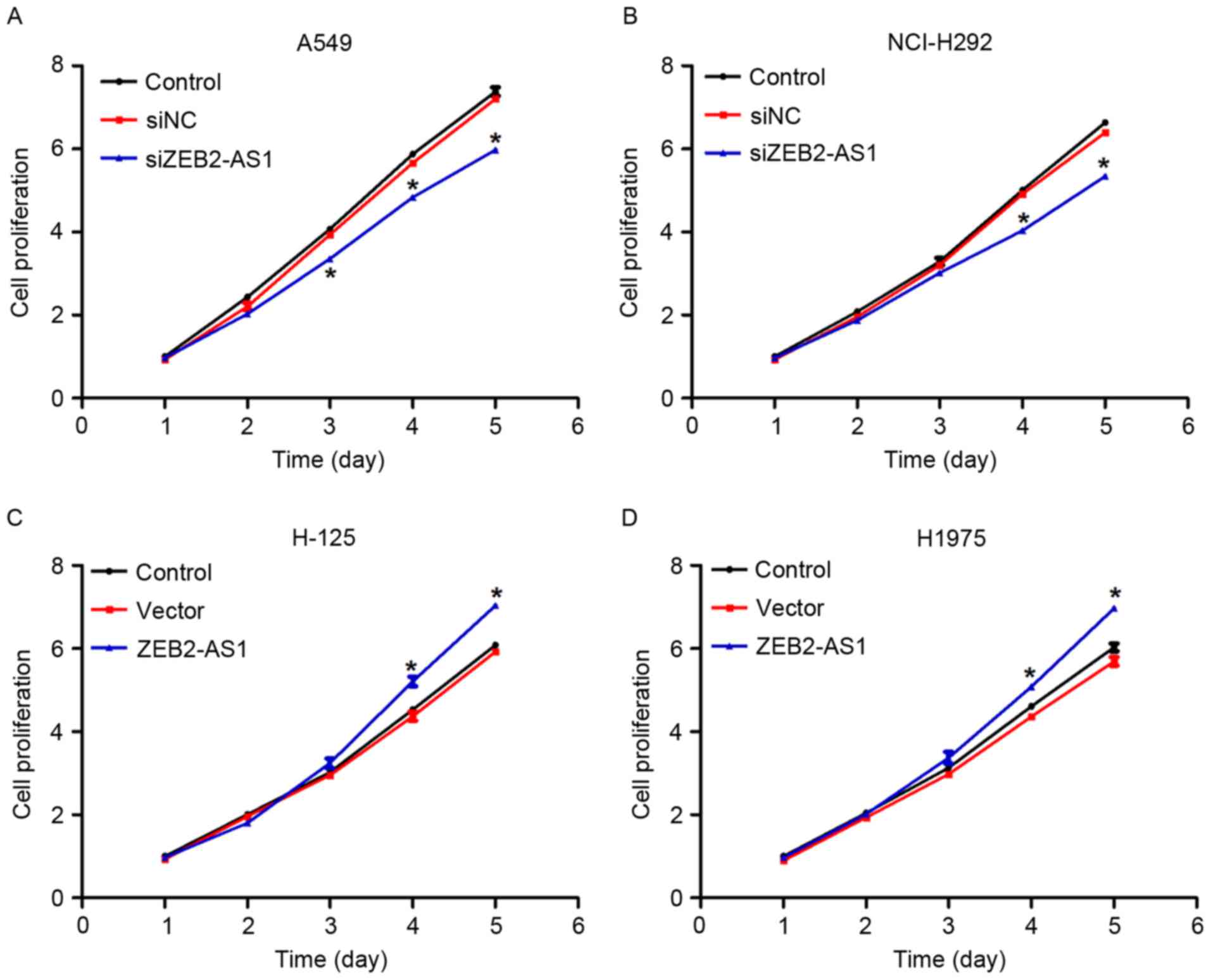
Subsequently, a cell proliferation assay was
performed in lung cancer cells. There were no notable differences
in the first 2 days between the control groups and the
overexpression or siZEB2-AS1-treated group in all four lung cancer
cell lines (Fig. 3). However, the
cell proliferative rate was decreased by 18, 20 and 22% in
siZEB2-AS1-treated A549 cells on days 3, 4 and 5, respectively
(Fig. 3A). A similar phenomenon was
also identified in NCI-H292 cells transfected with siZEB2-AS1
(Fig. 3B). Similarly, when cells were
transfected with ZEB2-AS1-expressing plasmids, cell proliferation
was increased by 14 and 10% on the fourth day in H-125 and H1975
cells, respectively, and a further increase was observed on day 5
in the two cell lines (Fig. 3C and
D). Together with Fig. 2, these
data indicated that ZEB2-AS1 promoted cell proliferation in human
lung cancer cell lines in vitro.
Expression of lncRNA ZEB2-AS1 is
associated with cell apoptosis in human lung cancer cells
Since ZEB2-AS1 served a significant function in cell
proliferation, the effect of ZEB2-AS1 on cell apoptosis was
evaluated in the four lung cancer cell lines. Bcl-2, Bax, caspase-3
and −9 may all be considered to be representative of the process of
apoptosis, and so were assessed using western blot analysis. As
presented in Fig. 4A and B, the
expression levels of the four proteins were all markedly increased
in A549 and NCI-H292 cells when they were transfected with
siZEB2-AS1. When H-125 and H1975 cells were treated with ZEB2-AS1
expressing plasmid, the levels of Bcl-2, Bax, caspase-3 and −9
protein expression were all decreased, whereas that of GAPDH
remained unchanged (Fig. 4C and
D).
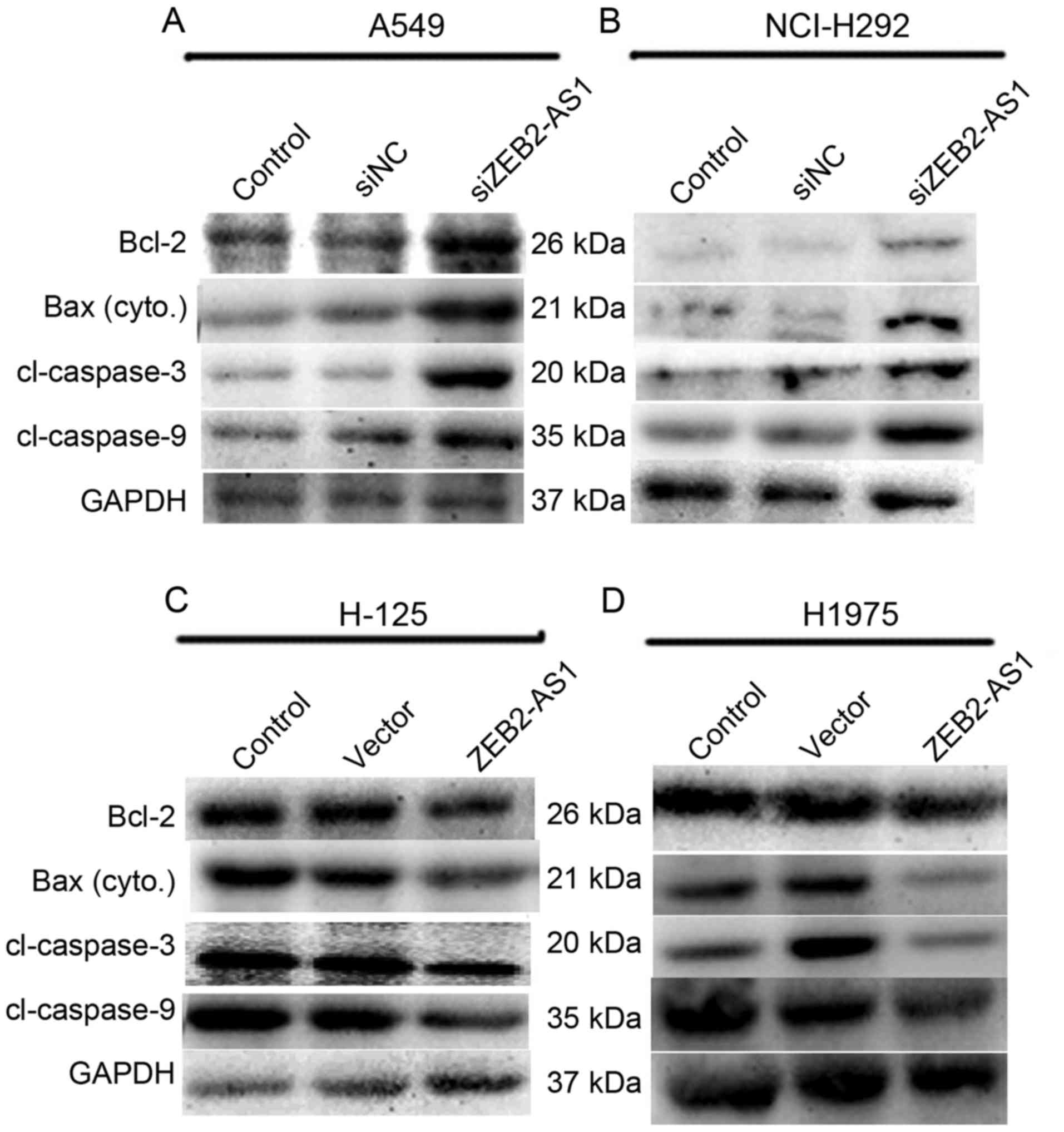 | Figure 4.Long non-coding RNA ZEB2-AS1 promotes
cell apoptosis in human lung cancer cells. (A) A549 cells and (B)
NCI-H292 cells were transfected with siZEB2-AS1 and total protein
was collected for western blot analysis 48 h after treatment. The
expression levels of Bcl-2, cytoplasmic Bax, caspase-3 and −9, with
molecular weights 26, 21, 20 and 35 kDa, respectively, were
investigated. GAPDH was used as an internal control. (C) H-125 and
(D) H1975 cells were treated with ZEB2-AS1 plasmid and the
expression levels of Bcl-2, Bax, caspase-3 and −9 were detected.
ZEB2-AS1, zinc finger E-box-binding homeobox 2 antisense RNA 1;
Bcl-2, B-cell lymphoma 2; Bax, Bcl-2-associated X protein. |
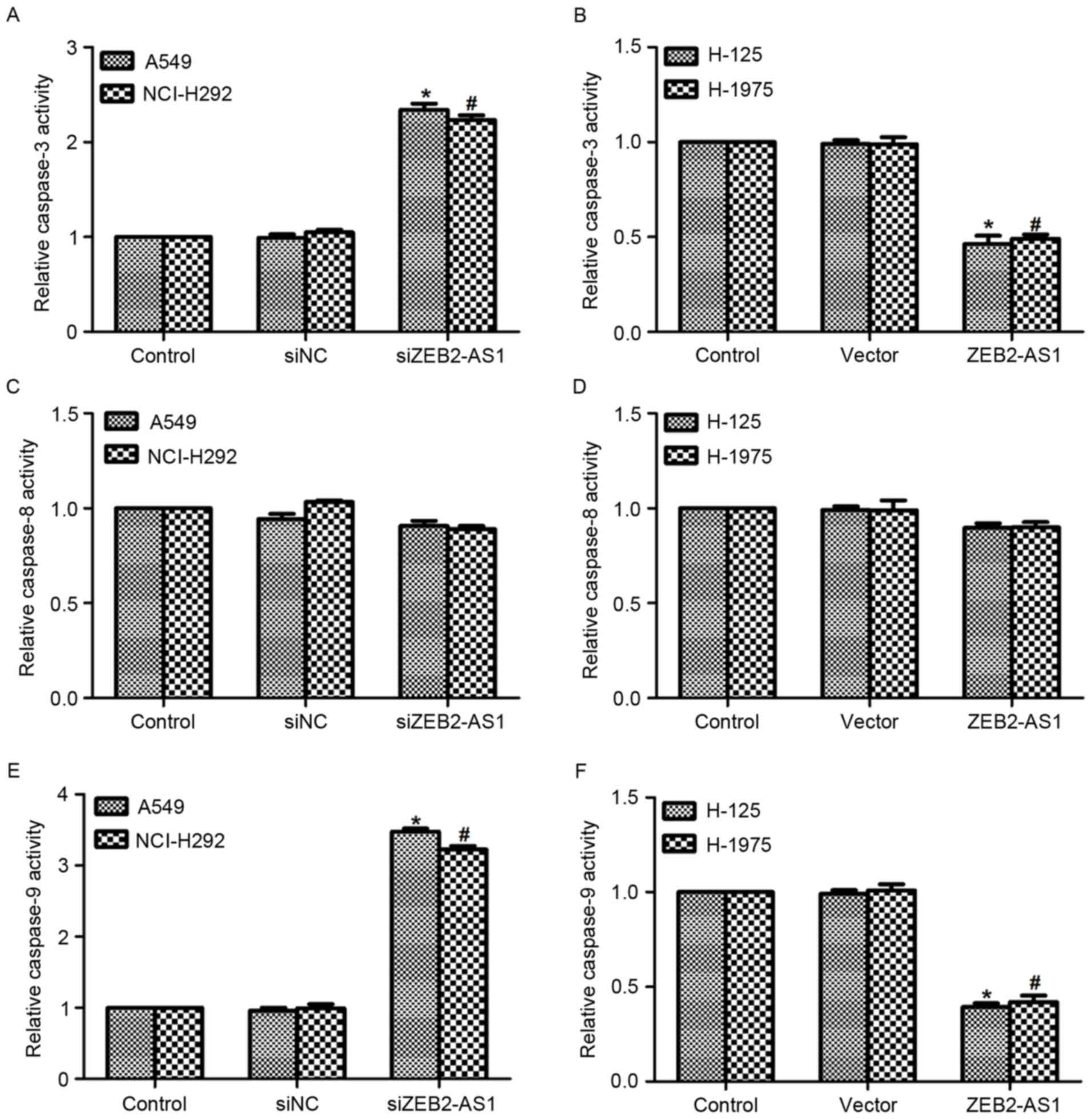
To assess the function of ZEB2-AS1 in lung cancer
further, the relative activity of caspase-3, −8 and −9 was detected
in the four cell lines. As presented in Fig. 5A, transfection with siZEB2-AS1 in A549
and NCI-H292 cells increased the activity of caspase-3 by 2.2-fold,
whereas the relative activity of caspase-3 was decreased by ~50%
when cells were treated with ZEB2-AS1 expressing plasmid in the two
cell lines (Fig. 5B). On the
contrary, the relative activity of caspase-8 remained stable when
cells were treated with specific siRNA against ZEB2-AS1 or ZEB2-AS1
expressing plasmid in four lung cancer cell lines (Fig. 5C and D). The relative activity of
caspase-9 was also detected in vitro. Similar to the
activity of caspase-3, transfection of A549 and NCI-H292 cells with
siZEB2-AS1 resulted in increased caspase-9 activity, whereas
treatment with ZEB2-AS1 plasmid in H-125 and H1975 cells decreased
the activity of caspase-9 (Fig. 5E and
F). Taken together, these results indicated that the inhibition
of ZEB2-AS1 promoted cell apoptosis in human lung cancer cells.
Discussion
Lung cancer is the most common type of cancer and
the leading cause of cancer-associated mortality among men and
women worldwide in 2005 (17).
Although great efforts have been made to improve the diagnosis and
treatment of patients with lung cancer, the 5-year survival rate
remains low worldwide. Genetic and epigenetic alterations have been
widely accepted as the driving factors of cancer (18). Research is presently focusing on the
identification of novel biomarkers for the early stages of lung
cancer.
ZEB2-AS1 is a recently identified lncRNA that has
been demonstrated to perform significant functions in HCC (14,15). It is
a non-coding antisense transcript from the promoters of ZEB2, which
is reported to function as a transcription factor and is associated
with a number of distinct types of cancer (19–21). It
was also demonstrated that ZEB2 was overexpressed in human HCC and
associated with HCC progression (22). ZEB2-AS1 was also observed to be
upregulated in human HCC and served as a prognostic factor
(14,15).
In the present study, the overexpression of ZEB2-AS1
in H-125 and H1975 cells promoted their proliferative rate and
colony forming ability, consistent with previously published
studies (14,15). However, the present study did not
evaluate the detailed function of ZEB2-AS1 in cell metastasis,
instead investigating the effects of ZEB2-AS1 on cell apoptosis. Li
et al (15) identified that
knockdown of ZEB2-AS1 in human HCC cells inhibited cell metastasis
using Transwell and wound-healing assays, which may represent the
next step in research involving lung cancer cell lines. Induction
of cell apoptosis in multicellular organisms is one of the most
effective ways to eliminate the harmful or unnecessary cells, and
its abnormal regulation maybe associated with tumorigenesis
(23). Apoptosis is primarily
initiated via two pathways: The intrinsic pathway (initiated by
stress stimulation), and the extrinsic pathway (initiated by
signals from other cells) (24,25).
Activation of the two pathways requires activation of initiator
caspases (caspase-9 and −8), which then activate effector caspases
(caspase-3), following which the cells undergo apoptosis (26,27). There
are two hypotheses of the direct initiation of extrinsic pathways
in mammals that have been suggested: The TNF-induced model and the
Fas-Fas ligand-mediated model (25,28), which
are all associated with the activation of caspase-8. The present
study demonstrated that the knockdown of ZEB2-AS1 in A549 and
NCI-H292 cells promoted cell apoptosis and increased the relative
activities of caspase-3 and caspase-9, eliciting no change in
caspase-8 activity.
To conclude, the results of the present study
demonstrated that the expression of ZEB2-AS1 is upregulated in
human lung cancer in vivo and in vitro. Knockdown of
ZEB2-AS1 in lung cancer A549 and NCI-H292 cell lines inhibited cell
proliferation, whereas overexpression of ZEB2-AS1 in lung cancer
H-125 and H1975 cells inhibited cell apoptosis, indicating that
ZEB2-AS1 may serve as a prognostic factor for the diagnosis and
treatment of patients with lung cancer in the clinic.
References
|
1
|
Jemal A, Bray F, Center MM, Ferlay J, Ward
E and Forman D: Global cancer statistics. CA Cancer J Clin.
61:69–90. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Pikor LA, Ramnarine VR, Lam S and Lam WL:
Genetic alterations defining NSCLC subtypes and their therapeutic
implications. Lung Cancer. 82:179–189. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Youlden DR, Cramb SM and Baade PD: The
international epidemiology of lung cancer: Geographical
distribution and secular trends. J Thorac Oncol. 3:819–831. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Dela Cruz CS, Tanoue LT and Matthay RA:
Lung cancer: Epidemiology, etiology, and prevention. Clin Chest
Med. 32:605–644. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Katayama S, Tomaru Y, Kasukawa T, Waki K,
Nakanishi M, Nakamura M, Nishida H, Yap CC, Suzuki M, Kawai J, et
al: Antisense transcription in the mammalian transcriptome.
Science. 309:1564–1566. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Cabili MN, Trapnell C, Goff L, Koziol M,
Tazon-Vega B, Regev A and Rinn JL: Integrative annotation of human
large intergenic noncoding RNAs reveals global properties and
specific subclasses. Genes Dev. 25:1915–1927. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Brown CJ, Ballabio A, Rupert JL,
Lafreniere RG, Grompe M, Tonlorenzi R and Willard HF: A gene from
the region of the human X inactivation centre is expressed
exclusively from the inactive X chromosome. Nature. 349:38–44.
1991. View
Article : Google Scholar : PubMed/NCBI
|
|
8
|
Rinn JL: lncRNAs: Linking RNA to
chromatin. Cold Spring Harb Perspect Biol. 6:a0186142014.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Goff LA and Rinn JL: Linking RNA biology
to lncRNAs. Genome Res. 25:1456–1465. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Li M, Qiu M, Xu Y, Mao Q, Wang J, Dong G,
Xia W, Yin R and Xu L: Differentially expressed protein-coding
genes and long noncoding RNA in early-stage lung cancer. Tumour
Biol. 36:9969–9978. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Yang Y, Li H, Hou S, Hu B, Liu J and Wang
J: The noncoding RNA expression profile and the effect of lncRNA
AK126698 on cisplatin resistance in non-small-cell lung cancer
cell. PLoS One. 8:e653092013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Graham TR, Zhau HE, Odero-Marah VA,
Osunkoya AO, Kimbro KS, Tighiouart M, Liu T, Simons JW and O'Regan
RM: Insulin-like growth factor-I-dependent up-regulation of ZEB1
drives epithelial-to-mesenchymal transition in human prostate
cancer cells. Cancer Res. 68:2479–2488. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Sanchez-Tilló E, de Barrios O, Siles L,
Amendola PG, Darling DS, Cuatrecasas M, Castells A and Postigo A:
ZEB1 promotes invasiveness of colorectal carcinoma cells through
the opposing regulation of uPA and PAI-1. Clin Cancer Res.
19:1071–1082. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Lan T, Chang L, Wu L and Yuan Y:
Downregulation of ZEB2-AS1 decreased tumor growth and metastasis in
hepatocellular carcinoma. Mol Med Rep. 14:4606–4612. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Li T, Xie J, Shen C, Cheng D, Shi Y, Wu Z,
Deng X, Chen H, Shen B, Peng C, et al: Upregulation of long
noncoding RNA ZEB1-AS1 promotes tumor metastasis and predicts poor
prognosis in hepatocellular carcinoma. Oncogene. 35:1575–1584.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Devesa SS, Bray F, Vizcaino AP and Parkin
DM: International lung cancer trends by histologic type: Male:
Female differences diminishing and adenocarcinoma rates rising. Int
J Cancer. 117:294–299. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zeng H, Zheng R, Guo Y, Zhang S, Zou X,
Wang N, Zhang L, Tang J, Chen J, Wei K, et al: Cancer survival in
China, 2003–2005: A population-based study. Int J Cancer.
136:1921–1930. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Song N, Liu H, Ma X and Zhang S: Placental
growth factor promotes ovarian cancer cell invasion via ZEB2. Cell
Physiol Biochem. 38:351–358. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Yi X, Shi S, Li X and Zhao L: Expression
and clinical significance of ZEB2 and E-cadherin in nasopharyngeal
carcinoma. Lin Chung Er Bi Yan Hou Tou Jing Wai Ke Za Zhi.
29:1648–1651. 2015.(In Chinese). PubMed/NCBI
|
|
21
|
Li J, Yuan J, Yuan X, Zhao J, Zhang Z,
Weng L and Liu J: MicroRNA-200b inhibits the growth and metastasis
of glioma cells via targeting ZEB2. Int J Oncol. 48:541–550. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Pang X, Huang K, Zhang Q, Zhang Y and Niu
J: miR-154 targeting ZEB2 in hepatocellular carcinoma functions as
a potential tumor suppressor. Oncol Rep. 34:3272–3279. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Kerr JF, Wyllie AH and Currie AR:
Apoptosis: A basic biological phenomenon with wide-ranging
implications in tissue kinetics. Br J Cancer. 26:239–257. 1972.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Dejean LM, Martinez-Caballero S, Manon S
and Kinnally KW: Regulation of the mitochondrial apoptosis-induced
channel, MAC, by BCL-2 family proteins. Biochim Biophys Acta.
1762:191–201. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Wajant H: The Fas signaling pathway: More
than a paradigm. Science. 296:1635–1636. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Bejarano I, Espino J, Gonzalez-Flores D,
Casado JG, Redondo PC, Rosado JA, Barriga C, Pariente JA and
Rodríguez AB: Role of calcium signals on hydrogen peroxide-induced
apoptosis in human myeloid HL-60 cells. Int J Biomed Sci.
5:246–256. 2009.PubMed/NCBI
|
|
27
|
Murphy KM, Ranganathan V, Farnsworth ML,
Kavallaris M and Lock RB: Bcl-2 inhibits Bax translocation from
cytosol to mitochondria during drug-induced apoptosis of human
tumor cells. Cell Death Differ. 7:102–111. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Chen G and Goeddel DV: TNF-R1 signaling: A
beautiful pathway. Science. 296:1634–1635. 2002. View Article : Google Scholar : PubMed/NCBI
|