Introduction
Melanomas are a rare but aggressive cutaneous type
of cancer in humans (1). At the
dissemination stage in a majority of cases, the disease is
resistant to treatment with cytostatics and radiotherapy (1). Therefore, the identification of novel
molecular mechanisms involved in the melanomagenesis process and
tumor progression have enabled the production of targeted therapies
that yield notable effects (1). The
basis for melanomagenesis is the accumulation of genetic disorders
in the melanocyte (the most frequent ones include the following
mutations: B-Raf proto-oncogene, serine/threonine kinase, N-Ras
proto-oncogene, GTPase and phosphatase and tensin homolog)
(1). However, only the interaction
between microenvironment elements and genetic changes in the
melanocyte result in the ultimate transformation of a dysplastic
melanocyte into a melanoma cell, and at further stages result in
the local invasion and dissemination of the primary lesion
(1). It is the microenvironment that
is one of the key elements of cancer formation and is being studied
at present.
A melanoma microenvironment is a markedly
heterogenic population of cells that involves fibroblasts,
macrophages, lymphocytes, other immune system cells, adipocytes and
cells that form the structural elements of cutaneous blood vessels
sunk in the extracellular matrix (2).
The aforementioned complex network of cellular associations are
constantly interacting through direct contact and active protein
substances including secretory proteins (e.g., metalloproteinases
or osteonectin) and growth factors [e.g., transforming growth
factor-β (TGF-β), Wnt, Hedgehog, epidermal growth factor (EGF),
hepatocyte growth factor and platelet-derived growth factor]
(2–5),
and is accompanied by hypoxia (6).
The present review provides a detailed overview on
the function of macrophages in the melanoma microenvironment.
General characteristics of macrophages
Clonal survival, malignant tumor heterogeneity and
resistance to systemic treatment are the features of cancer that,
in the light of a previous study, are largely shaped by the immune
system (7).
Of all the cells of the immune system, it is the
function of macrophages that has been explored most thoroughly.
They are a group of cells that are known for their plasticity,
which depends on signals from the external environment and thus
their cytophysiological functions are widely varied (8,9). There are
numerous different propositions on how to divide macrophages.
Depending on the immune response, there are three basic groups of
macrophages: i) Classically activated macrophages i.e., those being
an element of a cellular immune response, produced in the course of
inflammatory reaction and formed primarily in response to the
granulocyte-macrophage colony-stimulating factor (GM-CSF),
interferon-γ and tumor necrosis factor (TNF)-α, and themselves
producing pro-inflammatory cytokines [including interleukin
(IL)-12] and reactive forms of oxygen and carbon oxide; they
destroy and remove pathogens and abnormal cells and activate other
cells of the immune system, ii) wound-healing macrophages which are
induced by Il-4 and produce growth factors and proangiogenic
factors, and iii) regulatory macrophages which may be produced in
response to the excessive release of glucocorticosteroids in stress
situations, but which are also induced by the activation of
toll-like receptor (TLR) by, among others, the presence of
immunoglobulin G complexes; they produce Il-10 which functions as
an immunosuppressant, reduces the production of pro-inflammatory
factors and inhibits the activity of cytotoxic lymphocytes T
[resulting in the stimulation of programmed death ligand-1
(PD-L1)], and thus limits the inflammatory reaction (8,9). There is
also a fourth group of macrophages (trophic macrophages) which
phenotypically have the features of macrophages from groups 2 and
3, and are involved in tissue development and the maintenance of
homeostasis, regulated primarily by CSF-1 (9).
According to another widely used and much simpler
division, macrophages may be grouped as either activated
macrophages (M1/activated) or alternatively activated macrophages
(M2/trophic) (10). Within each of
the groups, macrophage subpopulations are observed, which differ
very little with regards to function and phenotype (8). This suggests that macrophages are best
understood as a continuum of cells that smoothly transit from one
subgroup to another (8,9).
Transcriptional profiling of resident macrophages
revealed that the populations are characterized by a high
transcriptional variety with minimal overlap, which indicates that
there are numerous unique classes of macrophages (11). Heterogeneity of macrophage classes
results in a wide range of their biological functions (11). Macrophages are involved in almost all
biological processes in an organism, and in addition to their
involvement with the immune response to pathogens, they also serve
a function in developmental, homeostatic and repair processes
(11). Their repair function ensures
proper embryogenesis, morphogenesis and organogenesis (11). It was revealed that the loss of
macrophages results in a cluster of developmental abnormalities
(11). Macrophages are involved in
the development of brain, bones, heart and vascular system
(11). Additionally, they maintain
metabolic homeostasis e.g., in the course of a bacterial infection,
they promote resistance to insulin through pro-inflammatory
cytokines to decrease nutrient accumulation (11). Macrophages additionally regulate
adipocyte responses to insulin (11).
Unfortunately, due to chronic irritation, the
notable repair and homeostatic functions of macrophages are lost,
which results in their involvement in the development of diseases
(11).
Macrophages-general function in
carcinogenesis
Data concerning the function of macrophages in the
first stage of initiation and promotion of transformed cancer cells
is contradictory. A previous in vitro study confirmed that
activated macrophages kill cancer cells (12), whereas other studies reveal that a
decreased number of macrophages have no influence on the
susceptibility of an organism to cancer; furthermore, in a number
of cases, macrophages contribute to the eventual transformation of
a given normotypic cell into a cancer cell (13,14). It
appears that these differences are due to different macrophage
phenotypes and consequently to the production of different
cytokines (9,10).
A separate issue is that of a chronic inflammation,
in which the activated macrophages serve key functions. It has been
hypothesized that the reactive forms of nitrogen and oxygen produce
a microenvironment that is conducive to mutagenesis (15). Free radicals damage the DNA of normal
cells, and the genome loses stability and results in a
transformation into cancer cells (15). Other notable stages in cancer
progression include cancer cell invasion, angiogenesis, metastasis
and suppression of adaptive anti-tumor immunity (7,9,16). Tumor-associated macrophages (TAMs) are
involved in these stages. TAMs are formed from monocytes
circulating in the blood (16). With
the help of multiple different cytokines including CSF-1, C-C motif
chemokine ligand 2 (CCL-2), IL-34, vascular endothelial growth
factor A (VEGFA) or C-X-C motif chemokine ligand 12 (CXCL12)
produced by cancer cells as they infiltrate the tumor through the
vessels (16). They accumulate in the
invasive tumor edge, cancer cell/stromal border, central tumor
mass, hypoxic/necrotic regions and perivascular areas (16). TAMs have features of a trophic
macrophage phenotype (M2), which means that they may remodel the
extracellular matrix and suppress the immune system (8,14,17). TAMs produce other mediators and
enzymes. Secreted Protein Acidic and Rich in Cystein, cathepsins
and metalloproteinase (MMP) 2 and 9 produced by TAMs decompose the
elements of the extracellular matrix, including type IV collagen,
which is the basic element of the basal membrane, which enables and
promotes invasion (9,11). Macrophages additionally regulate
angiogenesis and are associated with the density of microvessels
surrounding the tumor (16).
Proangiogenic macrophages are characterized by the increased
expression of tyrosine-protein kinase receptor 2 (TIE2) and the
production of VEGF. Their proangiogenic function is increased by
angiopoietin-2, produced by activated endothelial cells.
Subsequently, the EGF-CSF-1 paracrine loop between macrophages and
cancer cells with the CXCL12 chemokine promotes the development of
the tumor microenvironment of metastasis i.e., the micro-anatomical
site regulating the escape of cancer cells from the primary tumor
(8,16).
TAMs modulate the immune reaction by enhancing the
synthesis of TGF-β and prostaglandin E2 (PGE2), in addition to
reducing the synthesis of Il-12, Il-18 and the TLR signaling
pathway, which results in the reduced activation of other cells of
immune response (17). They markedly
influence the cytotoxic lymphocytes T on which they exert a direct
effect (by stimulating the synthesis of arginase and nitrogen oxide
that inhibit the effect of cytotoxic T lymphocytes) and an indirect
effect (through IL-10 they stimulate monocytes to express the
costimulatory molecule PD-L1 which suppresses these lymphocytes,
and through the CCL22 chemokine which affects the regulatory T
lymphocytes that additionally inhibit them) (9,18).
Another notable issue is the function of macrophages
in metastasis. Primary tumor cells produce chemoattractants (S100A8
and A9) for myeloid cells that settle on the tissues to prepare
space for the colonization by cancer cells and create the
pre-metastatic niche (9). The
settling cancer cells recruit macrophages from the surrounding
myeloid cells and these in turn stimulate the growth and further
dissemination of the primary tumor cells (9).
Macrophages in skin melanomas
The presence of macrophages in primary lesions was
revealed in cutaneous melanomas (19), uveal melanomas (20,21) and
sinonasal melanomas (22). In the
case of cutaneous lesions, they are predominantly located in the
primary foci, and to a lesser extent in metastatic foci (19). Elevated numbers of macrophages within
a melanoma is markedly associated with poor prognosis (23).
Tumorigenesis and growth
Melanoma cells are able to produce multiple factors
that modulate the activity of immune response cells. Autocrine
factors stimulate melanoma cells to continue non-controlled
proliferation, and those with paracrine activity modulate the
microenvironment in order for tumor growth and further invasion to
be promoted (24). The most notable
factors include GM-CSF, CCL2, IL-8/CXCL-8, TGF-β, IL-1, IL-6 and
IL-10, of which GM-CSF and CCL2 have the highest impact on melanoma
macrophages (25). GM-CSF inhibits
the cytotoxic effect of macrophages (25). However, presently available data from
studies on the effect of CCL2 on macrophage recruitment is
contradictory. The effect of CCL2 released by melanoma cells is
dependent on the extent of its secretion (26). With a high concentration of CCL2,
there is a substantial infiltration of the primary lesion by
macrophages, primarily by M1, which aims to destroy the tumor
(27). However, with the decreased
concentration of CCL2, M2 macrophages accumulate and result in the
promotion of tumor growth (27).
Contrary to the aforementioned studies, it has been proven that the
production of CCL2 by melanoma cells and the associated TAM
recruitment results in enhanced angiogenesis (28) and are associated with a more advanced
disease (29). It may be that the
discrepant results are due to the two-stage influence of CCL2 on
tumor growth. It has been revealed that CCL2 and GM-CSF drive
angiogenesis firstly by stimulating the expression of
hypoxia-inducible factor-1 α and hypoxia response element, and in
turn these factors increase the expression of VEGF-A (30). IL-6 produced by melanoma cells
additionally promotes the growth of TAMs, which at the subsequent
stage induce IL-10 expression that further drives the vicious
circle of immunosuppression (25).
IL-10 produced by tumor cells induces the expression of the
negative costimulatory molecule B7-H4 identified on TAMs, which on
contact with T cells inhibits their proliferation and cytokine
release (25). B7-H4 was identified
in melanoma cells and TAMs (31).
Immune suppression
IL-10 and TGFβ are produced by TAMs, which inhibit
the differentiation of bone marrow cells into dendritic cells,
determining their further differentiation into TAMs (25). Furthermore, the combination of factors
produced by melanoma-associated macrophages induce the activity of
the myeloid-derived suppressor cells that additionally inhibit the
response of the immune system (32).
It should be noted that within TAMs, similar to melanoma cells,
there is a high expression of PD-L1 that helps TAM and regulatory T
lymphocytes to form an immunosuppressive microenvironment (33,34)
(Fig. 1A).
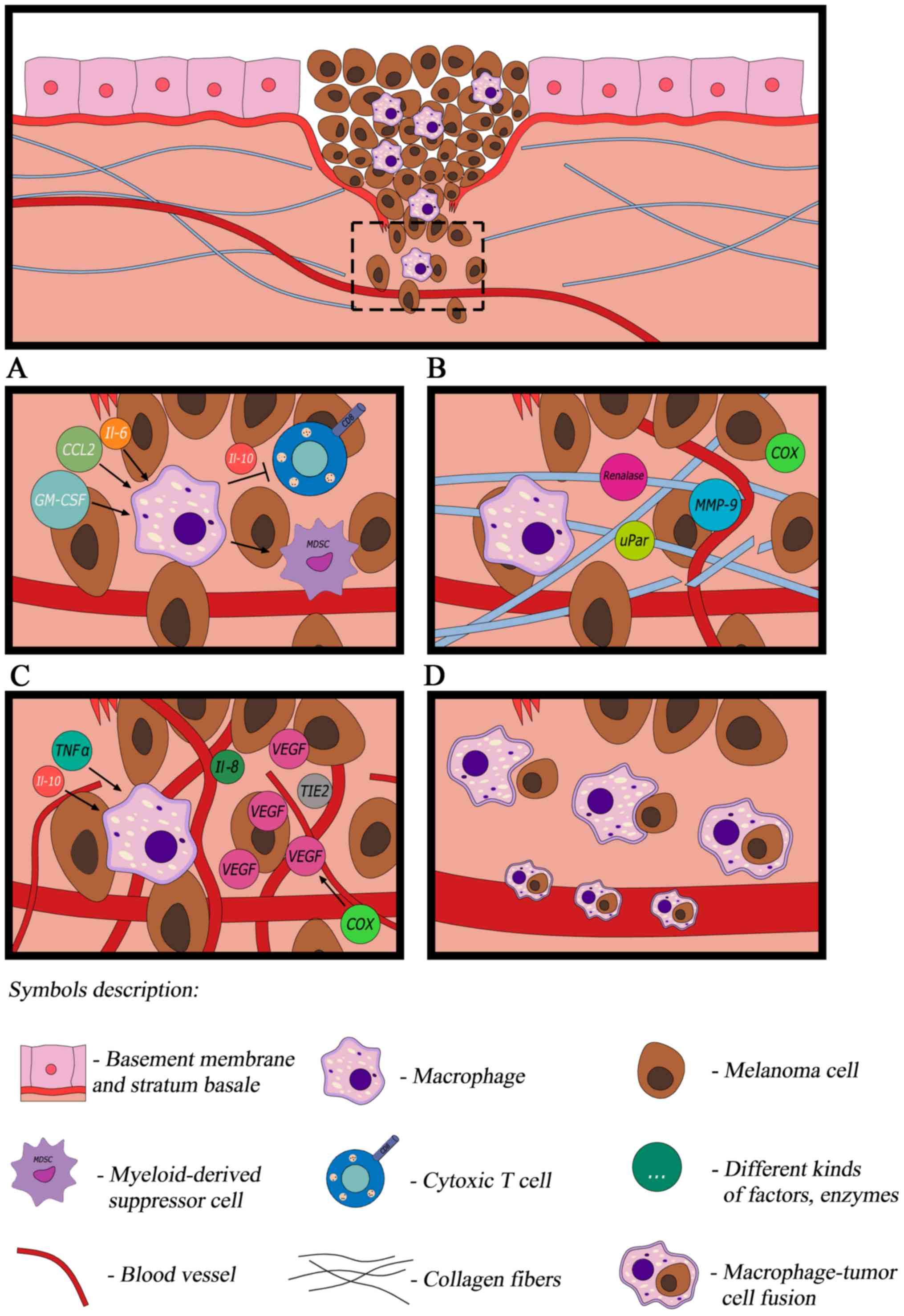 | Figure 1.In response to cytokines produced by
cancer cells, macrophages transform into TAMs. (A) TAMs modulate
the immune reaction, (B) are able to remodel the extracellular
matrix (C) promote angiogenesis and (D) contribute to melanoma
dissemination. TAM, tumor-associated macrophage; IL, interleukin;
CCL, C-C motif chemokine ligand; GM-CSF, granulocyte-macrophage
colony-stimulating factor; MMP9 matrix metalloproteinase 9; VEGF,
vascular endothelial growth factor; uPar, urokinase-type
plasminogen activator receptor; COX, cyclooxygenase; TNF-α, tumor
necrosis factor-α; TIE2, tyrosine-protein kinase receptor 2. |
The signal transducer and activator of transcription
3 (STAT3) has been identified as the master regulator of a number
of the aforementioned factors. STAT3 is a pro-tumorigenic
transcription factor. In melanoma, it regulates all facets of the
immune response and promotes production of M2 macrophages (25,35). In
melanoma, inhibition of the STAT3 pathway results in the increase
of inhibition and pro-apoptotic effects against malignant tumor
cells. Additionally, the improved recognition of malignant tumor
cells by the immune system has been revealed, in addition to
improved responses to the anti-tumor cytokine interferon-α (INF-α)
as a result of STAT3 inhibition (35).
Tumor microenvironment modulation
Molecular signals regulating the specific dialogue
between melanoma cells and their microenvironment remain unknown.
Kinases associated with the membrane lipids of melanoma cells
additionally affect the macrophages which form the microenvironment
of melanoma. Emerging reports demonstrate that the key function of
sphingosine 1-kinase (S1K) is that it produces a bioactive lipid
called lipid sphingosine 1-phosphate (23). Lower expression of S1K results in the
reduction of the number of M2 macrophages and increase of the
number of M1 macrophages (23), which
may be due to the inhibition of cancer progression. This
hypothesis, however, requires further study.
Macrophages also modulate the tumor microenvironment
through the production of various proteins, enzymes and oxides,
thus promoting proliferation, tumor growth and invasion (Fig. 1B) (9,36).
Melanoma-associated macrophages have been revealed to have a
distorted balance between the production of inducible nitric oxide
synthase (iNOS) and arginase (36).
Macrophages use arginine to produce NO by iNOS, or to produce
ornithine through arginase activity (36). NO is primarily cytotoxic and ornithine
production promotes the proliferation of tumor cells (36). It has been demonstrated that in
clinically less advanced melanomas, iNOS is more active compared
with arginase, and this is stimulated by contact with melanoma
cells (36). In the case of more
advanced primary lesions, a significant reduction in the number of
macrophages with a higher percentage of iNOS has been observed
(36). It is notable that NO released
by macrophages possesses anticancer properties only in the presence
of INFγ produced by natural killer cells (36).
Melanoma-associated macrophages are characterized by
expression of pro-inflammatory protein cyclooxygenase-2 (COX-2).
The percentage of COXs-positive TAMs is highest in in situ
and in thin melanomas, and lower in highly advanced and metastatic
melanomas. It has been demonstrated that the expression of this
thoroughly studied mediator of inflammation is a marker of melanoma
progression (19). Macrophages
stimulated by osteopontin from the melanoma microenvironment start
to synthesize the COX-2 protein (37). α9β1 integrin on macrophages is the
receptor for osteopontin, which stimulates COX-2 expression in
macrophages through the ERK and p38 pathways (37). In addition to maintaining
inflammation, COX-2 additionally promotes melanoma cell migration
and angiogenesis through the COX-2 dependent production of PGE2
(37). Osteopontin combined with PGE2
substantially increases the influence on angiogenesis by enhancing
the expression of MMP-9 (36).
Another study revealed that the increased expression of COX-2 in
melanoma tumor types is associated with increased VEGF expression,
density of micro vessels and inflammatory infiltration formed of
macrophages (37,38).
Another microenvironment protein that promotes
melanoma growth is renalase. This flavoprotein, that functions as
cell survival factor, is identified in melanoma cells and cluster
of differentiation 163-positive TAMs (39). Renalase activates the phosphoinositide
3-kinase/protein kinase B and mitogen-activated protein kinase
signaling pathways which are two of the most important signaling
pathways of the epithelial mesenchymal transition (EMT) (4,39).
Attenuation of renalase expression reduces melanoma cell survival
and inhibits tumor growth (39),
which may suggest a potential novel target of molecular targeted
anticancer therapies.
TAMs additionally produces protease-like proteins
which promote melanoma invasion (9,24,40). It has been demonstrated that
melanoma-associated macrophages synthesize large amounts of MMP-9
and urokinase-type plasminogen activator receptor (uPAR) as a
result of direct contact with melanoma cells (40). MMP-9 decomposes collagen IV, which is
main component of the basement membrane, and additionally
decomposes latent TGF-β complexes, which further intensifies the
EMT (2,40). uPAR is an element of the plasminogen
activation system and is involved in multiple proteolytic processes
that result in the reorganization and degradation of the
extracellular matrix (40).
Angiogenesis
Melanoma-associated macrophages are additionally
involved in the indirect promotion of angiogenesis (Fig. 1C) as they release TNF-α and IL-1α. In
response to stimulation with these cytokines, melanoma cells
produce angiogenic factors, including IL-8, VEGF, TIE2 and CD31,
which results in neoangiogenesis (29,41).
Furthermore, the proangiogenic factor released by pericytes-milk
fat globule-epidermal growth factor 8 stimulates the polarization
of M2 macrophages, suggesting that they also increase tumor
angiogenesis (42).
Hypoxia is yet another element of the
microenvironment notably associated with and affecting TAMs
(43). Hypoxia within the tumor
drives TAM accumulation in the melanoma microenvironment (43). Signals connecting TAM recruitment with
hypoxia have yet to be elucidated. It has been confirmed that
high-mobility group box 1 (HMGB1) protein is released by melanoma
cells under hypoxic conditions and promotes the accumulation of M2
macrophages and the production of immunosuppressive IL-10
surrounding the tumor (43). HMGB1
stimulates macrophages to produce IL-10, affecting the receptor for
advanced glycation end products, thereby inducing an inflammatory
response (43).
Interactions of macrophages with melanoma
cells
Interactions of M2 macrophages with the
subpopulations of melanoma cells are not well-known. Studies on
mice with spontaneous melanoma have revealed that CD34
tumor-initiating cells (TICs) and stem-like cells, i.e., the cells
that initiate tumor growth and determine certain features of a
tumor, including chemoresistance, are M2 macrophage dependent, and
the precise survival and proliferation of TICs are dependent on
TAMs (44). Furthermore, TICs are
stimulated in the presence of TAMs to form melanospheres i.e.,
non-adherent colonies of melanoma cells (44). It has been demonstrated that
CD34− TIC stimulation by TAMs is associated with
melanoma progression in vivo (44). The results additionally include a
notable observation concerning how chemotherapy was used to treat
melanoma (cisplatin, temozolamide), as it was demonstrated that
chemotherapy drives TAM recruitment in the tumor, stimulates the
growth of TAM-responsive TICs, and that TAMs themselves protect
TICs against chemotherapy effects (44). It was demonstrated that on the
molecular level, the simulation of TICs was a result of TAM-derived
TGF-β and polyamines (44,45). The function of TAM-derived TGF-β is to
autoregulate and stimulate arginase, which results in the
production of polyamines (which serve key functions in the growth
and differentiation of cancer cells) (44,45).
Dissemination
Another function of melanoma-associated macrophages
is their involvement in dissemination (Fig. 1D) which is the main reason for
cancer-associated mortality (5,9,16). One of the most widely accepted
hypotheses for cancer cell dissemination is the EMT allowing cancer
cells to acquire the ability to migrate (4,5). According
to an alternative theory, macrophages are involved in the
dissemination process. They are said to fuse with tumor cells
creating a hybridoma [a macrophage with tumor cell fusion (MTF)]
and then pass into the bloodstream (46). MTFs were identified in patients with
cutaneous melanoma (46). The
selected MTFs presented with features of macrophages and melanomas.
Morphologically, MTFs were large cells with pseudopodia and
lamellipodia. Expression of macrophage characteristic markers (CD14
and CD68) in addition to M2 macrophage-specific markers (CD163,
CD204, CD206) were identified (46).
Expression of characteristics of melanocytes [including activated
leukocyte cell adhesion molecule, protein melan-A and
pro-carcinogenic cytokine macrophage migration inhibitory factor
(MIF)] were additionally demonstrated (46). In one case MTFs possessed a B-Raf
proto-oncogene, serine/threonine kinase mutation (44). These results suggest that macrophages
are involved in melanoma dissemination through the formation of
MTFs which may escape to the bloodstream and are able to settle in
distant organs, and the released cytokines (including MIF) prepare
niches to be colonized by the TICs (46). Further studies demonstrated that the
endothelial cells next to the melanoma produce an increased number
of various chemokines including CCL21, CCL2 and CXCL8 (16,47). They
result in the endothelium becoming permeable, melanoma cells
expressing receptors for these chemokines intravasating, and then
through the blood reaching the pre-metastatic niches (16). It may be assumed that similarly to
breast cancer, they form micro-clots with blood platelets there and
remain in the blood vessels (16).
Pre-metastatic niches are a reservoir of monocytes recruited from
the circulatory system which then transform to metastatic
associated macrophages (16). They
promote metastatic cell survival by adhesion and survival signal
(namely, CCL3) (16).
Conclusion
As presented in this review, macrophages serve a key
function in the complex regulation of the network of interactions
and associations between melanoma cells and multiple subpopulations
of cells that form the tumor stroma. Improved knowledge of the
macrophages, namely the identification of their phenotypes and
their influence on promoting all stages of melanomagenesis, has
altered our perception of the macrophages. They are no longer
involved exclusively in phagocytosis and the specific clearance of
dead cells, but they have become a notable and active element of
melanomagenesis. Furthermore, the knowledge of signaling pathways,
protein substances and their ligands paved the way for breakthrough
targeted therapies against disseminated melanoma (e.g., using an
anti-PD-L1 antibody) (12). Targeted
therapies may be directly aimed at TAMs to eliminate them or
modulate their activity, and alternatively they may be used to
support the existing treatment methods. It has been revealed that
activation of macrophages induces their activity against melanoma
(48). It may be achieved by
administering immunomodulatory substances including GM-CSF,
galectin-9, vaccination with pathogens, nanoparticles
(polyhydroxylated fullerenols) or blocking melanoma inhibition of
macrophage migration by a macrophage inhibitory cytokine inhibitor
(48). Another therapeutic strategy
is to prevent the transformation of macrophages into TAMs. For this
strategy, antibodies neutralizing Il-4, Il-10 or TGF-β may be used
(48). Finally, TAM-targeted
therapies are being studied. There are encouraging preclinical
studies of inhibitor STAT-3, janus kinase-2 (35) or nanoparticles delivering small
interfering RNA to TAMs (49).
Multifunctional TAMs appear to be an attractive anti-melanoma
target, which may complete other treatments as part of an effective
and comprehensive anti-melanoma strategy.
Acknowledgements
The presented results of studies performed as part
of the subject as per records in the Simple system number
ST.C280.17.010 were financed through a statutory subsidy by the
Minister of Science and Higher Education.
Glossary
Abbreviations
Abbreviations:
|
COX
|
cyclooxygenase
|
|
EGF
|
epidermal growth factor
|
|
EMT
|
epithelial mesenchymal transition
|
|
GM-CSF
|
granulocyte-macrophage
colony-stimulating factor
|
|
Il
|
interleukin
|
|
iNOS
|
inducible nitric oxide synthase
|
|
MMP
|
matrix metalloproteinase
|
|
MTF
|
macrophages with tumor cells
fusion
|
|
PD-L1
|
programmed death ligand
|
|
PGE2
|
prostaglandin E2
|
|
S1K
|
sphingosine 1-kinase
|
|
STAT3
|
signal transducer and activator of
transcription 3
|
|
TAM
|
tumor-associated macrophage
|
|
TGF-β
|
transforming growth factor β
|
|
TIC
|
tumor-initiating cells
|
|
TLR
|
toll-like receptor
|
|
TNF
|
tumor necrosis factor
|
|
uPAR
|
urokinase-type plasminogen activator
receptor
|
|
VEGF
|
vascular endothelial cell growth
factor
|
References
|
1
|
Lin K, Baritaki S, Militello L, Malaponte
G, Bevelacqua Y and Bonavida B: The role of B-RAF mutations in
melanoma and the induction of EMT via dysregulation of the
NF-κB/Snail/RKIP/PTEN circuit. Genes Cancer. 1:409–420. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Taylor MA, Parvani JG and Schiemann WP:
The pathophysiology of epithelial-mesenchymal transition induced by
transforming growth factor-beta in normal and malignant mammary
epithelial cells. J Mammary Gland Biol Neoplasia. 15:169–190. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Ruiter D, Bogenrieder T, Elder D and
Herlyn M: Melanoma-stroma interactions: Structural and functional
aspects. Lancet Oncol. 3:35–43. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kalluri R and Weinberg RA: The basics of
epithelial-mesenchymal transition. J Clin Invest. 119:1420–1428.
2009. View
Article : Google Scholar : PubMed/NCBI
|
|
5
|
Wu Y and Zhou BP: New insights of
epithelial-mesenchymal transition in cancer metastasis. Acta
Biochim Biophys Sin (Shanghai). 40:643–650. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Bedogni B and Powell MB: Hypoxia,
melanocytes and melanoma-survival and tumor development in the
permissive microenvironment of the skin. Pigment Cell Melanoma Res.
22:166–174. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Palucka AK and Coussens LM: The basis of
oncoimmunology. Cell. 164:1233–1247. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Mosser DM and Edwards JP: Exploring the
full spectrum of macrophage activation. Nat Rev Immunol. 8:958–969.
2008. View
Article : Google Scholar : PubMed/NCBI
|
|
9
|
Qian BZ and Pollard JW: Macrophage
diversity enhances tumor progression and metastasis. Cell.
141:39–51. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Mantovani A and Sica A: Macrophages,
innate immunity and cancer: Balance, tolerance, and diversity. Curr
Opin Immunol. 22:231–237. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Wynn TA, Chawla A and Pollar JW:
Macrophage biology in development, homeostasis and disease. Nature.
496:445–455. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Klimp AH, de Vries EG, Scherphof GL and
Daemen T: A potential role of macrophage activation in the
treatment of cancer. Crit Rev Oncol Hematol. 44:143–161. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Teng MW, Swann JB, Koebel CM, Schreiber RD
and Smyth MJ: Immune-mediated dormancy: An equilibrium with cancer.
J Leukoc Biol. 84:988–993. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Lin EY and Pollard JW: Tumor-associated
macrophages press the angiogenic switch in breast cancer. Cancer
Res. 67:5064–5066. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Pang B, Zhou X, Yu H, Dong M, Taghizadeh
K, Wishnok JS, Tannenbaum SR and Dedon PC: Lipid peroxidation
dominates the chemistry of DNA adduct formation in a mouse model of
inflammation. Carcinogenesis. 28:1807–1813. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Lewis CE, Harney AS and Pollard JW: The
multifaceted role of perivascular macrophages in tumors. Cancer
Cell. 30:3652016. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Torroella-Kouri M, Silvera R, Rodriguez D,
Caso R, Shatry A, Opiela S, Ilkovitch D, Schwendener RA,
Iragavarapu-Charyulu V, Cardentey Y, et al: Identification of a
subpopulation of macrophages in mammary tumor-bearing mice that are
neither M1 nor M2 and are less differentiated. Cancer Res.
69:4800–4809. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Curiel TJ, Coukos G, Zou L, Alvarez X,
Cheng P, Mottram P, Evdemon-Hogan M, Conejo-Garcia JR, Zhang L,
Burow M, et al: Specific recruitment of regulatory T cells in
ovarian carcinoma fosters immune privilege and predicts reduced
survival. Nat Med. 10:942–949. 2004. View
Article : Google Scholar : PubMed/NCBI
|
|
19
|
Bianchini F, Massi D, Marconi C, Franchi
A, Baroni G, Santucci M, Mannini A, Mugnai G and Calorini L:
Expression of cyclo-oxygenase-2 in macrophages associated with
cutaneous melanoma at different stages of progression.
Prostaglandins Other Lipid Mediat. 83:320–328. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Bronkhorst IH, Ly LV, Jordanova ES,
Vrolijk J, Versluis M, Luyten GP and Jager MJ: Detection of M2
macrophages in uveal melanoma and relation with survival. Invest
Ophthalmol Vis Sci. 52:643–650. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Jager MJ, Ly LV, El Filali M and Madigan
MC: Macrophages in uveal melanoma and in experimental ocular tumor
models: Friends or foes? Prog Retin Eye Res. 30:129–146. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Shi L, Lei D, Ma C, Xu F, Li Y, Wang Y,
Cong N, Liu D and Pan XL: Clinicopathological implications of
tumour-associated macrophages and vascularization in sinonasal
melanoma. J Int Med Res. 38:1276–1286. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Mrad M, Imbert C, Garcia V, Rambow F,
Therville N, Carpentier S, Ségui B, Levade T, Azar R, Marine JC, et
al: Downregulation of sphingosine kinase-1 induces protective tumor
immunity by promoting M1 macrophage response in melanoma.
Oncotarget. 7:71873–71886. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Lázár-Molnár E, Hegyesi H, Tóth S and
Falus A: Autocrine and paracrine regulation by cytokines and growth
factors in melanoma. Cytokine. 12:547–554. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Ilkovitch D and Lopez DM: Immune
modulation by melanoma-derived factors. Exp Dermatol. 7:977–985.
2008. View Article : Google Scholar
|
|
26
|
Graves DT, Barnhill R, Galanopoulos T and
Antoniades HN: Expression of monocyte chemotactic protein-1 in
human melanoma in vivo. Am J Pathol. 140:9–14. 1992.PubMed/NCBI
|
|
27
|
Nesbit M, Schaider H, Miller TH and Herlyn
MJ: Low-level monocyte chemoattractant protein-1 stimulation of
monocytes leads to tumor formation in nontumorigenic melanoma
cells. J Immunol. 166:6483–6490. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Gazzaniga S, Bravo AI, Guglielmotti A, van
Rooijen N, Maschi F, Vecchi A, Mantovani A, Mordoh J and Wainstok
R: Targeting tumor-associated macrophages and inhibition of MCP-1
reduce angiogenesis and tumor growth in a human melanoma xenograft.
J Invest Dermatol. 127:2031–2041. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Torisu H, Ono M, Kiryu H, Furue M, Ohmoto
Y, Nakayama J, Nishioka Y, Sone S and Kuwano M: Macrophage
infiltration correlates with tumor stage and angiogenesis in human
malignant melanoma: Possible involvement of TNFalpha and IL-1alpha.
Int J Cancer. 85:182–188. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Varney ML, Olsen KJ, Mosley RL and Singh
RK: Paracrine regulation of vascular endothelial growth factor-a
expression during macrophage-melanoma cell interaction: Role of
monocyte chemotactic protein-1 and macrophage colony-stimulating
factor. J Interferon Cytokine Res. 25:674–683. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Swatler J and Kozłowska E: Immune
checkpointtargeted cancer immunotherapies. Postepy Hig Med Dosw
(Online). 70:25–42. 2016.(In Polish). View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Mao Y, Poschke I, Wennerberg E, Pico de
Coaña Y, Egyhazi Brage S, Schultz I, Hansson J, Masucci G,
Lundqvist A and Kiessling R: Melanoma-educated CD14+ cells acquire
a myeloid-derived suppressor cell phenotype through COX-2-dependent
mechanisms. Cancer Res. 73:3877–3887. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Hino R, Kabashima K, Kato Y, Yagi H,
Nakamura M, Honjo T, Okazaki T and Tokura Y: Tumor cell expression
of programmed cell death-1 ligand 1 is a prognostic factor for
malignant melanoma. Cancer. 116:1757–1766. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Kakizaki A, Fujimura T, Furudate S,
Kambayashi Y, Yamauchi T, Yagita H and Aiba S: Immunomodulatory
effect of peritumorally administered interferon-beta on melanoma
through tumor-associated macrophages. Oncoimmunology.
4:e10475842015. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Lesinski GB: The potential for targeting
the STAT3 pathway as a novel therapy for melanoma. Future Oncol.
9:925–927. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Massi D, Marconi C, Franchi A, Bianchini
F, Paglierani M, Ketabchi S, Miracco C, Santucci M and Calorini L:
Arginine metabolism in tumor-associated macrophages in cutaneous
malignant melanoma: Evidence from human and experimental tumors.
Hum Pathol. 38:1516–1525. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Kale S, Raja R, Thorat D, Soundararajan G,
Patil TV and Kundu GC: Osteopontin signaling upregulates
cyclooxygenase-2 expression in tumor-associated macrophages leading
to enhanced angiogenesis and melanoma growth via α9β1 integrin.
Oncogene. 33:2295–2306. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Gregório H, Raposo TP, Queiroga FL, Prada
J and Pires I: Investigating associations of cyclooxygenase-2
expression with angiogenesis, proliferation, macrophage and
T-lymphocyte infiltration in canine melanocytic tumours. Melanoma
Res. 26:338–347. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Hollander L, Guo X, Velazquez H, Chang J,
Safirstein R, Kluger H, Cha C and Desir GV: Renalase expression by
melanoma and tumor-associated macrophages promotes tumor growth
through a STAT3-mediated mechanism. Cancer Res. 76:3884–3894. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Marconi C, Bianchini F, Mannini A, Mugnai
G, Ruggieri S and Calorini L: Tumoral and macrophage uPAR and MMP-9
contribute to the invasiveness of B16 murine melanoma cells. Clin
Exp Metastasis. 25:225–231. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Kim OH, Kang GH, Noh H, Cha JY, Lee HJ,
Yoon JH, Mamura M, Nam JS, Lee DH, Kim YA, et al: Proangiogenic
TIE2(+)/CD31 (+) macrophages are the predominant population of
tumor-associated macrophages infiltrating metastatic lymph nodes.
Mol Cells. 36:432–438. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Yamada K, Uchiyama A, Uehara A, Perera B,
Ogino S, Yokoyama Y, Takeuchi Y, Udey MC, Ishikawa O and Motegi S:
MFG-E8 drives melanoma growth by stimulating mesenchymal stromal
cell-induced angiogenesis and M2 polarization of tumor-associated
macrophages. Cancer Res. 76:4283–4292. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Huber R, Meier B, Otsuka A, Fenini G,
Satoh T, Gehrke S, Widmer D, Levesque MP, Mangana J, Kerl K, et al:
Tumour hypoxia promotes melanoma growth and metastasis via high
mobility group box-1 and M2-like macrophages. Sci Rep. 6:299142016.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Tham M, Tan KW, Keeble J, Wang X, Hubert
S, Barron L, Tan NS, Kato M, Prevost-Blondel A, Angeli V and
Abastado JP: Melanoma-initiating cells exploit M2 macrophage TGFβ
and arginase pathway for survival and proliferation. Oncotarget.
5:12027–12042. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Chang CI, Liao JC and Kuo L: Macrophage
arginase promotes tumor cell growth and suppresses nitric
oxide-mediated tumor cytotoxicity. Cancer Res. 61:1100–1106.
2001.PubMed/NCBI
|
|
46
|
Clawson GA, Matters GL, Xin P,
Imamura-Kawasawa Y, Du Z, Thiboutot DM, Helm KF, Neves RI and
Abraham T: Macrophage-tumor cell fusions from peripheral blood of
melanoma patients. PLoS One. 10:e01343202015. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Somasundaram R and Herlyn D: Chemokines
and the microenvironment in neuroectodermal tumor-host interaction.
Semin Cancer Biol. 19:92–96. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Wang H and Zhang L, Yang L, Liu C, Zhang Q
and Zhang L: Targeting macrophage anti-tumor activity to suppress
melanoma progression. Oncotarget. 8:18486–18496. 2017.PubMed/NCBI
|
|
49
|
Qian Y, Qiao S, Dai Y, Xu G, Dai B, Lu L,
Yu X, Luo Q and Zhang Z: Molecular-targeted immunotherapeutic
strategy for melanoma via dual-targeting nanoparticles delivering
small interfering RNA to tumor-associated macrophages. ACS Nano.
11:9536–9549. 2017. View Article : Google Scholar : PubMed/NCBI
|















