Introduction
Renal cell carcinoma (RCC) is the third most common
urological neoplasm following prostate and bladder cancer, and
accounts for 2% of all cancer-associated mortality in the United
States of America (1). The disease is
comprised of >10 histological and molecular subtypes, of which
clear cell RCC (ccRCC) is the most common, accounting for ~70% of
all diagnosed cases (1,2). Prognosis is closely associated with the
stage of RCC at the point of diagnosis. The 5-year survival rate of
stage I RCC is ~95%, while that of stage IV is just 20% (2). Therefore, early detection of RCC is of
great importance for successful treatment. However, during early
stage RCC there are typically no clear symptoms, making diagnosis
difficult, and ~25–30% of patients with RCC are metastatic at
diagnosis (3). At present, no
standardized approaches to biomarker sampling or analysis have been
adopted for RCC since the majority of the putative tumor markers
themselves remain under active investigation for further validation
(4). Reliable biomarkers have not yet
been established for screening (2).
In addition, there are no data suggesting that adjuvant therapies
such as cytokines and vaccines were effective following RCC surgery
(5).
MicroRNAs (miRNAs/miRs) are short, non-coding single
stranded RNAs with 20–22 nucleotides that regulate the expression
of their target genes at the post-transcriptional level (6). An increasing body of evidence implicates
miRNAs in various aspects of tumorigenesis (1). In addition, certain miRNAs are
considered to be potential cancer biomarkers and anti-cancer
therapeutics (7). Previous studies
have demonstrated that several miRNAs are dysregulated in RCC, and
result in aberrant cell growth, angiogenesis, apoptosis and
autophagy (6,8–10).
Previous studies have demonstrated that miR-136-5p
is involved in a number of types of carcinoma, including glioma
(11), triple-negative breast cancer
(12) and non-small cell lung cancer
(NSCLC) (13). However, the function
of miR-136-5p in RCC remains to be explored. In the present study,
miR-136-5p was revealed to function as a tumor suppressor in RCC,
as it repressed migration, invasion, viability, proliferation and
promoted apoptosis in in vitro experiments.
Materials and methods
Sample collection
A total of 28 tumor samples and matched non-tumor
kidney tissues were obtained from Peking University Shenzhen
Hospital (Shenzhen, China) between January 2015 and January 2016.
The normal kidney tissue was obtained by sampling 2 cm away from
the tumor tissue. The specimens were frozen in liquid nitrogen
(−195.8°C) until RNA extraction. The present study was approved by
the local Ethics Committee of Peking University Shenzhen Hospital
(Shenzhen, China), and written informed consent was obtained from
all patients. The clinic pathological features of all patients are
listed in Table I.
 | Table I.Clinicopathological features of
patients with renal cell carcinoma. |
Table I.
Clinicopathological features of
patients with renal cell carcinoma.
| Characteristic | No. of
patients |
|---|
| Mean age, range
(years) | 45 (25–62) |
| Sex |
|
|
Male | 9 |
|
Female | 19 |
| Histological
type |
|
| Clear
cell | 24 |
|
Papillary | 4 |
| pT-stage |
|
| T1 | 19 |
| T2 | 7 |
|
T3+4 | 2 |
| Fuhrmann grade |
|
| I | 7 |
| II | 18 |
|
III | 2 |
| IV | 1 |
| AJCC clinical
stage |
|
| I | 8 |
| II | 18 |
| III +
IV | 2 |
RNA extraction and
reverse-transcription quantitative polymerase chain reaction
(RT-qPCR)
Total RNA was extracted from the RCC tissue and
adjacent tissue using TRIzol reagent (Invitrogen; Thermo Fisher
Scientific, Inc., Waltham, MA, USA) and was purified using the
RNeasy Maxi kit (Qiagen GmbH, Hilden, Germany) following the
manufacturer's protocols. The concentration of the RNA was measured
using a NanoDrop 2000/2000c spectrophotometer (NanoDrop
Technologies; Thermo Fisher Scientific, Inc., Pittsburgh, PA, USA).
The miScript Reverse Transcription kit (Qiagen GmbH) was used to
prepare cDNA from 1 µg RNA. The reverse transcription process
consisted of 37°C for 60 min, 95°C for 5 min and then storage at
4°C. To analyze the expression of miR-136-5p, qPCR was conducted
using the resultant cDNA with the miScript SYBR® Green
PCR kit (Qiagen GmbH) following the manufacturer's protocol, on the
Roche Light Cycler 480 Real-Time PCR System (Roche Diagnostics,
Basel, Switzerland). The thermocycler conditions were as follows:
95°C for 1 min, 40 cycles of 95°C for 15 sec, 55°C for 30 sec and
72°C for 30 sec. The sequences of the primers for miR-136-5p and
the internal control, U6, are presented in Table II. The 2−ΔΔCq method was
used to analyze the expression of miR-136-5p (14).
 | Table II.Sequences of primers and miRs. |
Table II.
Sequences of primers and miRs.
| Primer | Sequence
(5′-3′) |
|---|
| miR-136-5p |
|
|
Forward |
ACTCCATTTGTTTTGATGATGGA |
|
Reverse | Reverse provided by
the miScript SYBR® Green kit |
| U6 |
|
|
Forward |
CTCGCTTCGGCAGCACA |
|
Reverse |
ACGCTTCACGAATTTGCGT |
| miR-136-5p
mimics |
|
|
Forward |
ACUCCAUUUGUUUUGAUGAUGGA |
|
Reverse |
CAUCAUCAAAACAAAUGGAGUUU |
| NC |
|
|
Forward |
UUCUCCGAACGUGUCACGUTT |
|
Reverse |
ACGUGACACGUUCGGAGAATT |
| miR-136-5p
inhibitor |
UCCAUCAUCAAAACAAAUGGAGU |
| NC inhibitor |
CAGUACUUUUGUGUAGUACAA |
Cell culture and transfection
A normal human embryo kidney cell line (293T) and
two RCC cell lines (786-O and ACHN) were obtained from the
Guangdong and Shenzhen Key Laboratory of Male Reproductive Medicine
and Genetics (Shenzhen, China). Cells were cultured in Dulbecco's
modified Eagle's medium (DMEM; Gibco; Thermo Fisher Scientific,
Inc.) supplemented with 10% fetal bovine serum (FBS; Gibco; Thermo
Fisher Scientific, Inc.), 1% penicillin-streptomycin (Gibco; Thermo
Fisher Scientific, Inc.) and 1% glutamine (Gibco; Thermo Fisher
Scientific, Inc.) at 37°C in a 5% CO2 humidified
incubator. Transfection was performed with miR-136-5p mimics,
miR-136-5p inhibitors, negative control (NC) miRNA and inhibitor
negative control (inhibitor NC) miRNA (5 pmol; Shanghai GenePharma
Co., Ltd., Shanghai, China) using Lipofectamine 2000 reagent
(Invitrogen; Thermo Fisher Scientific, Inc.) according to the
manufacturer's protocol when cells were at 70% confluence. The
sequences of mimics, inhibitor, NC and inhibitor NC are presented
in Table II. Following transfection,
RT-qPCR was used to assess transfection efficiency and the
expression of miR-136-5p.
Cell Counting Kit-8 (CCK-8) assay
Cells (5×103) transfected with 5 pmol
miR-136-5p mimics, inhibitors, NC or inhibitor NC were seeded in
96-well plates and then incubated at 37°C. After 0, 24, 48 and 72
h, 10 µl CCK-8 reagent (Beyotime Institute of Biotechnology,
Haimen, China) was added into each well of a 96-well plate for 30
min at 37°C, and then the absorbance values of the experimental
wells were read at 490 nm.
MTT assay
Following transfection with 5 pmol miR-136-5p
mimics, NC, miR-136-5p inhibitors or inhibitor NC,
~5×103 cells were seeded into each well of a 96-well
plate and incubated for 4 days. Following addition of 20 µl MTT (5
mg/ml; Sigma-Aldrich; Merck KGaA, Darmstadt, Germany) to the plate,
the cells were incubated for a further 4 h at 37°C. The medium was
discarded, 100 µl DMSO was added into each well, and the plates
were shaken on a reciprocating decolorization shaking table
(TSB-108 Qilinbeier, Jiangsu, China; http://www.qilinbeier.cn/tsb-108.html) for 10 min in
the dark. Then, the absorbance values were read at 595 nm using an
ELISA microplate reader (680; Bio-Rad Laboratories, Inc., Hercules,
CA, USA).
Wound healing assay
Cells (5×105) were seeded into each well
of a 12-well plate, and incubated until they formed a monolayer of
~80% confluent cells. Cells were then transfected with 100 pmol
chemically synthesized miRNA-136-5p inhibitors, mimics, NC or
inhibitor NC, as aforementioned. A wound was created with a sterile
200 µl pipette tip, and floating cells were washed away using
phosphate-buffered saline (PBS). Cell images were taken at 0 and 12
h after making the scratch using a digital camera system, and the
temperature of incubation subsequent to making the scratch was
37°C.
Transwell assay
Transwell chamber inserts (BD Biosciences, Franklin
Lakes, NJ, USA) with or without Matrigel (for invasion assays) were
used. Serum-free medium (200 µl DMEM) containing ~1×104
transfected cells was seeded into upper chamber of the insert.
Medium mixed with 10% FBS was added to the lower chamber of the
inserts. 786-O cells and ACHN cells were incubated at 37°C for 36 h
for the migration assay, and were incubated for 48 and 60 h,
respectively, for the invasion assay. All non-migrated cells were
removed by a cotton swab. The cells that had migrated or invaded to
the other side of the membrane were stained with 0.1% crystal
violet for 25 min at 25°C and counted for in three fields of view
using a microscope (Leica DMIRB Inverted Fluorescence Microscope,
Leica Microsystems GmbH, Wetzlar, Germany).
Flow cytometry assay
Cells were seeded in a 6-well plate
(3×105 cells/well) and maintained in a humidified
incubator with 5% CO2 at 37°C until they formed an ~80%
confluent monolayer. Cells were then transfected with 200 pmol
miR-136-5p mimics, inhibitors, NC or inhibitor NC. Following
transfection for 48 h, cells were gathered and washed twice with
cold PBS. Then, the cells were re-suspended in 100 µl 1X binding
buffer (Alexa Fluor 488 Annexin V/Dead Cell Apoptosis kit,
Invitrogen; Thermo Fisher Scientific, Inc.) to create a single-cell
suspension. Cell suspension (50 µl), 5 µl Annexin V-fluorescein
isothiocyanate and 5 µl propidium iodide (Alexa Fluor 488 Annexin
V/Dead Cell Apoptosis kit) were mixed and then incubated in room
temperature for 15 min in the dark Following the addition of 400 µl
binding buffer, the samples were analyzed using a flow cytometer
(EPICS, Xl-4; Beckman Coulter, Inc., Brea, CA, USA), and the
software used for data analysis was FlowJo v 10 (TreeStar, Inc.,
Ashland, OR, USA).
Bioinformatics analysis
Potential targets of miR-136-5p were predicted
through the combination of four public algorithms, including
miRWalk (15) (www.umm.uni-heidelberg.de/apps/zmf/mirwalk), miRanda
(16) (www.microrna.org), PicTar (pictar.mdc-berlin.de) and TargetScan (17) (www.targetscan.org). All four algorithms accepted the
predicted genes, which were selected based on gene function.
Statistical analysis
Each experiment was performed in triplicate and
repeated at least three times. The data are presented as the mean ±
standard deviation. MiR-136 expression levels between groups were
analyzed using paired Student's t-tests, with the exception of
relative expression of miR-136-5p in cells as presented in Fig. 1, which was analyzed using one-way
analysis of variance followed by Dunnett's post hoc test. A paired
Student's t-test was also used to analyze the results of assays to
characterize cell phenotype. All statistical calculations were
performed using SPSS 19.0 software (IBM Corp., Armonk, NY, USA).
P<0.05 was considered to indicate a statistically significant
difference.
Results
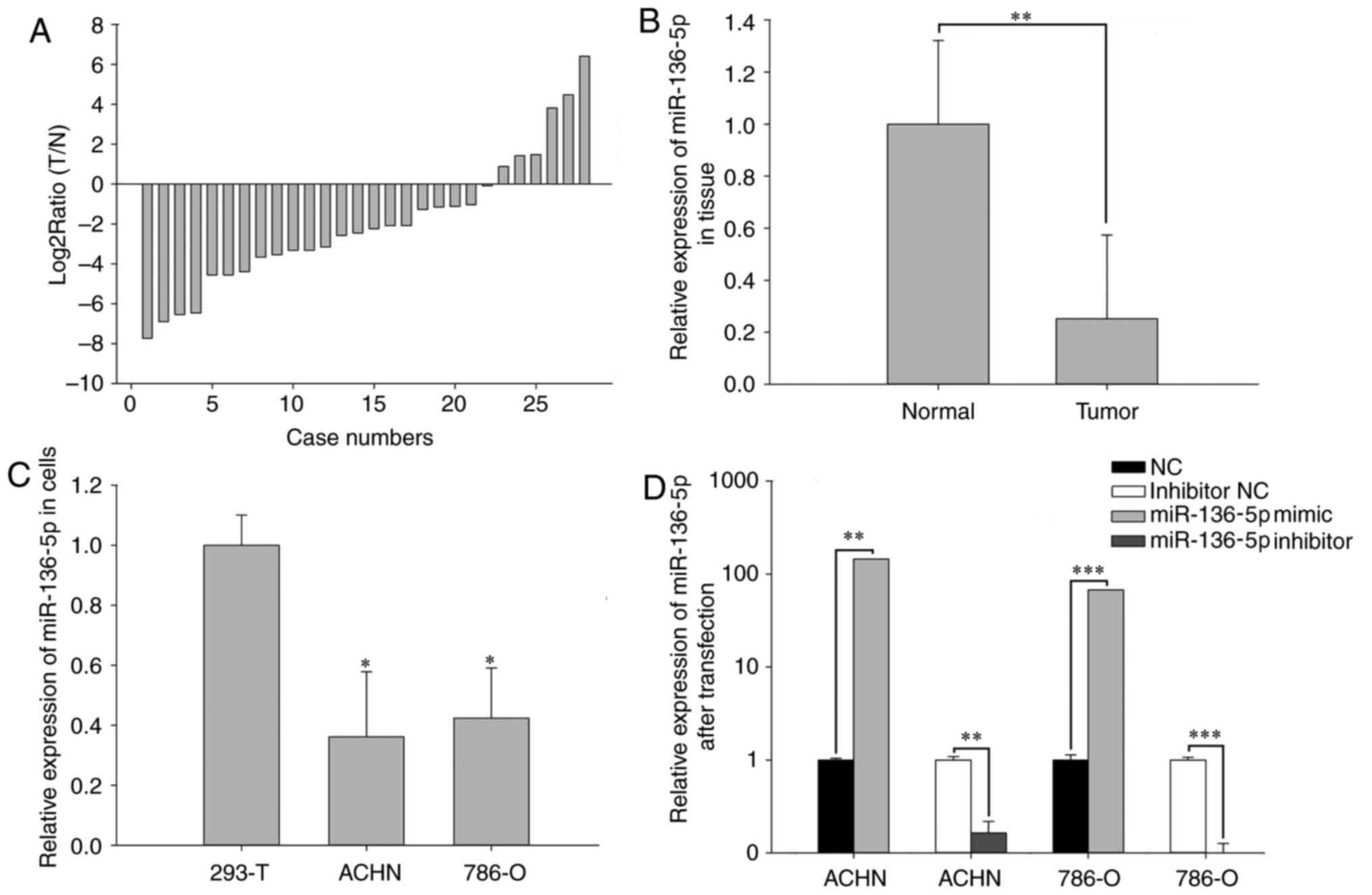
miR-136-5p is downregulated in RCC
tissues and cell lines
RT-qPCR was performed to examine the expression
levels of miR-136-5p in 28 paired RCC tissues and cell lines. As
presented in Fig. 1A, the ratio of
miR-136-5p expression [log2Ratio (T/N)] was revealed to
be downregulated in the RCC tissues from 22/28 patients. Mean
expression levels are presented in Fig.
1B, and revealed that the expression of miR-136-5p in RCC
tissues was significantly decreased compared with adjacent normal
tissues (P<0.01). Furthermore, miR-136-5p expression levels were
significantly decreased in ACHN (P<0.05) and 786-O (P<0.05)
RCC cell lines compared with the 293T normal kidney human embryo
kidney cell line, as presented in Fig.
1C.
Validation of cell transfection
efficiency
qPCR was performed to assess the transfection
efficiency of miR-136-5p mimics or NC and miR-136-5p inhibitors or
inhibitor NC. Compared with the NC group, the expression levels of
miR-136-5p were 145.00 times higher in ACHN cells (P<0.01) and
67.33 times higher in 786-O cells (P<0.001) transfected with
miR-136-5p. Compared with the inhibitor NC group, miR-136-5p
expression levels were 0.16 times that in ACHN cells (P<0.01)
and 0.09 times that in 786-O cells (P<0.001) following
transfection with miR-136-5p inhibitors (Fig. 1D).
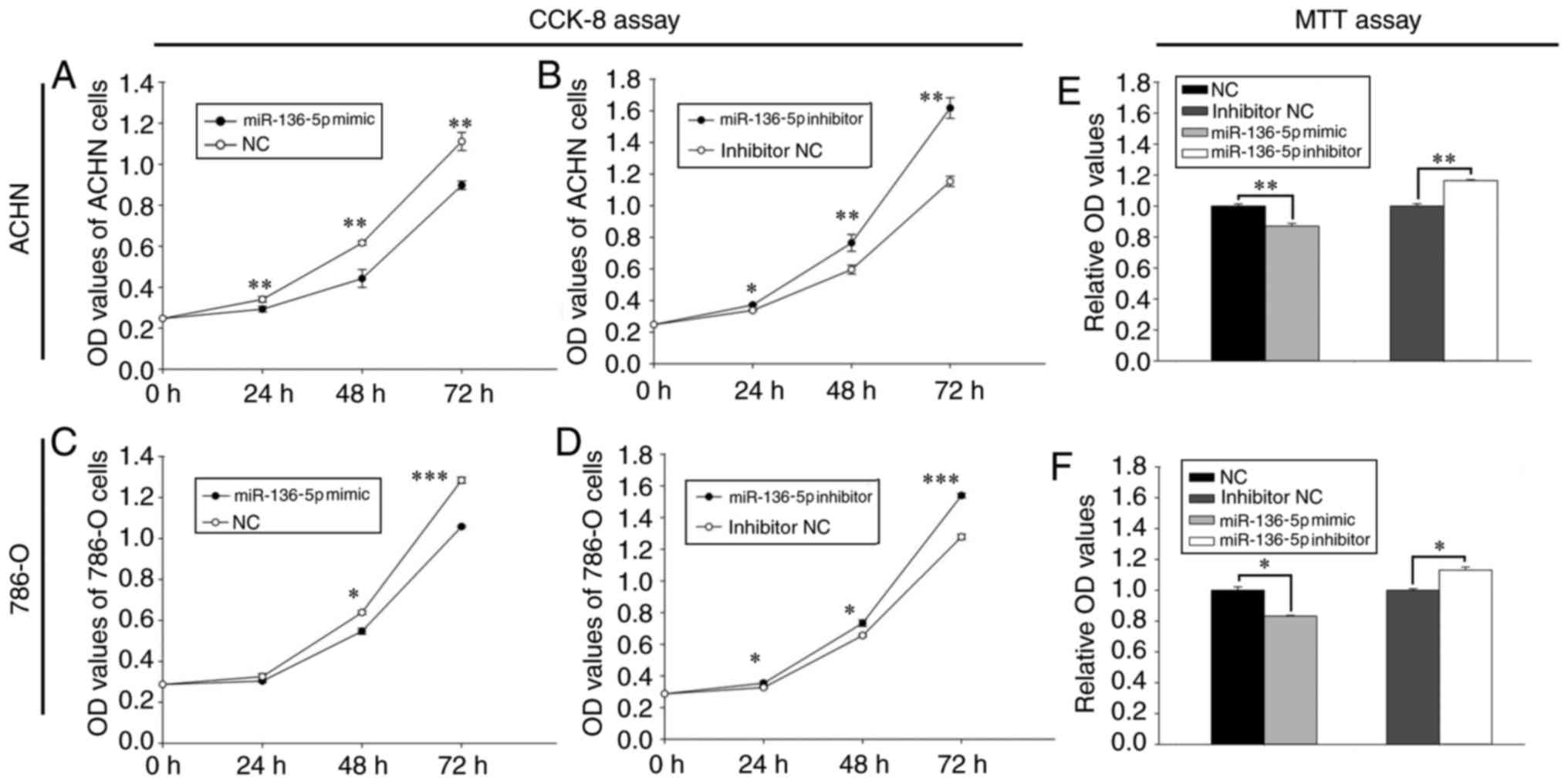
Upregulation of miR-136-5p inhibits
RCC cell proliferation and downregulation of miR-136-5p promotes
RCC cell proliferation
The effect of miR-136-5pon proliferation was
determined using a CCK-8 assay (Fig.
2). Following transfection with miR-136-5p mimics, the
proliferation of ACHN cells was decreased by 13.97% (24 h;
P<0.01), 28.15% (48 h; P<0.01) and 19.21% (72 h; P<0.01),
while that of 786-O cells was decreased by 6.67% (24 h), 14.35% (48
h; P<0.05) and 17.62% (72 h; P<0.001), respectively, compared
with the NC group (Fig. 2A and C).
Furthermore, following transfection with miR-136-5p inhibitors, the
proliferation of ACHN cells was increased by 10.15% (24 h;
P<0.05), 28.30% (48 h; P<0.01) and 40.19% (72 h; P<0.01),
while that of 786-O cells was increased by 8.29% (24 h; P<0.05),
11.97% (48 h; P<0.05) and 20.45% (72 h; P<0.001),
respectively, compared with the inhibitor NC group (Fig. 2B and D).
Upregulation of miR-136-5p inhibits
RCC cell viability and downregulation of miR-136 promotes RCC cell
viability
The effect of miR-136-5p on cell viability was
determined using an MTT assay. As presented in Fig. 2E, the viability of ACHN cells
transfected with the miR-136-5p mimic was reduced by 12.89%
(P<0.01) compared with the NC group, while the viability of
cells transfected with the miR-136-5p inhibitor was increased by
16.39% (P<0.01) compared with the inhibitor NC group. Similarly,
the viability of 786-O cells transfected with the miR-136-5p mimic
was decreased by 16.71% (P<0.05) compared with the NC group,
while the viability of cells transfected with the miR-136-5p
inhibitor was increased by 13.15% (P<0.05) compared with the
inhibitor NC group (Fig. 2F).
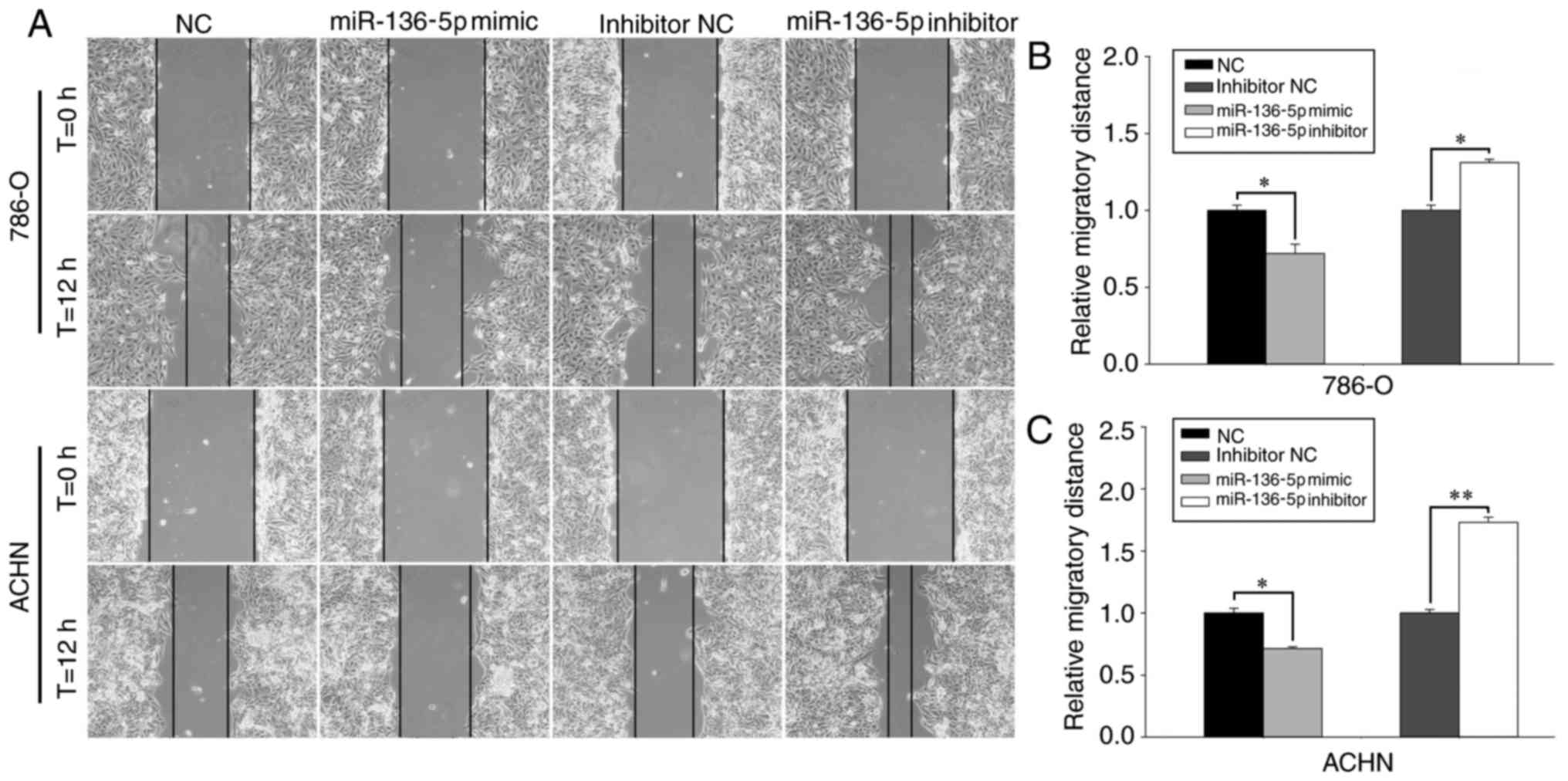
Upregulation of miR-136-5p inhibits
RCC cell migration and invasion, while downregulation of miR-136-5p
promotes RCC cell migration and invasion
The effect of miR-136-5p on RCC cell mobility was
determined using wound healing assays and Transwell assays
(Figs. 3 and 4). Representative images of the wound
healing assay are presented in Fig.
3A. The wound healing assay revealed that, compared with the NC
group, the migration of the mimic group was reduced by 28.53%
(P<0.05; Fig. 3C) in ACHN cells
and by 27.99% (P<0.05; Fig. 3B) in
786-O cells 12 h following the initial scratch. In contrast,
compared with the inhibitor NC group, the migration of the
inhibitor group was increased by 73.14% (P<0.01; Fig. 3C) in ACHN cells and by 31.08%
(P<0.05; Fig. 3B) in 786-O cells
12 h following the initial scratch.
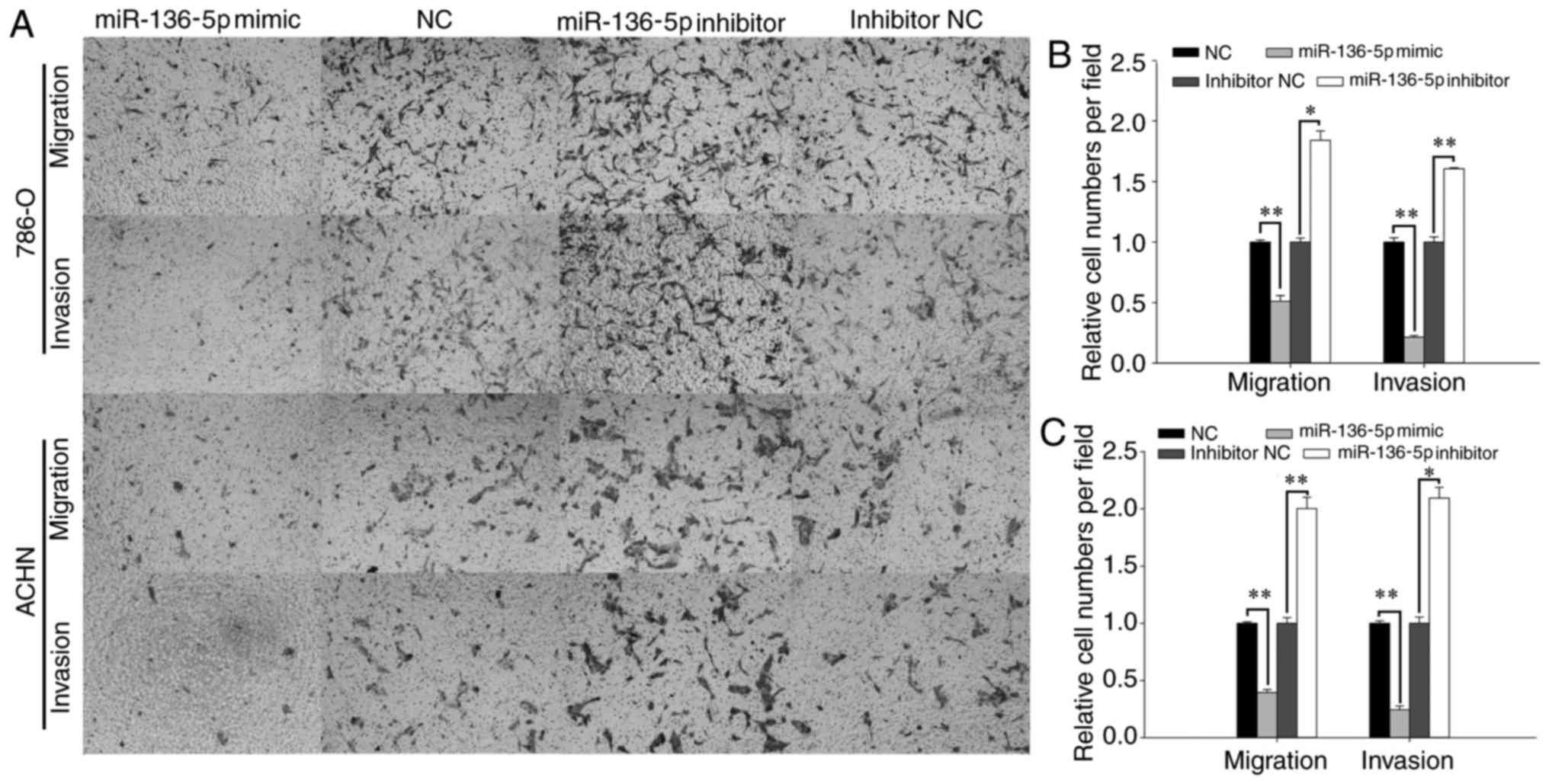
As presented in Fig.
4C, the Transwell assay revealed that, following transfection
with miR-136-5p mimics, ACHN cell migration was reduced by 60.34%
compared with the NC group (P<0.01), while the cells transfected
with miR-136-5p inhibitors was increased by 100.26% compared with
the inhibitor NC group (P<0.01). The invasion of ACHN cells
transfected with miR-136-5p mimics was reduced by 75.52% compared
with the NC group (P<0.01), while the invasion of cells
transfected with miR-136-5p inhibitors was increased by 109.52%
compared with the inhibitor NC group (P<0.05; Fig. 4C). Similarly, the migration of 786-O
cells transfected with miR-136-5p mimics was reduced by 49.02%
compared with the NC group (P<0.01), while the migration of
cells transfected with miR-136-5p inhibitor was increased by 84.09%
compared with the inhibitor NC group (P<0.05; Fig. 4B). The invasion of 786-O cells
transfected with miR-136-5p mimics was reduced by 78.41% compared
with the NC group (P<0.01), while the cells transfected with
miR-136-5p inhibitors was promoted by 60.63% compared with the
inhibitor NC group (P<0.01; Fig.
4B).
Upregulation of miR-136-5p induces
apoptosis and downregulation of miR-136-5p suppresses cell
apoptosis
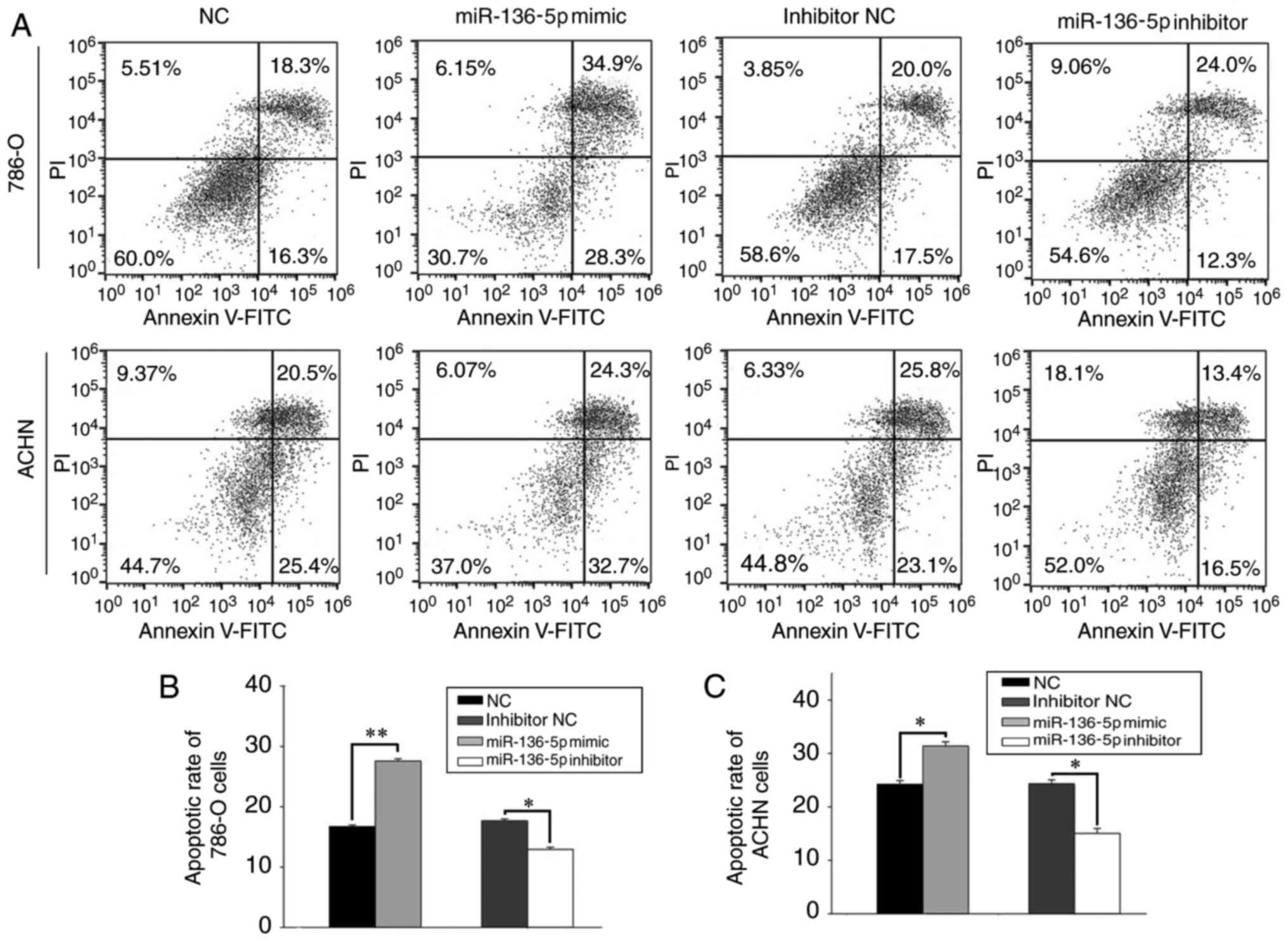
Flow cytometry was used to assess the effect of
miR-136-5p on apoptosis (Fig. 5A).
The results revealed that the early apoptosis rate of 786-O cells
transfected with miR-136-5p mimics and NC was 27.56±0.40 and
16.73±0.26%, respectively (P<0.01; Fig. 5B), while the early apoptosis rate of
786-O cells transfected with miR-136-5p inhibitors and inhibitor NC
was 12.93±0.34 and 17.66±0.32%, respectively (P<0.05; Fig. 5B). Similarly, the early apoptosis rate
of ACHN cells transfected with miR-136-5p mimics and NC was
31.4±0.78 and 24.26±0.66%, respectively (P<0.05; Fig. 5C), while the early apoptosis rate of
ACHN cells transfected with miR-136-5p inhibitors and inhibitor NC
was 15.03±0.93 and 24.33±0.72%, respectively (P<0.05; Fig. 5C).
Target gene prediction
Four algorithms were combined to predict the
putative target genes of miR-136-5p. All four algorithms
simultaneously predicted that dedicator of cytokinesis 5 (DOCK5)
was a potential target. The complementary site for the seed
sequences of miR-136-5p was 5′-AAUGGAGA-3′ in the DOCK5
3′-untranslated region.
Discussion
MiRNAs may be critical in the development,
proliferation, communication and death of cells, as well as in
tissue differentiation (18).
Emerging evidence suggests that miRNAs are involved in tumor
development and progression (19). In
the present study, miR-136-5p was revealed to be downregulated in
RCC tissues and cell lines compared with adjacent non-tumor tissues
and cells in vitro. Furthermore, cell proliferation,
migration and invasion were demonstrated to be suppressed following
upregulation of miR-136-5p. Apoptosis was also induced by
upregulation of miR-136-5p.
Previous studies have provided evidence that
miR-136-5p serves either an oncogenic or a tumor-suppressing
function in the development of carcinomas. For example, in NSCLC,
expression of miR-136-5p promoted cell proliferation by promoting
extracellular signal-regulated kinase (ERK)1/2 phosphorylation via
the targeting of protein phosphatase 2 regulatory subunit Bα
(PPP2R2A) (13). This result supports
the hypothesis that miR-136 acts as an oncogene in NSCLC.
In contrast, Jeong et al (11) demonstrated that miR-136-5p targeted
the Notch3 oncogene and functions as an ovarian cancer suppressor
(11). miR-136-5p was also reported
to be downregulated in triple-negative breast cancer by targeting
RAS protein activator like 2, and act as a tumor suppressor
(12). Yang et al (20) also described miR-136-5p as a tumor
suppressor in lung adenocarcinoma, and revealed that this occurred
through the targeting of SMAD family member (Smad)2 and Smad3. In a
previous study, miR-136-5p was revealed to be downregulated in
metastatic giant cell bone tumors compared to non-metastatic giant
cell bone tumors, by promoting nuclear factor I B expression
(21). In another study concerning
glioma, miR-136-5p was revealed to serve a tumor-suppressive
function in human glioma by upregulating the expression of
astrocyte elevated gene-1 and B cell lymphoma-2 (BCL2) (22). In another study, overexpression of
miR-136-5p negatively impacted proliferation of the LN229
glioblastoma cell line by downregulating matricellular cysteine
rich angiogenic inducer 61 protein expression (23). miR-136-5p was also reported to be
serve as an anti-oncogene in epithelial ovarian cancer (24). Gao et al (25) demonstrated that colorectal neoplasia
differentially expressed is a target of miR-136-5p in colorectal
cancer cells, and high levels of miR-136-5p may inhibit the
migration and invasion of colorectal cancer cells. Consistent with
these results, the present study demonstrated that transfection
with miR-136-5p mimics inhibited proliferation, invasion, and
migration and induced apoptosis in the 780-O and ACHN cell lines,
supporting the hypothesis that miR-136-5p is a tumor-suppressor in
RCC. Together, these data indicate that the biological function of
miR-136 is involved in the tumorigenesis and progression of various
types of human cancer.
In addition, miRNAs may be used as biomarkers to
improve our knowledge on diagnosis, prognosis and drug resistance,
and may be used as therapeutic approaches in certain types of
cancer. For example, miR-136-5p may be a potential biomarker for
ccRCC (26). Overexpression of
miR-136-5p may be associated with poor prognosis in giant cell bone
tumors (18). Wu et al
(27) reported that miR-136-5p
functions as a predictor of the response to temozolomide therapy,
and serves as a novel potential maker for glioma therapy. Chen
et al (28) demonstrated that
miR-136-5p was associated with cisplatin resistance and functions
as a tumor suppressor in glioma. miR-136-5p has also been reported
to inhibit cancer stem cell activity and increase the anti-tumor
effect of paclitaxel on ovarian cancer chemotherapy tolerance
(11). Zhao et al (29) reported that miR-136-5p may have
therapeutic potential in hepatitis B virus-associated
hepatocellular carcinoma. Therefore, miR-136-5p has the potential
to be a diagnostic or a prognostic biomarker for RCC, and this
should be confirmed by further research.
In addition to being associated with tumors,
miR-136-5p is also associated with multiple non-neoplastic
diseases. Ji et al (30)
demonstrated that high level of miR-136-5p suppressed cell
proliferation and promoted apoptosis of mesenchymal stem cells
through targeting BCL2, which is a potential causal factor of
preeclampsia. Zhang et al (31) demonstrated that miR-136-5p is involved
in keratinocyte growth through targeting PPP2R2A, and may be a
novel treatment target for the improvement of skin wound healing
(31). Aberrant miR-136-5p
upregulation in atherosclerosis contributes to abnormal vascular
smooth muscle cell proliferation via the ERK1/2 signaling pathway
by targeting PPP2R2A (32).
The mechanism underlying the effect of miR-136-5p in
RCC requires further exploration, and improved understanding of the
cellular function of ectopic miRNAs provides novel insights into
RCC (33). In the present study,
miR-136-5p was demonstrated to functions as a tumor suppressor in
RCC, and may consequently serve as a therapeutic target for
RCC.
In conclusion, the present study demonstrated that
miR-136-5p served as a tumor suppressor, inhibited growth,
viability, migration, invasion and induced apoptosis of RCC cells.
Therefore, it may be associated with the development and
progression of RCC. In addition, these results underscore the
clinical potential of miR-136-5p in RCC treatment, and support the
development of effective therapeutic strategies that target
miR-136-5p.
Acknowledgements
The present study was supported by the National
Natural Science Foundation of China (grant no. 81101922), the
Science and Technology Development Fund Project of Shenzhen (grant
nos. JCYJ20150403091443329 and JCYJ20170307111334308), the San-ming
Project of Medicine in Shenzhen (grant no. SZSM201612066) and the
Guangdong Key Medical Subject Fund.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Wang Z, Qin C, Zhang J, Han Z, Tao J, Cao
Q, Zhou W, Xu Z, Zhao C, Tan R and Gu M: MiR-122 promotes renal
cancer cell proliferation by targeting Sprouty 2. Tumour Biol.
39:10104283176911842017.PubMed/NCBI
|
|
2
|
Hsieh JJ, Purdue MP, Signoretti S, Swanton
C, Albiges L, Schmidinger M, Heng DY, Larkin J and Ficarra V: Renal
cell carcinoma. Nat Rev Dis Primers. 3:170092017. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Bharthuar A, Pandey H and Sood S:
Management of metastatic renal cell carcinoma-mini review. J Kidney
Cancer VHL. 2:75–83. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Teixeira AL, Dias F, Gomes M, Fernandes M
and Medeiros R: Circulating biomarkers in renal cell carcinoma: The
link between microRNAs and extracellular vesicles, where are we
now? J Kidney Cancer VHL. 1:84–98. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Ljungberg B, Bensalah K, Canfield S,
Dabestani S, Hofmann F, Hora M, Kuczyk MA, Lam T, Marconi L,
Merseburger AS, et al: EAU guidelines on renal cell carcinoma: 2014
update. Eur Urol. 67:913–924. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Aguiari G: MicroRNAs in clear cell renal
cell carcinoma: Biological functions and applications. J Kidney
Cancer VHL. 2:140–152. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ling H, Girnita L, Buda O and Calin GA:
Non-coding RNAs: The cancer genome dark matter that matters! Clin
Chem Lab Med. 55:1–714. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Liang T, Hu XY, Li YH, Tian BQ, Li ZW and
Fu Q: MicroRNA-21 regulates the proliferation, differentiation, and
apoptosis of human renal cell carcinoma cells by the mTOR-STAT3
signaling pathway. Oncol Res. 24:371–380. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Xiao H, Xiao W, Cao J, Li H, Guan W, Guo
X, Chen K, Zheng T, Ye Z, Wang J and Xu H: miR-206 functions as a
novel cell cycle regulator and tumor suppressor in clear-cell renal
cell carcinoma. Cancer Lett. 374:107–116. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Liu F, Chen N, Xiao R, Wang W and Pan Z:
miR-144-3p serves as a tumor suppressor for renal cell carcinoma
and inhibits its invasion and metastasis by targeting MAP3K8.
Biochem Biophys Res Commun. 480:87–93. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Jeong JY, Kang H, Kim TH, Kim G, Heo JH,
Kwon AY, Kim S, Jung SG and An HJ: MicroRNA-136 inhibits cancer
stem cell activity and enhances the anti-tumor effect of paclitaxel
against chemoresistant ovarian cancer cells by targeting Notch3.
Cancer Lett. 386:168–178. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Yan M, Li X, Tong D, Han C, Zhao R, He Y
and Jin X: miR-136 suppresses tumor invasion and metastasis by
targeting RASAL2 in triple-negative breast cancer. Oncol Rep.
36:65–71. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Shen S, Yue H, Li Y, Qin J, Li K, Liu Y
and Wang J: Upregulation of miR-136 in human non-small cell lung
cancer cells promotes Erk1/2 activation by targeting PPP2R2A.
Tumour Biol. 35:631–640. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Dweep H, Sticht C, Pandey P and Gretz N:
miRWalk-database: Prediction of possible miRNA binding sites by
‘walking’ the genes of three genomes. J Biomed Inform. 44:839–847.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Enright AJ, John B, Gaul U, Tuschl T,
Sander C and Marks DS: MicroRNA targets in Drosophila. Genome Biol.
5:R12003. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Lewis BP, Burge CB and Bartel DP:
Conserved seed pairing, often flanked by adenosines, indicates that
thousands of human genes are microRNA targets. Cell. 120:15–20.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kotlabova K, Doucha J and Hromadnikova I:
Placental-specific microRNA in maternal circulation-identification
of appropriate pregnancy-associated microRNAs with diagnostic
potential. J Reprod Immunol. 89:185–191. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Tusong H, Maolakuerban N, Guan J, Rexiati
M, Wang WG, Azhati B, Nuerrula Y and Wang YJ: Functional analysis
of serum microRNAs miR-21 and miR-106a in renal cell carcinoma.
Cancer Biomarker. 18:79–85. 2017. View Article : Google Scholar
|
|
20
|
Yang Y, Liu L, Cai J, Wu J, Guan H, Zhu X,
Yuan J, Chen S and Li M: Targeting Smad2 and Smad3 by miR-136
suppresses metastasis-associated traits of lung adenocarcinoma
cells. Oncol Res. 21:345–352. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Mosakhani N, Pazzaglia L, Benassi MS,
Borze I, Quattrini I, Picci P and Knuutila S: MicroRNA expression
profiles in metastatic and non-metastatic giant cell tumor of bone.
Histol Histopathol. 28:671–678. 2013.PubMed/NCBI
|
|
22
|
Yang Y, Wu J, Guan H, Cai J, Fang L, Li J
and Li M: MiR-136 promotes apoptosis of glioma cells by targeting
AEG-1 and Bcl-2. FEBS Lett. 586:3608–3612. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Jeansonne D, Pacifici M, Lassak A, Reiss
K, Russo G, Zabaleta J and Peruzzi F: Differential effects of
MicroRNAs on glioblastoma growth and migration. Genes (Basel).
4:46–64. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Zhao H, Liu S, Wang G, Wu X, Ding Y, Guo
G, Jiang J and Cui S: Expression of miR-136 is associated with the
primary cisplatin resistance of human epithelial ovarian cancer.
Oncol Rep. 33:591–598. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Gao H, Song X, Kang T, Yan B, Feng L, Gao
L, Ai L, Liu X, Yu J and Li H: Long noncoding RNA CRNDE functions
as a competing endogenous RNA to promote metastasis and oxaliplatin
resistance by sponging miR-136 in colorectal cancer. Onco Targets
Ther. 10:205–216. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Hao JF, Ren KM, Bai JX, Wang SN, Shao B,
Cao N and Li X: Identification of potential biomarkers for clear
cell renal cell carcinoma based on microRNA-mRNA pathway
relationships. J Cancer Res Ther. 10 Suppl:C167–C177. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Wu H, Liu Q, Cai T, Chen YD, Liao F and
Wang ZF: MiR-136 modulates glioma cell sensitivity to temozolomide
by targeting astrocyte elevated gene-1. Diagn Pathol. 9:1732014.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Chen W, Yang Y, Chen B, Lu P, Zhan L, Yu
Q, Cao K and Li Q: MiR-136 targets E2F1 to reverse cisplatin
chemosensitivity in glioma cells. J Neurooncol. 120:43–53. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Zhao J, Wang W, Huang Y, Wu J, Chen M, Cui
P, Zhang W and Zhang Y: HBx elevates oncoprotein AEG-1 expression
to promote cell migration by downregulating miR-375 and miR-136 in
malignant hepatocytes. DNA Cell Biol. 33:715–722. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Ji L, Zhang L, Li Y, Guo L, Cao N, Bai Z,
Song Y, Xu Z, Zhang J, Liu C and Ma X: MiR-136 contributes to
pre-eclampsia through its effects on apoptosis and angiogenesis of
mesenchymal stem cells. Placenta. 50:102–109. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Zhang D, Wang J, Wang Z, Zhang T, Shi P,
Wang X, Zhao F, Liu X, Lin X and Pang X: miR-136 modulates
TGF-beta1-induced proliferation arrest by targeting PPP2R2A in
keratinocytes. Biomed Res Int. 2015:4535182015.PubMed/NCBI
|
|
32
|
Zhang CF, Kang K, Li XM and Xie BD:
MicroRNA-136 promotes vascular muscle cell proliferation through
the ERK1/2 pathway by targeting PPP2R2A in atherosclerosis. Curr
Vasc Pharmacol. 13:405–412. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Zhang B, Pan X, Cobb GP and Anderson TA:
microRNAs as oncogenes and tumor suppressors. Dev Biol. 302:1–12.
2007. View Article : Google Scholar : PubMed/NCBI
|