Introduction
Cavernous angioma (CA) is an uncommon benign
vascular malformation, consisting of dilated thin-walled sinusoidal
vascular spaces lacking intervening nervous tissue (1). These lesions are usually located in the
intracranial structure (2). In the
spine, CA predominantly affects the vertebral bodies with or
without epidural space extension (3).
Solitary epidural CAs not originating from the vertebral bone are
relatively rare. With the aid of magnetic resonance imaging (MRI),
solitary epidural CAs are being discovered in increasing numbers;
nevertheless, most previous studies are case reports with an
associated literature review and large series studies are very
limited in the literature (2–17). Because of the risk of spontaneous and
intraoperative bleeding, solitary epidural CAs need to get
neurosurgeons more attention. In this series, we present the data
of 12 patients with intraoperatively and histologically proven
solitary epidural CAs from a single center. The clinical
presentations, radiological features and surgical outcomes are
presented and discussed.
Materials and methods
Between April 2011 to August 2017, 12 patients with
solitary epidural CAs underwent microsurgery in Department of
Neurosurgery, Anhui Province Hospital. A patient was included in
the study based on the following criteria: i) a solitary epidural
lesion present on MRI; ii) intraoperative confirmation of a
solitary epidural lesion; and iii) a postoperative pathological
diagnosis of CA. Clinical data were collected with institutional
review board approval. Surgery was performed in all cases through
posterior approach. Histological specimens were sent to the
Department of Pathology for histological confirmation. Follow-up
data for all patients were obtained during individual office visits
or telephone interviews. Modified Japanese Orthopedic Association
(JOA) scores (Table I) were applied
to assess neurological function (18).
 | Table I.Modified Japanese Orthopedic
Association scale (18). |
Table I.
Modified Japanese Orthopedic
Association scale (18).
| Section | Score (points) |
|---|
| Motor function of
upper extremity |
|
| Unable to
feed oneself | 0 |
| Unable to
use knife and fork; able to | 1 |
| eat with
a spoon |
|
| Able to
use knife and fork with much difficulty | 2 |
| Able to
use knife and fork with slight difficulty | 3 |
|
Normal | 4 |
| Motor function of
lower extremity |
|
| Unable to
walk | 0 |
| Can walk
on flat floor with walking aid | 1 |
| Can walk
up and/or down stairs with handrail | 2 |
| Lack of
stability and smooth gait | 3 |
|
Normal | 4 |
| Sensory function of
upper extremity |
|
| Severe
sensory loss or pain | 0 |
| Mild
sensory loss | 1 |
|
Normal | 2 |
| Sensory function of
lower extremity |
|
| Severe
sensory loss or pain | 0 |
| Mild
sensory loss | 1 |
|
Normal | 2 |
| Sensory function of
trunk extremity |
|
| Severe
sensory loss or pain | 0 |
| Mild
sensory loss | 1 |
|
Normal | 2 |
| Bladder function |
|
| Unable to
void | 0 |
| Marked
difficulty in micturition (retension) | 1 |
|
Difficulty in micturition
(frequency, hesitation) | 2 |
|
Normal | 3 |
Results
Clinical presentations
The patients were 7 females and 5 males with the
mean age of 52.1 years (range, 25–73 years). The mean duration of
symptoms was 8.1 months (range, 12 h-2 years). The clinical course
showed two patterns: A chronically progressive course (n=10), and
an acute onset (n=2). The presentations included anesthesia (11
cases, 92.7%), motor deficit (9 cases, 75%), pain (3 cases, 25%),
and sphincter dysfunction (2 cases, 16.7%). The preoperative JOA
score was 10.5±1.75 (range, 7–13). The detailed clinical profiles
are summarized in Table II.
 | Table II.Characteristics of 12 patients with
solitary spinal epidural cavernous angiomas. |
Table II.
Characteristics of 12 patients with
solitary spinal epidural cavernous angiomas.
|
|
|
|
|
|
|
|
|
| Modified JOA
scores |
|
|---|
|
|
|
|
|
|
|
|
|
|
|
|
|---|
| Case no. | Age
(years)/sex | Clinical symptom
and sign | Duration of
illness | Level | Emergency
surgery | Extent of
resection | Surgical
method | Blood loss
(ml) | Pre- | Post- | Last-FU | FU (months) |
|---|
| 1 | 57/F | Paralysis | 1 month | T3-4 | No | GTR | Piecemeal
resection | 300 | 10 | 14 | 15 | 76 |
| 2 | 43/M | Anesthesia;
paralysis | 20 months | T3-6 | No | GTR | En bloc
resection | 200 | 10 | 12 | 15 | 67 |
| 3 | 61/F | Low back pain;
radiating pain anesthesia; paralysis | 6 months | C7-T1 | No | GTR | Piecemeal
resection | 400 | 11 | 14 | 16 | 65 |
| 4 | 49/M | Anesthesia;
paralysis | 8 months | T7-8 | No | GTR | En bloc
resection | 100 | 11 | 14 | 16 | 56 |
| 5 | 49/F | Anesthesia;
paralysis | 6 months | T1-3 | No | GTR | En bloc
resection | 200 | 9 | 12 | 15 | 50 |
| 6 | 49/F | Anesthesia;
paralysis; sphincter disturbances | 8 days | T1-3 | Yes | GTR | En bloc
resection | 100 | 7 | 7 | 7 | 47 |
| 7 | 73/M | Radiating pain;
anesthesia | 2 months | L3-4 | No | GTR | Piecemeal
resection | 300 | 12 | 15 | 15 | 16 |
| 8 | 45/F | Anesthesia | 6 months | T2-3 | No | STR | Piecemeal
resection | 500 | 12 | 15 | 16 | 16 |
| 9 | 47/M | Anesthesia;
paralysis; sphincter disturbances | 6 h | T1-2 | Yes | GTR | En bloc
resection | 100 | 7 | 7 | 13 | 12 |
| 10 | 25/M | Radiating pain;
anesthesia | 2 years | C7-T1 | No | GTR | En bloc
resection | 200 | 13 | 11 | 15 | 11 |
| 11 | 65/F | Anesthesia;
paralysis; | 1 year | T1-3 | No | GTR | En bloc
resection | 200 | 12 | 14 | 15 | 11 |
| 12 | 62/F | Anesthesia;
paralysis; | 1 year | T4-5 | No | GTR | Piecemeal
resection | 400 | 12 | 14 | 15 | 4 |
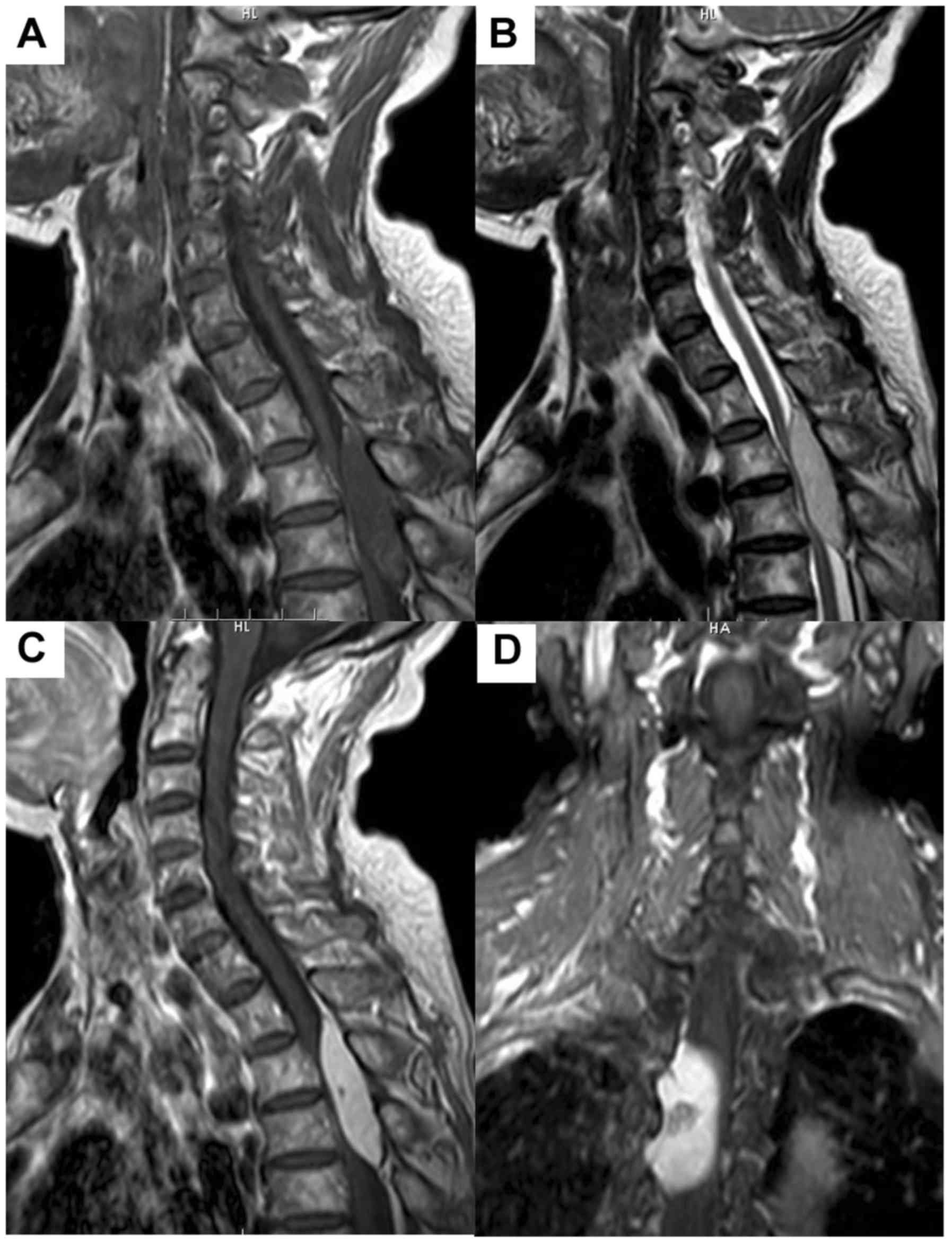
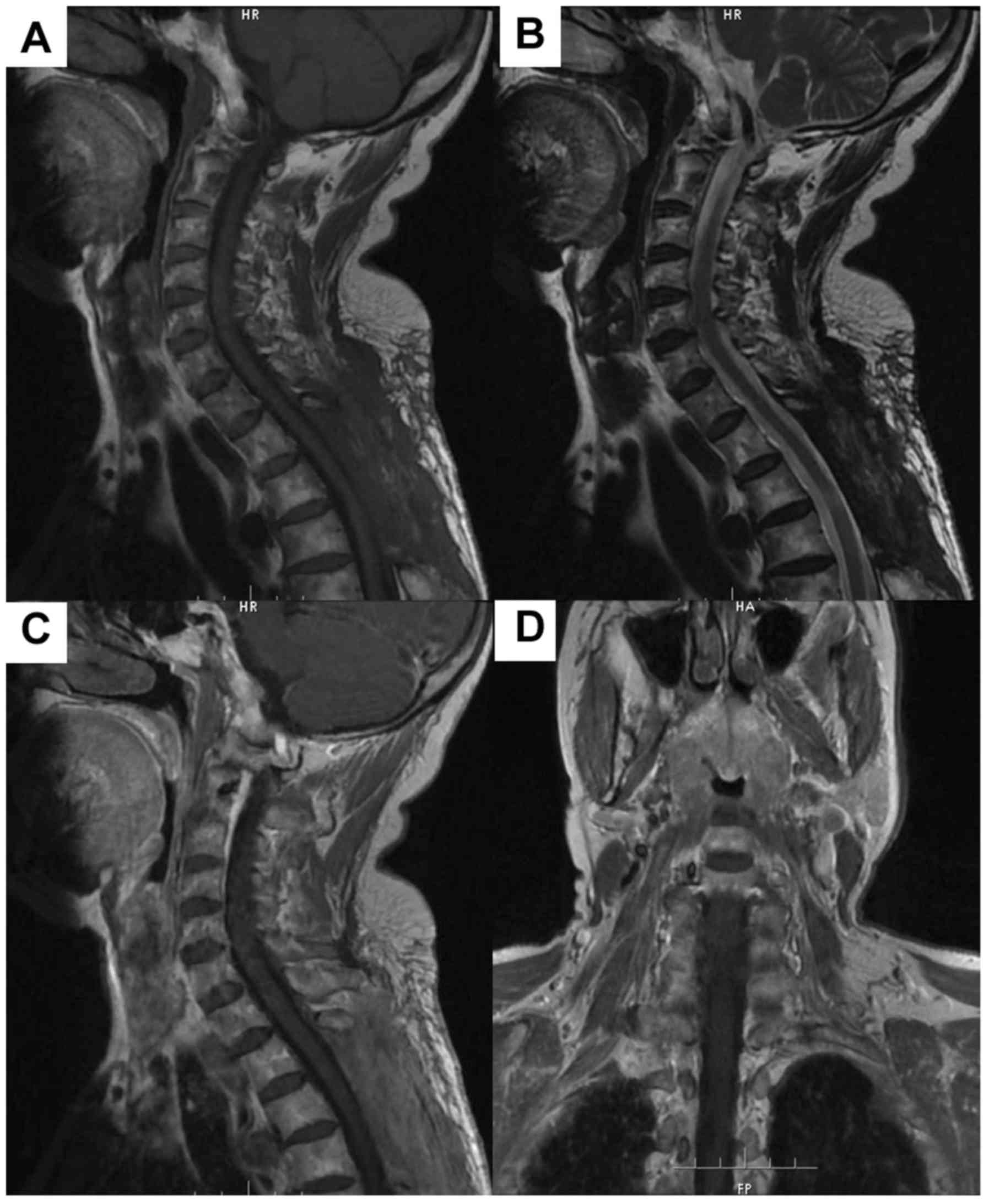
Radiological features
CAs were located in cervicothoracic (1 case, 8.3%),
thoracic (9 cases, 75%), and lumbar (2 cases, 16.7%) spine. The
lesion was isointense in 10 cases, and hypo-and isointense in 2
cases on T1-weighted images (WI). The T2-WI revealed the lesion was
hyperintense in 10 cases, iso- to hyperintense in 2 cases. No
lesion was surrounded by a hypointense hemosiderin ring on T2-WI.
Contrast-enhanced T1-WI revealed homogeneous markedly enhancement
in 9 cases and heterogeneous markedly enhancement in 3 cases. Eight
lesions were located in the dorsal spinal canal and 4 were in the
lateral canal. In 6 cases, the lesion extended into the
intervertebral foramen, and none had extended to the paravertebral
space. Widening of intervertebral foramen was not seen in the 6
cases. According to the preoperative MRI, only one case was
diagnosed as CA. The differential diagnosis included schwannomas (6
cases, 50%), meningiomas (3 cases, 25%) and epidural hemangioma (2
cases, 16.7%). The detailed radiological features are summarized in
Table III. An illustrative example
of case 11 is illustrated in Figs. 1
and 2.
 | Table III.Magnetic resonance imaging of 12
patients with solitary spinal epidural cavernous angiomas. |
Table III.
Magnetic resonance imaging of 12
patients with solitary spinal epidural cavernous angiomas.
|
|
| MRI findings |
|
|
|
|---|
|
|
|
|
|
|
|
|---|
| Case | Position | T1-WI | T2-WI | +GA | Hypointense
hemosiderin ring | Intervertebral
foramen extension | Preoperative
diagnosis |
|---|
| 1 | Left | Isointense | Hyperintense | Heterogeneous;
markedly | No | Yes | Schwannoma |
| 2 | Dorsal | Isointense | Hyperintense | Homogeneous;
markedly | No | No | Cavernous
angiomas |
| 3 | Dorsal | Isointense | Hyperintense | Homogeneous;
markedly | No | Yes | Schwannoma |
| 4 | Dorsal | Isointense | Hyperintense | Homogeneous;
markedly | No | No | Meningioma |
| 5 | Dorsal | Isointense | Hyperintense | Homogeneous;
markedly | No | No | Meningioma |
| 6 | Dorsal |
Hypo-isorintense | Hyperintense | Heterogeneous;
markedly | No | No | Hematoma |
| 7 | Left | Isointense | Hyperintense | Homogeneous;
markedly | No | Yes | Schwannoma |
| 8 | Right | Isointense | Hyperintense | Homogeneous;
markedly | No | Yes | Schwannoma |
| 9 | Dorsal |
Hypo-isorintense | Hyperintense | Heterogeneous;
markedly | No | No | Hematoma |
| 10 | Right | Isointense | Hypo- and
hyperintense | Homogeneous;
markedly | No | Yes | Schwannoma |
| 11 | Dorsal | Isointense | Hyperintense | Homogeneous;
markedly | No | Yes | Schwannoma |
| 12 | Dorsal | Isointense | Hyperintense | Homogeneous;
markedly | No | No | Meningioma |
Surgical outcomes and pathological
findings
After admission to the neurosurgical department, 2
patients with acute onset were treated surgically within 3 h. All
lesions were resected through the posterior approach using an
operative microscope. Intraoperatively, the lesions were
purple-reddish, lobulated or nodular in shape, and soft in texture.
The lesions were highly vascular and easily bloody when touching.
In 6 cases, the lesions extended into adjacent neural foramen and
nerve roots. Most lesions were well demarcated from the dura and
facilitated their exposure and dissection. Gross total resection
(GTR) was achieved in 11 cases (91.6%), and subtotal resection
(STR) was achieved in one case (8.3%). En bloc resection was
performed in 7 cases (5 cases without intervertebralforamen
extension, 2 cases with intervertebralforamen extension). Piecemeal
resection was achieved in 5 cases (4 cases with
intervertebralforamen extension, 1 case without
intervertebralforamen extension). Blood loss during en bloc
resection was 157.14±49.49 ml (range, 100–200 ml) and that during
piecemeal resection was 380±82.46 ml (range, 300–500 ml). The blood
loss during en bloc resection was significantly less (P<0.05)
than that in piecemeal resection group.
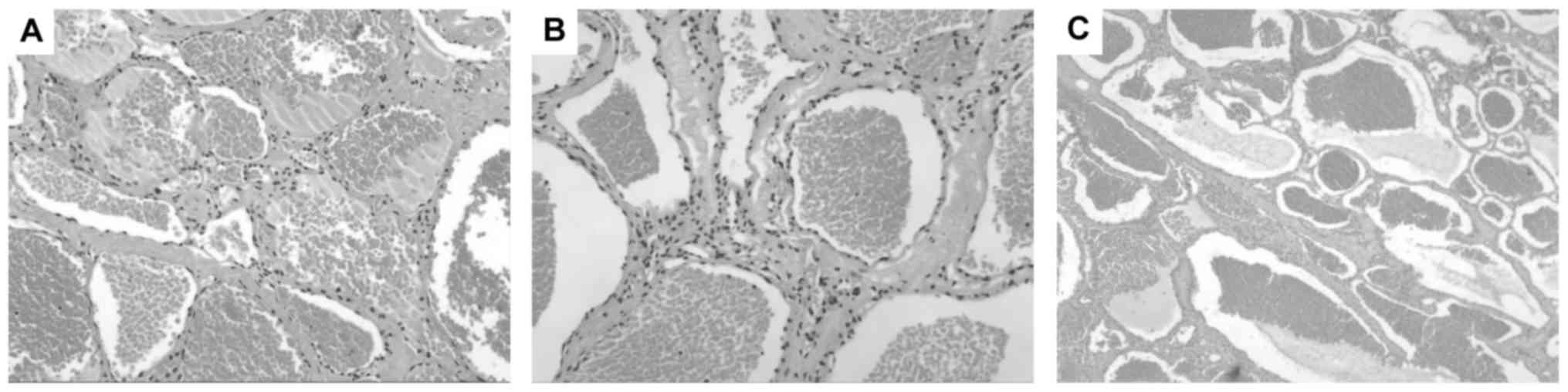
Histopathological examination revealed each lesion
consisted of a large number of thin-walled vessels in collagenous
connective tissue, lined by a single layer of endoththelial cells
(Fig. 3). Some of vessels were filled
with blood or thrombi.
Follow-up
After surgery immediately, 9 patients with
progressive course experienced improvement, 2 patients with acute
onset remained unchanged from the preoperative condition. A mild
worsening of neurological deficits was seen in one patient with
2-year history of illness, but improved later to a better status
than preoperatively. During a mean follow-up period of 35.9 months,
neurological status had markedly improved in 11 patients and was
stable in one patient compared with their preoperative neurological
deficit. At the last assessment, the postoperative JOA score was
14.42±2.36 (range, 7–16). The final JOA score was significantly
improved (P<0.05) over the preoperative JOA score. Postoperative
MRI results showed no tumor recurrence in all cases. Surgical
outcomes and assessment of neurological function are summarized in
Table II.
Discussion
Spinal CAs are relatively rare, accounting for 12%
of spinal vascular anomalies (2,5). Solitary
epidural CAs are extremely rare. Our series adds 12 cases, which is
a substantial addition to the existing literature. In our group,
the age ranged from 25 to 73 years, with a mean age of 52.1 years,
which accorded with the literature report (15,17). And
besides, a mild female predilection was noted (female/male ratio of
1.4:1). Some authors suggest that reason for the female
predominance of spinal CAs may be hormonal effects (15,19).
However, this hypothesis has not yet been proven. According to the
literature on intramedullary CAs, 45% of tumors were in the
cervical region, 20% were in the cervicothoracic region, and 35%
were in the thoracic region (1). In
our study, the most common location is thoracic spine in about 75%
of cases, followed by lumbar region in fewer than 20%; a cervical
location is extremely rare.
The presentations of solitary epidural CAs can be
classified as two patterns: a chronically progressive type (83.3%)
and an acute type (16.7%). The chronically progressive type with
progressive spinal cord or spinal nerve root compression, is
usually caused by slow enlargement of the lesion, which was thought
to be caused by small repeated intralesional hemorrhages and
embolisms, the proliferation of blood vessels (4). In the chronically progressive type, the
symptom at onset was anesthesia or radiculopathy. Motor deficit
eventually appeared in the late stages. The acute type, with a
sudden onset of neurological deterioration, was usually caused by a
large amount of acute hemorrhage (3,6,8). In this type, the initial symptom was a
sudden onset of aesthesia, followed by rapid development of
paralysis and sphincter disturbances. All these findings
corresponded to the previous reports (3,4,6,8).
The origin of solitary epidural CAs is still
unknown. Caruso et al (6)
postulated that the specific epidural localization may be explained
by the embryological development of dura mater; the vascular
elements from the primordial plexus may have some influence in the
postnatal development of a cavernous lesion. This theory is
attractive because it provides an easily understandable mechanism.
However, CAs are not necessarily congenital, as some reports
describe acquired cavernous malformation (20,21). Thus,
various pathogenic mechanisms may cause these lesions.
Compared with common epidural tumors, the diagnosis
of solitary epidural CAs is very critical in preventing unnecessary
operative bleeding. Spinal angiography has no role in diagnosis as
CAs have no communication with the spinal arteries (4,10). MRI is
the most reliable diagnostic tool for spinal epidural CAs (5). On MRI, epidural CAs are usually creeping
growth and showed lobulated-spindle shaped with taper ends, which
may be due to the softer texture of the CA and the limited epidural
space (14). Owing to stagnant blood
and slow blood flow, they are generally isointense on T1-WI and
hyperintense on T2-WI (14,22). Contrast-enhanced T1-WI shows
homogeneous enhancement because of much sinusoid structure in the
tumor (4); nevertheless, sometimes
heterogeneous signal can also be noticed due to intralesional
hemorrhage or thrombus (10,14). The most striking difference in the MRI
characteristics between epidural and intramedullary CAs is the lack
of a ring of hypointensity from hemosiderin deposit (9,16). This
may be result of more rapid removal of blood products outside the
blood-brain barrier (2). In our
group, most of the lesions grow dorsally within the spinal canal.
The larger available epidural space and the lower resistance in the
posterior portion of the spinal canal may be explanations (5,14). In 6
cases, the tumors grew into the intervertebral foramen, which may
be due to the loose tissue structure inside the neuroforamen
(15,23). Solitary epidural CAs should be
differentiated from epidural contrast-enhancing lesions, such as
schwannomas, meningiomas, lymphomas and angiolipomas. Schwannomas
always have a smooth contour, cystic changes and necrosis,
extending into the paraspinal region with the enlargement of
intervertebral foramen, which could be the clue to the differential
diagnosis of CAs (3,5,13,24). However, some reports describe
intervertebral foramen widening in epidural CAs (3,14), which
make CAs difficult to differentiate from schwannomas. Meningiomas
are rarely located in epidural space. Nevertheless, isointense
signal to the spinal cord with frequent broad dural attachment
(dural tail sign) on the contrast-enhanced T1-WI favors the
diagnosis of epidural meningiomas (25). Lymphomas are characterized by
isointense or hyperintense signal on T2-WI, less frequent
paravertebral extension and intervertebral foramen widening
(13). An angiolipoma is typically
hyperintense on T1-and T2-WI because of fat content, while the fat
in a CA is usually absent (26).
Although solitary epidural CAs are considered to
have typical imaging features, definitive preoperative diagnosis
may still be challenging based only on MRI. An accurate diagnosis
depends on pathology. Histologically, CAs must be distinguished
from arteriovenous malformations and capillary hemangiomas
(27,28). The arteriovenous malformation shows
with a cluster of abnormal arteries and veins and vessel walls
containing elastin, and smooth muscle (28). The characteristic features of
capillary hemangioma are thin irregular capillary-sized vessels
caught in low attenuating fibroses, lobular architecture and the
presence of a lining of a continuous basal lamina (29). Typical histological features of CAs
include large number of sinusoidal channels in collagenous tissue,
sometimes with thrombosis, blood, calcification, and perivascular
hemosiderin deposition (2,7,11,12). In the present study, all histological
characteristics were consistent with CAs.
Given the tendency of hemorrhage and histologically
benign, complete resection should be currently the best treatment
for symptomatic epidural CAs. According to the literature, the GTR
rate is 57.1–100% in solitary epidural CAs (5,16). In our
series, 91.6% of the tumors showed well-demarcated dissection plane
and no tight adhesion to the dura mater, and GTR was achieved.
However, diffuse dural attachment associated with intervertebral
foramen extension is impossible to resect completely. Thus, STR is
acceptable to improve the neurologic function to avoid severe
complications (17). Because of the
excessive vascularity of CAs, en bloc resection was advocated and
tumor biopsy should be avoided (17).
We used bipolar coagulation to disrupt the abnormal proliferation
of feeding arteries and draining veins sequentially. Shrinkage of
the tumor through extended of its surface avoids massive bleeding
and permits safer handling of the lesion. In our study, en bloc
resection was achieved in 58.3% of the patients. However, if CAs
are densely adhered to attached nerve rootlets or extend beyond the
intervertebral foramen, en bloc resection was difficult to achieve
and piecemeal resection has to be done. Although intraoperative
bleeding in piecemeal resection group was significantly more
(P<0.05) than that in en bloc resection group, it still could be
controlled by using careful microsurgical techniques such as
low-power bipolar coagulation and tightly packed Gelfoam roll
compression. Embolization is always useless for epidural CAs
because of their slow blood flow (4,15).
At the last evaluation, the JOA scores of most
patients had significantly improved (P<0.05); moreover, no tumor
recurrence was observed. Although underwent a emergency surgery,
one patient with a sudden onset did not have any improvement due to
delayed admission after neurological deterioration. A large amount
of acute hemorrhage and spinal cord compression could cause severe
neurologic damage such as sphincter dysfunction. Thus, for
symptomatic patients with sudden neurological deterioration, early
surgical decompression should be performed to prevent further
neurological damage.
Thus far, the efficacy of radiotherapy has not been
reliably evaluated since the natural history of epidural CAs is
still controversial (5). In our
study, the STR case did not receive radiotherapy, and had no tumor
recurrence. Some authors advocate radiotherapy as an adjuvant
therapy for residual tumor after STR (30), but others have thought that the
procedure may be ineffective and produce radiation damage to the
spinal cord (15). Recently, Sohn
et al (7) suggested that
image-guided stereotactic radiosurgery (SRS) enable accurate
targeting of specific lesions with relatively high doses and
minimal collateral risk. They determined that the equivalent
hypofractionated dose of 32 Gy in 4 fractions would be the most
appropriate and effective treatment while spinal cord irradiation
(total volume, 2.4 cm3) was kept to less than 4 Gy in
the partial volume of 0.7 cm3 (7). This treatment protocol delivered rapid
clinical benefits and long-term tumor control in one case of spinal
epidural CA in the thoracic spine (7). However, therapeutic effect of SRS for
the local control of epidural CAs should be investigated further
for this limited number of cases.
In conclusion, solitary epidural CAs should be
considered in the differential diagnosis of a middle-aged patient
with an epidural tumor involving the thoracic regions, if the
lesion has dorsal localization and homogeneous enhancement on MRI.
Chronically progressive spinal cord and spinal nerve root
compression are main clinical symptoms. Early surgery is advocated
to prevent irreversible neurological deficits. When aggravated by a
large amount of acute hemorrhage, neurological deterioration is
usually acute and prompt surgical decompression is the best choice.
Because of the excessive vascularity of CAs, en bloc resection is
recommended and piecemeal resection should be avoided. When GTR
cannot be achieved, STR of the lesions for releasing cord
compression is advised, and radiological follow-up is required. In
addition, a good clinical outcome after GTR can be expected, and
the risk of long-term recurrence is low.
Acknowledgements
This study was supported by the National Natural
Science Foundation of China (grant no. 81502141) and the Science
and Technology Project grant from Anhui Province (grant no.
1606c08235).
References
|
1
|
Tong X, Deng X, Li H, Fu Z and Xu Y:
Clinical presentation and surgical outcome of intramedullary spinal
cord cavernous malformations. J Neurosurg Spine. 16:308–314. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Zevgaridis D, Büttner A, Weis S, Hamburger
C and Reulen HJ: Spinal epidural cavernous hemangiomas. Report of
three cases and review of the literature. J Neurosurg. 88:903–908.
1998. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Talacchi A, Spinnato S, Alessandrini F,
Iuzzolino P and Bricolo A: Radiologic and surgical aspects of pure
spinal epidural cavernous angiomas. Report on 5 cases and review of
the literature. Surg Neurol. 52:198–203. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Aoyagi N, Kojima K and Kasai H: Review of
spinal epidural cavernous hemangioma. Neurol Med Chir (Tokyo).
43:471–475. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Hatiboglu MA, Iplikcioglu AC and Ozcan D:
Epidural spinal cavernous hemangioma. Neurol Med Chir (Tokyo).
46:455–458. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Caruso G, Galarza M, Borghesi I, Pozzati E
and Vitale M: Acute presentation of spinal epidural cavernous
angiomas: Case report. Neurosurgery. 60:E575–E576. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Sohn MJ, Lee DJ, Jeon SR and Khang SK:
Spinal radiosurgical treatment for thoracic epidural cavernous
hemangioma presenting as radiculomyelopathy: Technical case report.
Neurosurgery. 64:E1202–E1203. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Sarikaya-Seiwert S, Gierga K, Wessalowski
R, Steiger HJ and Hänggi D: Solitary spinal epidural cavernous
angiomas in children presenting with acute neurological symptoms
caused by hemorrhage. J Neurosurg Pediatr. 5:89–93. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Floeth F, Riemenschneider M and Herdmann
J: Intralesional hemorrhage and thrombosis without rupture in a
pure spinal epidural cavernous angioma: A rare cause of acute
lumbal radiculopathy. Eur Spine J. 19 Suppl 2:S193–S196. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Sanghvi D, Munshi M, Kulkarni B and Kumar
A: Dorsal spinal epidural cavernous hemangioma. J Craniovertebr
Junction Spine. 1:122–125. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Saracen A and Kotwica Z: Thoracic spinal
epidural cavernous haemangioma with an acute onset: Case report and
the review of the literature. Clin Neurol Neurosurg. 115:799–801.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Sharma MS, Borkar SA, Kumar A, Sharma MC,
Sharma BS and Mahapatra AK: Thoracic extraosseous, epidural,
cavernous hemangioma: Case report and review of literature. J
Neurosci Rural Pract. 4:309–312. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Bayri Y, Ekşi MŞ, Yalçınkaya Koç D and
Konya D: Spinal epidural cavernous angioma: Two case reports and
review of the literature. Acta Orthop Traumatol Turc. 49:459–464.
2015.PubMed/NCBI
|
|
14
|
Feng J, Xu YK, Li L, Yang RM, Ye XH, Zhang
N, Yu T and Lin BQ: MRI diagnosis and preoperative evaluation for
pure epidural cavernous hemangiomas. Neuroradiology. 51:741–747.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zhong W, Huang S, Chen H, Sun H, Cai B,
Liu Y and You C: Pure spinal epidural cavernous hemangioma. Acta
Neurochir (Wien). 154:739–745. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Khalatbari MR, Abbassioun K and
Amirjmshidi A: Solitary spinal epidural cavernous angioma: Report
of nine surgically treated cases and review of the literature. Eur
Spine J. 22:542–547. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Li TY, Xu YL, Yang J, Wang J and Wang GH:
Primary spinal epidural cavernous hemangioma: Clinical features and
surgical outcome in 14 cases. J Neurosurg Spine. 22:39–46. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Chiles BW III, Leonard MA, Choudhri HF and
Cooper PR: Cervical spondylotic myelopathy: Patterns of
neurological deficit and recovery after anterior cervical
decompression. Neurosurgery. 44:762–770. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Labauge P, Bouly S, Parker F, Gallas S,
Emery E, Loiseau H, Lejeune JP, Lonjon M, Proust F, Boetto S, et
al: Outcome in 53 patients with spinal cord cavernomas. Surg
Neurol. 70:176–181. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Barker FG III, Amin-Hanjani S, Butler WE,
Lyons S, Ojemann RG, Chapman PH and Ogilvy CS: Temporal clustering
of hemorrhages from untreated cavernous malformations of the
central nervous system. Neurosurgery. 49:15–24. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Gao W, Qiao X, Ma S, Ma J, Dong X, Qin T
and Fang Q: Contribution of skin trauma to infantile skin
hemangioma. Med Hypotheses. 76:512–513. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Demir MK, Ozdemir H, Unlu E, Temizöz O and
Genchellac H: Differential diagnosis of spinal epidural meningioma
and hemangioma at MR imaging. Radiology. 244:9332007. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Uchida K, Yayama T, Nakajima H, Hirai T,
Kobayashi S, Chen K, Guerrero AR and Baba H: Microsurgical
resection of cavernous haemangioma around the thoracic
neuroforamen: A case report. J Orthop Surg (Hong Kong). 18:370–373.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Demachi H, Takashima T, Kadoya M, Suzuki
M, Konishi H, Tomita K, Yonezawa K and Ubukata A: MR imaging of
spinal neurinomas with pathological correlation. J Comput Assist
Tomogr. 14:250–254. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Wu L, Yang T, Deng X, Yang C, Zhao L, Yao
N, Fang J, Wang G, Yang J and Xu Y: Spinal extradural en plaque
meningiomas: Clinical features and long-term outcomes of 12 cases.
J Neurosurg Spine. 21:892–898. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Gelabert-González M and García-Allut A:
Spinal extradural angiolipoma: Report of two cases and review of
the literature. Eur Spine J. 18:324–335. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Caroli E, Acqui M, Trasimeni G, Di Stefano
D and Ferrante L: A case of intraroot cauda equina cavernous
angioma: Clinical considerations. Spinal Cord. 45:318–321. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Lee JW, Cho EY, Hong SH, Chung HW, Kim JH,
Chang KH, Choi JY, Yeom JS and Kang HS: Spinal epidural
hemangiomas: Various types of MR imaging features with
histopathologic correlation. AJNR Am J Neuroradiol. 28:1242–1248.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Gencpinar P, Açıkbaş SC, Nur BG, Karaali
K, Arslan M, Gurer EI, Duman O and Haspolat S: Epidural capillary
hemangioma: A review of the literature. Clin Neurol Neurosurg.
126:99–102. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Padovani R, Acciarri N, Giulioni M,
Pantieri R and Foschini MP: Cavernous angiomas of the spinal
district: Surgical treatment of 11 patients. Eur Spine J.
6:298–303. 1997. View Article : Google Scholar : PubMed/NCBI
|