Introduction
Acute promyelocytic leukemia (APL), a subtype of
acute myeloid leukemia (AML), accounts for 46% of leukemia cases
and is frequently associated with dizziness, fever, nausea,
hematochezia and anemia (1,2). Currently, the standard treatment for APL
is combination chemotherapy, which may cause gastrointestinal
effects, multi-organ impairment and alopecia. Therefore, new
therapeutic options for APL are urgently needed. Near-infrared
light can easily penetrate the skin and is absorbed deeply into
tissues (3). Accordingly, the ability
of a near-infrared (NIR) laser and hollow gold nanospheres (HAuNSs)
to modulate the release of anticancer agents was tested using the
antitumor drug paclitaxel, which exhibits surface plasmon
absorbance in the NIR region and significant anti-tumor effects,
demonstrated strong cytotoxicity and reduced tumorigenesis in nude
mice (4). Another study observed
survival rates no higher than 30% among human lung carcinoma
epithelial cells (A549) exposed to photodynamic therapy for 4 h
(5). Recently, our group has
successfully confirmed that blue light can enhance the
proliferation inhibition of HL60 cells treated by all-trans
retinoic acid/nanodiamonds (6).
However, the pro-apoptotic effects of blue light on promyelocytic
leukemia cells and related mouse models, as well as the possible
mechanisms, have not yet been reported.
Pro-apoptotic factors activate mitochondrial
apoptotic signaling pathways, which lead to the excessive opening
of mitochondrial permeability transition pores (MPTPs). This
excessive pore opening causes the dissipation of mitochondrial
membrane potential (MMP) across the inner mitochondrial membrane,
efflux of Ca2+, uncoupling of the respiratory chain and
production of superoxide (7,8). Subsequently, these changes enhance the
release of cytochrome c, apoptosis-inducing factor and
B-cell lymphoma-2 (Bcl-2) family proteins from the mitochondria
into the membrane space, thus triggering the cell to enter
apoptosis.
Bcl-2 family proteins localize within the
mitochondria and appear to regulate mitochondrial outer membrane
permeability by binding to mitochondrial channels (9). Bcl-2-associated X protein (Bax) induces
the cytoplasmic release of mitochondrial mediators such as
cytochrome c, which serves as a marker of mitochondrial
injury and is involved in the caspase-dependent apoptosis pathway
(10). Moreover, the mitochondrion is
the major site of reactive oxygen species (ROS) generation
(11). Apoptosis can be triggered
both by ROS-induced abnormal gene expression and the blockage of
cell communication (12).
This study aimed to investigate the potential
systematic effects of blue light therapy for APL. Using in
vitro and in vivo experiments, we successfully explored
the pro-apoptotic effects of blue light and the underlying
mechanisms related to the mitochondrial apoptotic pathway.
Materials and methods
Cell culture
HL60 human promyelocytic leukemia cells (American
Type Culture Collection, Manassas, VA, USA) were cultured in
Roswell Park Memorial Institute (RPMI)-1640 medium supplemented
with 10% fetal bovine serum (FBS), 100 U/ml penicillin and 100
µg/ml streptomycin in a humidified atmosphere containing 5/95%
CO2/air at 37°C. All cell culture reagents were obtained
from Invitrogen (Thermo Fisher Scientific, Inc., Waltham, MA,
USA).
Cell viability assessment
Cells were exposed to blue light (wavelength: 456
nm; storage battery power supply: 12 V; radiation power: 0.25
mW/cm2 (Jiangmen Weigu Lighting Technology Co., Ltd.,
Jiangmen, China) for 10–56 h and to 50 µM of PTX (Jiangsu Yew
Pharmaceutical Co., Ltd., Jiangsu, China) for 24 h. Cell viability
was analyzed using a quantitative colorimetric assay and the Cell
Counting Kit-8 (CCK-8; BestBio, Shanghai, China) according to the
manufacturer's instructions.
Lactate dehydrogenase (LDH) release
assessment
Cells were exposed to blue light and 50 µM of PTX
for 24 h, after which the concentrations of LDH in the culture
supernatants were detected using an in vitro Toxicology
Assay kit (C0017; Beyotime Biotechnology, Jiangsu, China). Data
were expressed in terms of cell mortality, according to the
instruction manual.
Cell apoptosis assessment
Cells were exposed to blue light and 50 µM of PTX
for 24 h. Subsequently, suspended cells were incubated with Annexin
V-FITC and propidium iodide for 10 min at 25°C in darkness, and
then analyzed using a Muse™ Cell Analyzer (EMD
Millipore, Billerica, MA, USA) according to the manufacturer's
instructions.
MMP assessment
Cells were exposed to blue light and 50 µM of PTX
for 24 h, and subsequently incubated with 2 µM of 5,5′,
6,6′-tetrachloro-1,1′, 3,3′tetraethylbenzimidazol-ylcarbocyanine
iodide (JC-1; Sigma-Aldrich; Merck KGaA, Darmstadt, Germany) for 20
min at 25°C in darkness. Changes in the levels of red and green
fluorescence were detected using a Muse™ Cell Analyzer
(EMD Millipore).
ROS assessment
Cells were exposed to blue light and 50 µM of PTX
for 24 h, followed by 10 µM of 2′-7′-dichlorodihydrofluorescein
diacetate (DCFH-DA; Sigma-Aldrich; Merck KGaA) at 37°C for 10 min
in darkness. Changes in the levels of green fluorescence were
detected using a Muse™ Cell Analyzer (EMD
Millipore).
HL60-xenografted tumor mouse
model
The animal experimental protocol was approved by the
Animal Ethics Committee of Jilin University, and all experiments
were performed in accordance with the National Institutes of Health
Guide for the Care and Use of Laboratory Animals (NIH Publications
no. 8023, 1978 revision). Five-week-old male BALB/c nude mice
(Vital River Laboratory Animal Technology Co., Ltd., Beijing,
China) were housed in groups of three in cages exposed to a 12-h
light/dark cycle (lighted from 7:00 a.m. to 7:00 p.m.) at 23±1°C.
Water and food were available ad libitum.
HL60 cells were harvested from mid-log-phase
cultures, and 0.1 ml of cell suspension (1×108 cells/ml)
was subcutaneously (s.c.) injected into the right abdomen of each
mouse. When the tumor diameters reached 3–5 mm, the mice were
randomly divided into two groups (n=3) and exposed to normal (CTRL)
or blue light for nine days. The body weights and tumor dimensions
were measured daily, and the tumor volumes (mm3) were
calculated as follows: Length × (width)2 ×0.5. Finally,
the mice were sacrificed via injection with 200 mg/kg of
pentobarbital, after which the tumors were dissected and liver and
kidney tissues were collected.
Histopathological study
Hematoxylin and eosin (H&E) staining was used to
assess hepatic and renal histology. Briefly, liver and kidney
tissues were fixed in 4% paraformaldehyde for 24 h, and
subsequently dehydrated in a stepwise manner using an ethanol
gradient (50, 70, 80, 90, 95 and 100%). The dehydrated samples were
immersed in xylene for 30 min and incubated overnight in paraffin
at 65°C. Once embedded, the specimens were cut into 4-µm-thick
slices using a microtome (Leica, Wetzlar, Germany) and placed on
microscopy slides. The sections were deparaffinized with fresh
xylene for 10 min, hydrated using an ethanol gradient (100, 90, 80
and 70%) and washed thrice with distilled/deionized water. After
staining with H&E, the sections were examined using an IX73
inverted microscope with a ×40 objective (Olympus, Tokyo,
Japan).
Western blot
Tumor tissues were lysed using RIPA buffer
(Sigma-Aldrich; Merck KGaA) containing a 1% protease inhibitor
cocktail (Sigma-Aldrich; Merck KGaA) and 2% PMSF (Sigma-Aldrich;
Merck KGaA). Forty micrograms of protein per sample were separated
on a 12% SDS-PAGE gel. The proteins were then transferred
electrophoretically to 0.45 µm nitrocellulose membranes, which were
then incubated overnight with primary antibodies against Bcl-2,
Bax, Bcl extra-long (Bcl-xL), cytochrome c, cleaved
caspases-3 and −9 and glyceraldehyde-3-phosphate dehydrogenase
(GAPDH) (Cell Signaling Technology, Inc., Danvers, MA, USA) at 4°C
(all dilutions: 1:1,000). Subsequently, the membranes were
incubated with horseradish peroxidase-conjugated secondary
antibodies (Santa Cruz Biotechnology, Inc., Dallas, TX, USA) at
room temperature for 4 h. An enhanced chemiluminescence detection
kit (GE Healthcare, Little Chalfont, UK) was then used to detect
the labeled protein bands. The band intensities were quantified
using Image J software (National Institutes of Health, Bethesda,
MD, USA).
Statistical analysis
The data are expressed as means ± standard
deviations (SD). Statistical significance was detected using a
one-way variance analysis (ANOVA), followed by Dunn's test, using
SPSS version 16.0 software (SPSS, Inc., Chicago, IL, USA).
P<0.05 was considered to indicate a statistically significant
difference.
Results
Blue light induced apoptosis in HL60
cells
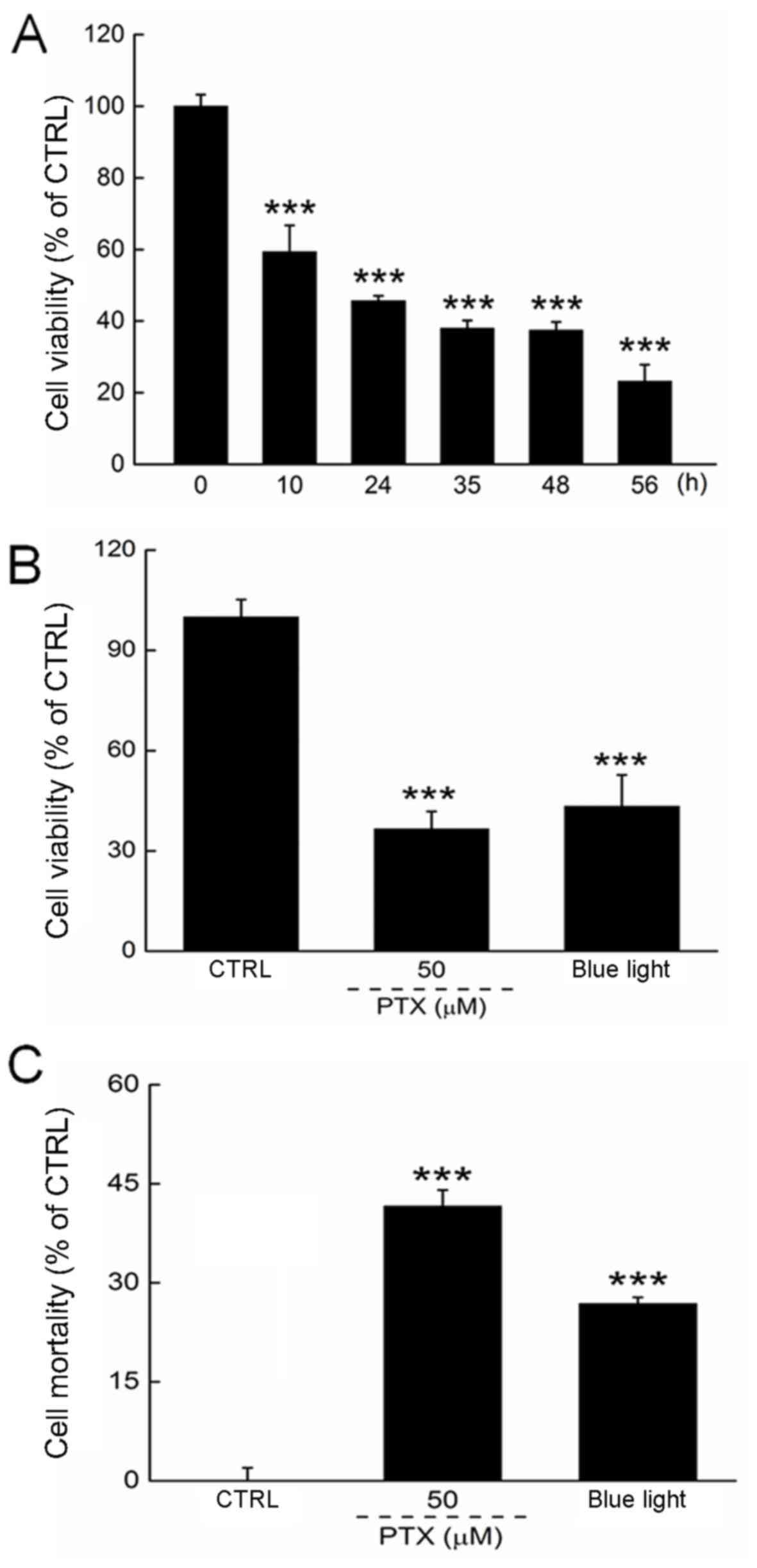
We first determined the optimal experimental blue
light dose by controlling the exposure duration. Blue light reduced
the viability of HL60 cells in a time-dependent manner from 12 to
56 h of exposure (P<0.001; Fig.
1A). Both PTX and a 24-h exposure to blue light reduced cell
viability by >50% (P<0.001; Fig.
1B). The cell mortality rate was determined by LDH release,
which was enhanced significantly in HL60 cells exposed to blue
light for 24 h (Fig. 1C).
Apoptosis rates of 81.8 and 68.3% were observed
among cells exposed to PTX (50 µM) and blue light, respectively,
for 24 h (Fig. 2A). Moreover,
compared with the control cells, PTX or blue light treatment led to
MMP depolarization rates of 72.5% (63.65 vs. 17.45%) and 65.6%
(50.85 vs. 17.45%) (Fig. 2B), which
further promoted the apoptosis of HL60 cells.
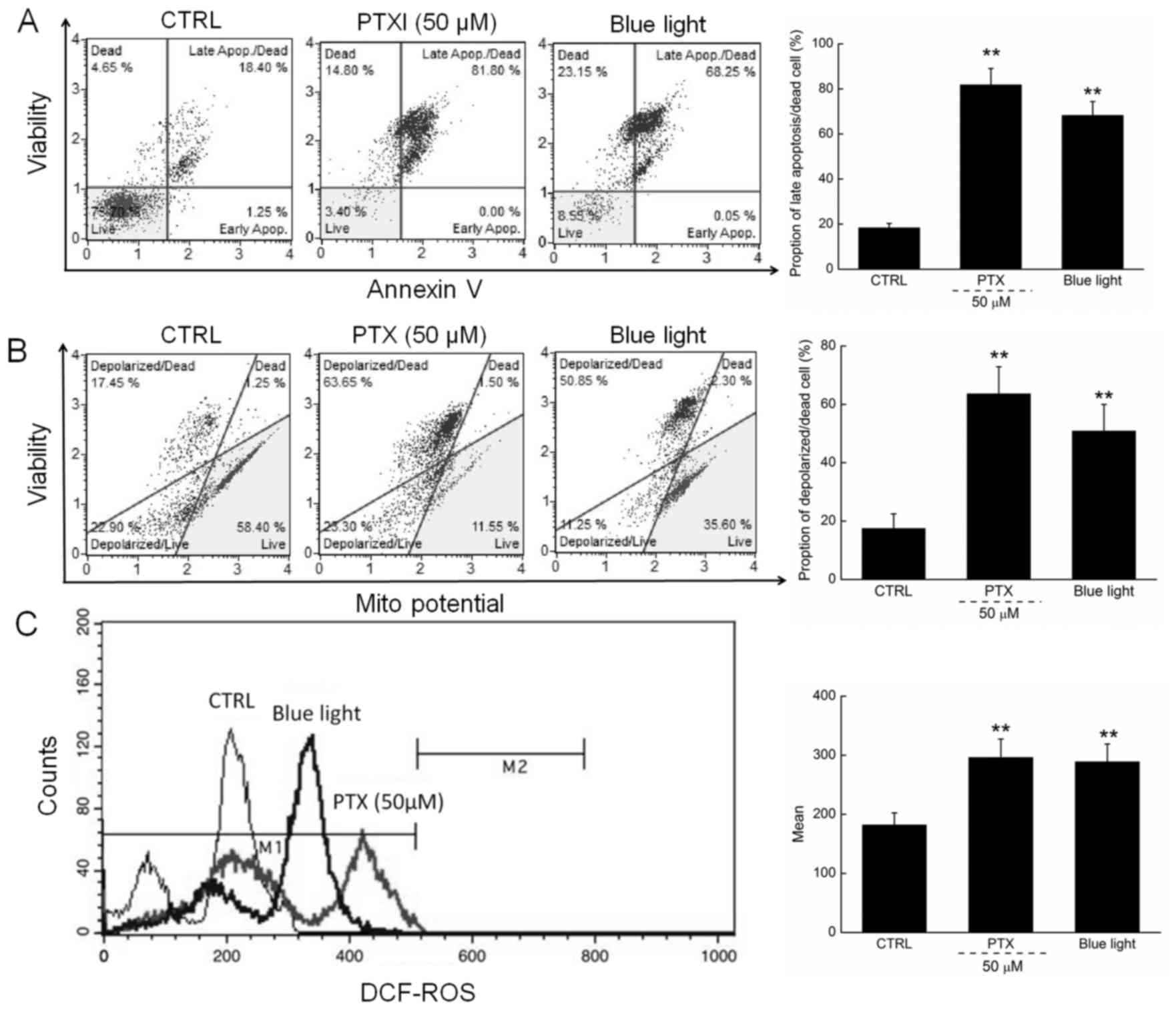 | Figure 2.(A) 24-h exposure to PTX and blue
light markedly enhanced the apoptotic rate of HL60 cells. (B) PTX
and blue light induced the dissipation of mitochondrial membrane
potential, detected via JC-1 staining. (C) 24-h exposure to PTX and
blue light significantly enhanced intracellular ROS levels in HL60
cells. Data are expressed as the mean ± standard deviation (n=6).
**P<0.01 vs. control cells. PTX, paclitaxel; ROS, reactive
oxygen species; JC-1, 5,5′,
6,6′-tetrachloro-1,1′,3,3′tetraethylbenzimidazol-ylcarbocyanine
iodide; CTRL, control. |
In addition, ROS-mediated late mitochondrial events
(13) were found to be strongly
enhanced in HL60 cells exposed to blue light and PTX treatment
(Fig. 2C).
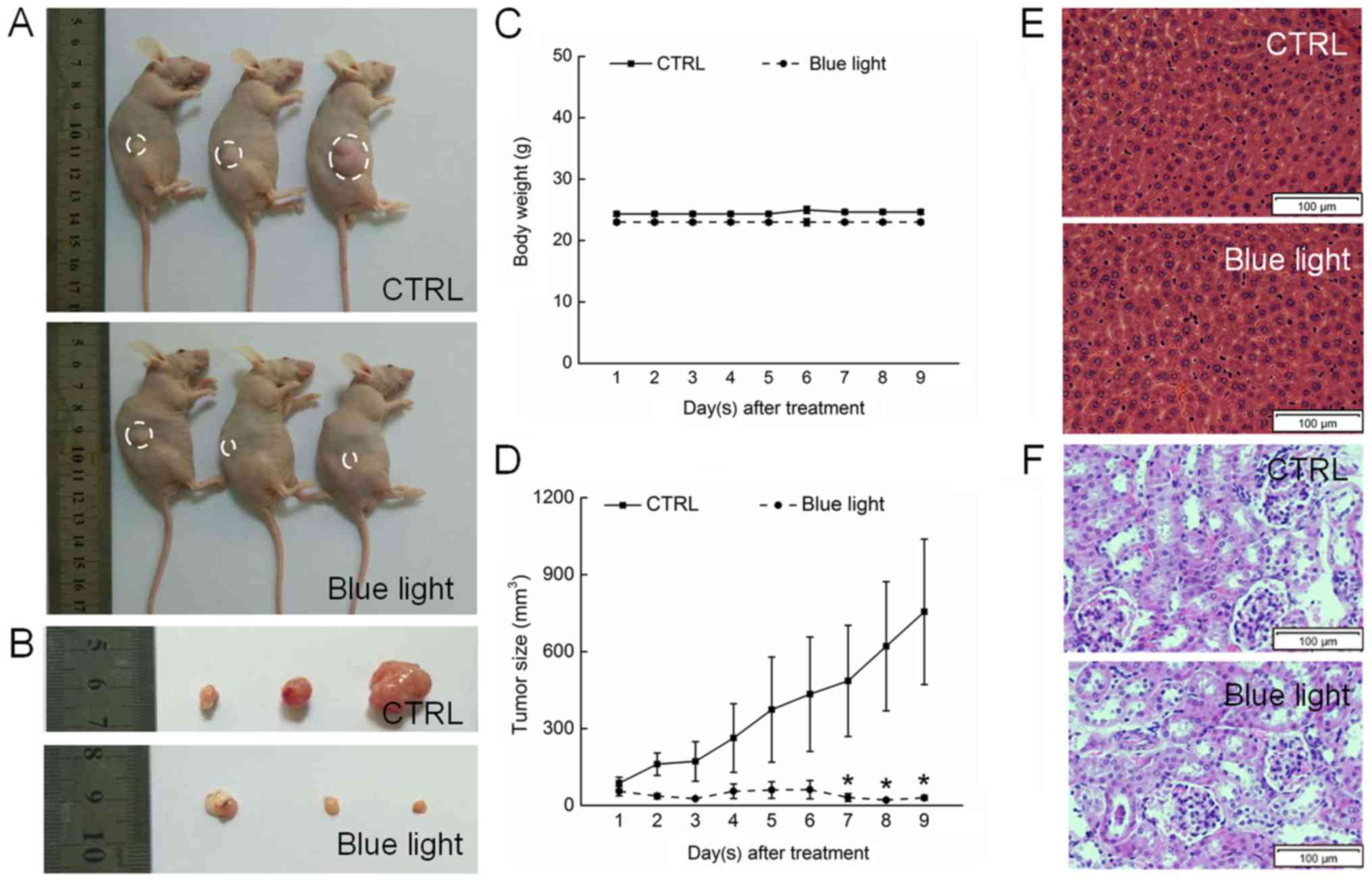
Blue light suppressed tumor growth in
nude mice
Blue light exhibited significant antitumor activity
against HL60-xenografted tumors in a nude mouse model. A 9-day
exposure to blue light strongly suppressed tumor growth without
affecting the body weights of the mice, indicating that blue light
has few side effects in normal tissues (Fig. 3A-C). A histopathological study in
which few pathologic changes were observed between the livers and
kidneys of blue-light exposed and non-exposed mice further
confirmed the safety of blue light therapy (Fig. 3E and F). Sixteen days after tumor
implantation, the tumors began to grow rapidly in the non-treated
control mice, reaching a mean tumor volume of 975.5±283.4
mm3. In contrast, tumor growth was strongly inhibited in
blue light-treated mice, beginning on day 7 (P<0.05; Fig. 3D).
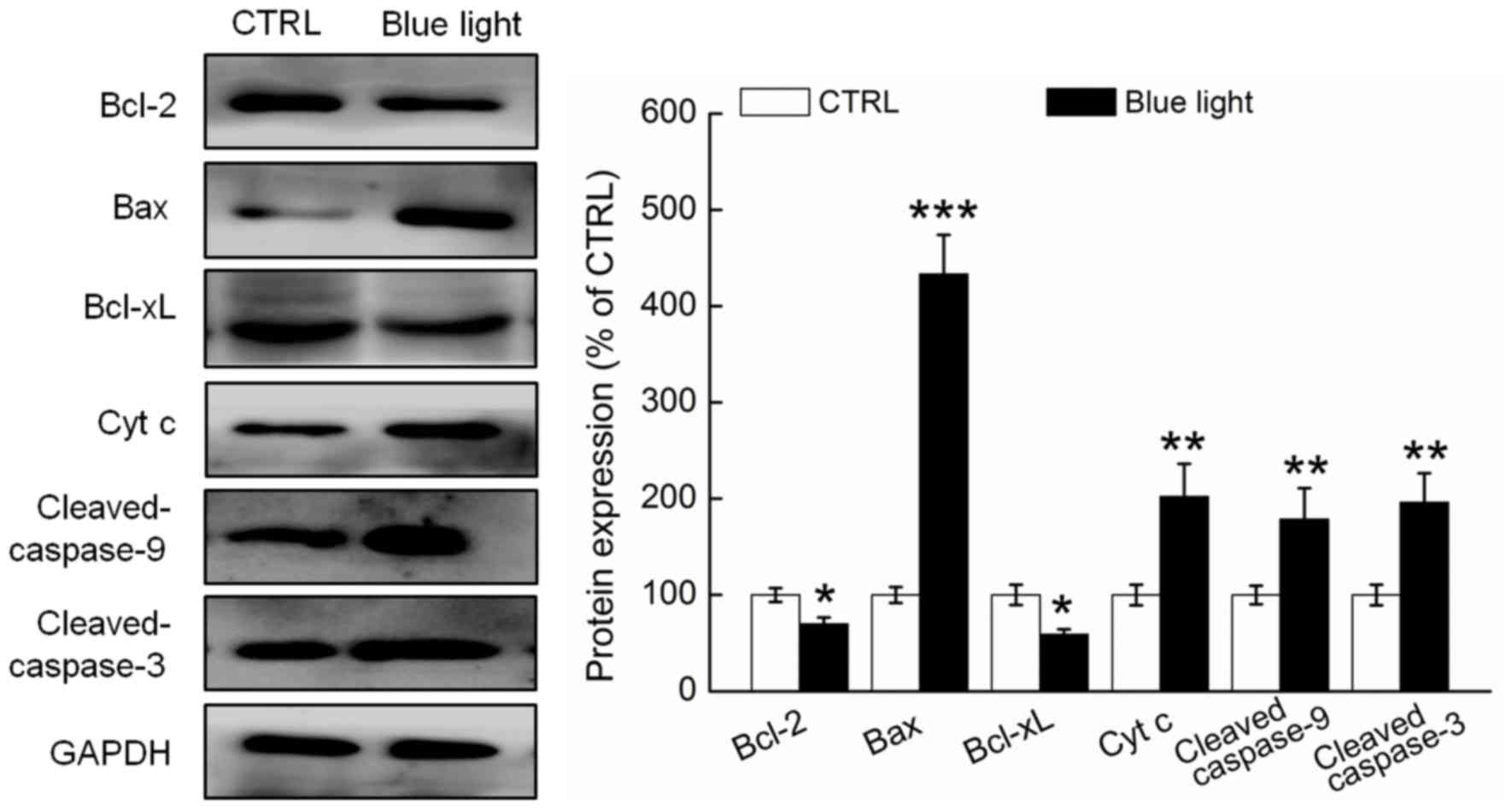
Blue light regulated the expression of
pro- and anti-apoptotic proteins
Compared with the non-treated mice, the tumor
tissues of the blue light-treated mice exhibited significantly
reduced expression of Bcl-2 and Bcl-xL and enhanced expression of
Bax, cytochrome c and cleaved caspases-3 and −9 (P<0.05;
Fig. 4). The Bcl-2/Bax ratio was
13.8% higher in the tumor tissues of the blue light-exposed mice,
compared to the non-exposed mice (Fig.
4).
Discussion
In the present study, we have clarified the
pro-apoptotic effects of blue light therapy and the underlying
mechanisms in HL60 cells. We selected a 24-h exposure duration to
investigate the mechanism underlying blue light-induced apoptosis
in these cells. LDH is enriched in the cytoplasm and normally
cannot pass through the membrane; however, cell damage causes a
release of LDH into the extracellular space (14). We observed that blue light reduced the
viability of HL60 cells in a time-dependent manner from 12 to 56 h,
as indicated by the release of LDH.
A normal MMP is required to maintain oxidative
phosphorylation and adenosine triphosphate (ATP) formation
(7). A stable MMP thus confers a
survival advantage upon the cell. Changes characteristic of
mitochondrial apoptosis are commonly observed during cell
apoptosis, which is characterized by the disruption of the MMP
(15). In our study, blue light
treatment caused depolarization of the MMP and thus promoted HL60
cell apoptosis. This process occurred before deoxyribonucleic acid
fragmentation. As reported previously, ROS and mitochondria are
linked by a short feedback loop as indicated by the cytoplasmic
overproduction of ROS during mitochondria-dependent apoptosis,
which further promotes the opening of the MPTP (16,17). Our
data suggest that blue light-mediated apoptosis in HL60 cells may
involve the ROS-mediated mitochondrial apoptosis pathway.
Moreover, our in vivo data are consistent
with those of in vitro investigations. The antitumor
activity of blue light was further confirmed by our finding that
blue light exposure strongly suppressed tumor growth without
affecting the body weights of mice. Caspase-3, an important
effector related to cell morphology and certain biochemical events
associated with apoptosis, is catalyzed by activated caspase-9
(18), and ROS was identified as
necessary for the full activation of the caspase cascade (13). Additionally, Bcl-2 family proteins,
which serve as biomarkers of mitochondrial function, have become
targets for anti-tumor agents. Specifically, the expression levels
of Bcl-2 and Bax are used to determine whether the mitochondrial
apoptosis pathway has been activated (19), and a heterodimer of Bcl-2/Bax is known
to regulate the MMP (20). Our
related data further confirm that the pro-apoptotic effects of blue
light in HL60 cells may be associated with the mitochondrial
pathway.
There are still several limitations in our present
study. Firstly, the effects of blue light were not measured in
normal cells; however, the histopathological study on livers and
kidneys of nude mice has confirmed the safety of blue light
therapy. Secondly, although the APL mouse model has been
successfully established in our group, it failed to perform in the
present study. The tumor bearing mice model established by HL60
cells applied in this experiment has been used for other previous
studies (21), which can also certify
the pro-apoptotic effects of blue light in human promyelocytic
leukemia. Moreover, our present data failed to systemically discuss
the correlation between MMP depolarization and ROS production
during blue light mediated pro-apoptosis in HL60 cells. Blue light
caused the changes on the expression levels of other Bcl-2 family
members such as Bim and Bak will be detected in further
experiments. N-Acetyl-L-cysteine (NAC), a ROS scavenger, will be
applied to investigate its influence on the pro-apoptotic effects
of blue light, and the translocation of Cytoplasma C from
mitochondria to cytosol will be detected in cells in our ongoing
experiments related to the effects of blue light.
In conclusion, our experimental findings verified
the pro-apoptotic effects of blue light in both in vitro and
in vivo models. Blue light exposure induced the excess
release of LDH and depolarization of the MMP in HL60 cells,
suppressed the growth of HL60-xenografted tumors and regulated the
expression of both pro- and anti-apoptotic proteins in tumor
tissues. Taken together, our data suggest that blue light induces
apoptosis in tumor cells via the mitochondrial apoptosis pathway.
Our findings provide pharmacological evidence of the potential
usefulness of blue light as an adjuvant therapy for leukemia.
Acknowledgements
Not applicable.
Funding
This work was supported by the Natural Science
foundation of China (Grant No. 81402955).
Availability of data and materials
All data generated and analysed during the present
study are included in this published article.
Authors' contributions
DW and HL designed the experiments, and wrote and
revised the manuscript; JZ, YL and QY performed the experiments and
drafted the manuscript; JL and YL analyzed the data.
Ethics approval and consent to
participate
The animal experimental protocol was approved by the
Animal Ethics Committee of Jilin University (Jilin, China; no.
2015-003).
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Shakor AB, Atia M, Ismail IA, Alshehri A,
El-Refaey H, Kwiatkowska K and Sobota A: Curcumin induces apoptosis
of multidrug-resistant human leukemia HL60 cells by complex
pathways leading to ceramide accumulation. Biochim Biophys Acta.
1841:1672–1682. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Zhang Y, Zeng C, Lu S, Qin T, Yang L, Chen
S, Chen J and Li Y: Identification of miR-125b targets involved in
acute promyelocytic leukemia cell proliferation. Biochem Biophys
Res Commun. 478:1758–1763. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Yu M, Guo F, Wang J, Tan F and Li N: A
pH-Driven and photoresponsive nanocarrier: Remotely-controlled by
near-infrared light for stepwise antitumor treatment. Biomaterials.
79:25–35. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
You J, Shao R, Wei X, Gupta S and Li C:
Near-infrared light triggers release of Paclitaxel from
biodegradable microspheres: Photothermal effect and enhanced
antitumor activity. Small. 6:1022–1031. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Penjweini R, Loew HG, Breit P and Kratky
KW: Optimizing the antitumor selectivity of PVP-Hypericin re A549
cancer cells and HLF normal cells through pulsed blue light.
Photodiagnosis Photodyn Ther. 10:591–599. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Zhuang J, Liu J, Liu Y, Li H, Wang D and
Teng L: Enhanced proliferation inhibition of HL60 cells treated by
synergistic all-trans retinoic acid/blue light/nanodiamonds. RSC
Adv. 7:38895–38901. 2017. View Article : Google Scholar
|
|
7
|
Mohan V, Agarwal R and Singh RP: A novel
alkaloid, evodiamine causes nuclear localization of cytochrome-c
and induces apoptosis independent of p53 in human lung cancer
cells. Biochem Biophys Res Commun. 477:1065–1071. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Indran IR, Tufo G, Pervaiz S and Brenner
C: Recent advances in apoptosis, mitochondria and drug resistance
in cancer cells. Biochim Biophys Acta. 1807:735–745. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kim BM, Choi YJ, Han Y, Yun YS and Hong
SH: N, N-dimethyl phytosphingosine induces caspase-8-dependent
cytochrome c release and apoptosis through ROS generation in human
leukemia cells. Toxicol Appl Pharmacol. 239:87–97. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Wen Q, Zhang X, Cai J and Yang PH: A novel
strategy for real-time and in situ detection of cytochrome c and
caspase-9 in Hela cells during apoptosis. Analyst. 139:2499–2506.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Jia G, Wang Q, Wang R, Deng D, Xue L, Shao
N, Zhang Y, Xia X, Zhi F and Yang Y: Tubeimoside-1 induces glioma
apoptosis through regulation of Bax/Bcl-2 and the ROS/Cytochrome
C/Caspase-3 pathway. Onco Targets Ther. 8:303–311. 2015.PubMed/NCBI
|
|
12
|
Di Giovanni S, Mirabella M, Papacci M,
Odoardi F, Silvestri G and Servidei S: Apoptosis and ROS
detoxification enzymes correlate with cytochrome c oxidase
deficiency in mitochondrial encephalomyopathies. Mol Cell Neurosci.
17:696–705. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
McManus MJ, Murphy MP and Franklin JL:
Mitochondria-derived reactive oxygen species mediate
caspase-dependent and -independent neuronal deaths. Mol Cell
Neurosci. 63:13–23. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Hsing CH, Chen CL, Lin WC and Lin CF:
Propofol treatment inhibits constitutive apoptosis in human primary
neutrophils and granulocyte-differentiated human HL60 cells. PLoS
One. 10:e01296932015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Mayer B and Oberbauer R: Mitochondrial
regulation of apoptosis. News Physiol Sci. 18:89–94.
2003.PubMed/NCBI
|
|
16
|
Esposti Degli M and McLennan H:
Mitochondria and cells produce reactive oxygen species in virtual
anaerobiosis: Relevance to ceramide-induced apoptosis. FEBS Lett.
430:338–342. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Green DR and Reed JC: Mitochondria and
apoptosis. Science. 281:1309–1312. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Hu Q, Wu D, Chen W, Yan Z and Shi Y:
Proteolytic processing of the caspase-9 zymogen is required for
apoptosome-mediated activation of caspase-9. J Biol Chem.
288:15142–15147. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Raisova M, Hossini AM, Eberle J, Riebeling
C, Wieder T, Sturm I, Daniel PT, Orfanos CE and Geilen CC: The
Bax/Bcl-2 ratio determines the susceptibility of human melanoma
cells to CD95/Fas-mediated apoptosis. J Invest Dermatol.
117:333–340. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Ding J, Mooers BH, Zhang Z, Kale J,
Falcone D, McNichol J, Huang B, Zhang XC, Xing C, Andrews DW and
Lin J: After embedding in membranes antiapoptotic Bcl-XL protein
binds both Bcl-2 homology region 3 and helix 1 of proapoptotic Bax
protein to inhibit apoptotic mitochondrial permeabilization. J Biol
Chem. 289:11873–11896. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zhu B, Zhang H and Yu L: Novel transferrin
modified and doxorubicin loaded Pluronic 85/lipid-polymeric
nanoparticles for the treatment of leukemia: In vitro and in vivo
therapeutic effect evaluation. Biomed Pharmacother. 86:547–554.
2017. View Article : Google Scholar : PubMed/NCBI
|