Introduction
Breast cancer is a common malignancy that causes
cancer-associated mortality in females worldwide (1,2). Despite
the improved examination and therapeutic interventions in early
breast cancer, the prognosis for patients with metastatic breast
cancer continues torequire significant improvement (3). Although numerous approaches have been
applied to the treatmentof patients with breast cancer, their
clinical efficiency remains unsatisfactory (4,5).
Therefore, novel potential adjuvants, particularly those able to
increase the curative rates in these patients, should be
developed.
Traditional Chinese Medicines (TCMs) are widely used
in the United States and Western Europe. TCMs are considered safe
and effective, since TCM formulas have been utilized for thousands
of years in China (6). Although the
precise assessment of TCM is difficult, it is administered to
patients with chronic diseases, including cancer (7), autoimmune diseases (8), asthma (9)
and acquired immune deficiency syndrome (10). Chan Su (Venenumbufonis), a TCM
originating from the dried white secretions of the auricular and
skin glands of toads, has been used in China to treat numerous
conditions, including sore throat, palpitations and even cancer
(11,12). Telocinobufagin (TBG), which is
isolated from Chan Su, possesses enriched pharmacological
characteristics, including immunoregulation (13), anticancer (14)and inhibition of Na+/K+-ATPase activity
(15). Furthermore, TBG also exhibits
crucial antitumor characteristics, including restraining cell
proliferation, inhibiting cell differentiation, disrupting the cell
cycle, dominating tumor angiogenesis and accommodating immune
repercussion (15).
Epithelial-mesenchymal transition (EMT) is a
fundamental process in normal embryonic growth (16) and is associated with the stimulation
of migration in cancer (17).
However, EMT also serves a function in breast cancer and may induce
the migration and invasion of cancer cells (18). During EMT, cells lose their epithelial
features and gain mesenchymal phenotypes. For instance, their
adhesion, apico-basal polarity and E-cadherin levels are decreased,
while their N-cadherin, vimentin, and fibronectin levels are
increased. Consequently, phenotypic characteristics, including
anchorage-independent growth and motility, are developed. EMT is
often associated with the gene zinc-finger transcriptional
repressor Snail (19). The Snail
family of transcription factors comprises Snail, Slug, and Smuc
(20). Snail, Slug, Twist, Zeb1 and
Zeb2 are important transcription factors that regulate EMT and
promote the formation and development of breast cancer (12,21). In
the present study, Snail was decreased by TBG treatment, but Slug,
Twist, Zeb1, and Zeb2 remained unchanged following administration
of TBG.
In the present study, TBG inhibited the invasion and
migration of breast cancer cells, triggered the collapse of F-actin
filaments, downregulated the mesenchymal markers, including
vimentin and fibronectin, and upregulated epithelial markers
including E-cadherin, through the phosphoinositide 3-kinase
(PI3K)/protein kinase B (Akt)/extracellular signal-related kinase
(ERK)/Snail signaling pathway. TBG also downregulated Snail, a
crucial transcriptional factor of EMT. The anti-metastatic activity
of TBG was verified by establishing a spontaneous metastatic model
of BALB/c mice.
Materials and methods
Cell lines
The 4T1 mouse cell line was obtained from the
American Type Culture Collection (Manassas, VA, USA). The 4T1 cells
were cultured in RPMI-1640 medium (Sigma-Aldrich; MerckKGaA,
Darmstadt, Germany), supplemented with 10% fetal bovine serum (FBS;
Sigma-Aldrich; Merck KGaA). The 4T1 cells were cultured in a
humidified atmosphere of 95% air and 5% CO2 at 37°C.
MTT assays
Cell viability was measured by MTT assay. 4T1
(5,000) cells were plated onto 96-well plates overnight. The cells
were treated with various concentrations of TBG (0, 0.05, 0.1, 0.5,
1, 1.25 and 1.5 µg/ml) for 48 h. The cells were incubated with MTT
solutions for 4 h at 37°C and DMSO was added to each well. The
absorbance was measured at 570 nm using a multifunctional
microplate reader (SpectraMax M5; Molecular Devices, LLC,
Sunnyvale, CA, USA).
Wound healing assay
A total of 5×105 4T1 cells were plated
onto 6-well plates overnight. Wounds were created by scratching
with a 10-µl white pipette tip. Detached cells were washed using
phosphate-buffered saline (PBS). TBG (Biopurify Phytochemicals
Ltd., Chengdu, China) was dissolved in 0.05 µg/ml dimethyl
sulfoxide (DMSO) concentration. A control group with an equal DMSO
concentration of RPMI-1640 medium (Sigma-Aldrich; Merck KGaA) was
created. The different concentrations of TBG (0, 0.05 and 0.5
µg/ml) were used to culture cells for 24 h. The wound distances
were measured under afluorescence microscope (LSM710;
magnification, ×20; Carl Zeiss GmbH, Jena, Germany). The wound area
was imaged using ImageJ software 1.8.0 (National Institutes of
Health, Bethesda, MD, USA). The same fields were measured again to
survey the wound gap. The experiments were repeated at least three
times.
Cell migration assay
In this procedure, 4×104 4T1 cells were
added to the upper chamber with 0.05 µg/ml TBG and RPMI-1640 medium
(Sigma-Aldrich; Merck KGaA), or the control treatment without FBS.
A total of 0.5 ml RPMI-1640 medium with 10% FBS was added to the
lower chamber. The chambers were incubated at 37°C for 48 h. The
transmigrated cells were stained with 0.2% crystal violetat room
temperature for 10 min. The invaded cells were imaged and counted
under afluorescence microscope (magnification, ×200). The
experiments were repeated at least three times.
Cell invasion assay
The cell invasion assay was conducted using a BD
PET-track-etched membrane invasion chamber with 50 µl diluted
Matrigel (BD Biosciences, San Jose, CA, USA) overnight. A total of
4×104 4T1 cells were added to the upper chamber with
0.05 µg/ml TBG and RPMI-1640 medium (Sigma-Aldrich; Merck KGaA), or
the control treatment without FBS. A total of 0.5 ml RPMI-1640
medium with 10% FBS was added to the lower chamber. The chambers
were incubated at 37°C for 48 h. The invaded cells were stained
with 0.2% crystal violetat room temperature for 10 min. The invaded
cells were imaged and counted under a fluorescence microscope
(magnification, ×200). The experiments were repeated at least three
times.
Immunofluorescence
In this procedure, 4×104 4T1 cells were
cultured in 24-well plates overnight, prior to 0.05 µg/ml TBG being
added. The control group was cultured in RPMI-1640 medium
(Sigma-Aldrich; Merck KGaA), with 10% FBS (Sigma-Aldrich; Merck
KGaA) for 24 h. 4T1 cells were washed three times with PBS, fixed
with 4% paraformaldehyde for 15 min at the room temperature and
permeabilized with 0.5% Triton X-100 for 20 min at the room
temperature. Next, the cells were blocked with 1% bovine serum
albumin (BSA) for 1 h at the room temperature. Cells were
subsequently incubated with primary antibodies against the
following: E-cadherin (1:100; UM870076), vimentin (1:100; UM870054)
and Fibronectin (1:100; AM06754SU-N; all from OriGene Technologies,
Inc., Rockville, MD, USA) at 4°C overnight, prior tobeing washed
three times with PBS. Cells were then incubated with Dylight 488
(1:100; 200-482-211) and Dylight 649 (1:100; A23620), secondary
antibodies including DyLight 488 AffiniPure Goat Anti-Mouse IgG
(H+L) (1:50; A23210), and DyLight 649 AffiniPure Goat Anti-Mouse
IgG (H+L) (1:50, A23610) in the dark for 1 h at 37°C. They were
purchased from Amy Jet Scientific, Inc., Hubei, China. The cell
nuclei were labeled with 4′,6-diamidine-2-phenylindole (DAPI).
Images was captured under a fluorescence microscope (LSM710;
magnification, ×100; Carl Zeiss GmbH, Jena, Germany).
Western blot analysis
4T1 cells were cultured overnight, prior to the
addition of 0.05 or 0.5 µg/ml TBG for 24 h at 37°C. Te control
cells were cultured in aRPMI-1640 medium (Sigma-Aldrich; Merck
KGaA), with 10% FBS (Sigma-Aldrich; Merck KGaA) for 24 h at 37°C.
Cells were lysed in phenylmethanesulfonyl fluoride (100 mM,
Beyotime Institute of Biotechnology, Haimen, China) in the fridge
at 4°C, and then centrifuged at 4°C at 12,000 × g for 5 min. The
protein concentration was determined using a BCA Protein Assay kit
(AmyJet Scientific, Inc.). Proteins (20 µg) were separated by 10%
SDS-PAGE followed by electro transfer onto polyvinylidene fluoride
membranes. After blocking with 5% non-fat dry milk in TBST buffer
at 37°C for 1 h, the membranes were incubated in TBST buffer with
the antibodies Akt (1:1,000; 4685), P-Akt (1:1,000; 4060), m-TOR
(1:1,000; 2983), P-mTOR (1:1,000; 5536), P-ERK (1:1,000; 4370) and
Snail (1:1,000; 3879) overnight at 4°C, which were purchased from
Cell Signaling Technology, Inc., (Danvers, MA, USA). The membranes
were incubated in TBST buffer with the antibodies against ERK
(1:500; sc-135900), Slug (1:500; sc-166476), Twist1 (1:500;
sc-6269), Zeb1 (1:500; sc10572) and Zeb2 (1:500; sc-271984)
overnight at 4°C, which were obtained from Santa Cruz
Biotechnology, Inc. (Dallas, TX, USA). The membranes were incubated
in TBST buffer with the antibodies E-cadherin (1:1,000; ab76055),
Fibronectin (1:1,000; ab2413) and vimentin (1:1,000; ab92547),
β-actin (1:2,000; ab8266) overnight at 4°C, which were obtained
from Abcam (Cambridge, UK). Membranes were then incubated in TBST
buffer with the Anti-Rabbit IgG VHH Single Domain HRP (1:3,000;
ab191866) secondary antibodies and Anti-Mouse IgG1 VHH Single
Domain HRP (1:3,000; ab193651) secondary antibodiesfor 1 h at room
temperature. All Membranes were developed with ECL reagents. by ECL
Plus Western Blot Detection System kit (Amersham, Piscataway, NJ,
USA). The blots were analyzed by IPP6.0 (Media Cybernetics, Inc.,
Rockville, MD, USA).
Observation of F-actin filaments
4T1 cells (4×104) were plated onto
sterile climbing plates in 24-well plates and were cultured in
RPMI-1640 medium (Sigma-Aldrich; Merck KGaA), supplemented with 10%
FBS (Sigma-Aldrich; Merck KGaA). The cells were treated with 0.05
µg/ml TBG at 1, 3, 6 and 12 h to observe the collapse of F-actin
filaments. 4T1 cells were washed three times with PBS, fixed with
4% paraformaldehyde for 15 min at room temperature and
permeabilized with 0.1% Triton X-100 for 20 min at room
temperature. Subsequently, the cells were blocked with 10% BSA for
1 h at room temperature. The cells were incubated with 5 µM
fluorescein isothiocyanate (FITC) phalloidin at 37°C for 1 h to
reveal F-actin. The cell nuclei were labeled with DAPI for 2 min at
room temperature and were then measured using a confocal microscope
(magnification, ×200; Carl Zeiss AG).
Tumor xenografts
A total of 30 Six-week-old female BALB/c nude mice
(18–22 g) were obtained from Qingdao Daren Fortune Animal
Technology Co., Ltd. (Qingdao, China). Mice were housedat ~20°C,
55–60% humidity, with a 12-h light/dark cycle. Food and water were
provided ad libitum. All animal experiments were implemented
under the guidelines approved by the Institutional Animal Care and
Use Committee (IACUC) of the company. Animal protocols were
approved by the guidelines built by the Animal Care Committee at
Weifang Medical University. The mice were acclimatized for one week
prior to the start of the study. A total of 30 BALB/c nude mice
were divided randomly into three groups. 4T1 cells
(2×106) were injected into the mammary fat pads of
female BALB/c nude mice. One week after the injection, three groups
of mice were treated with vehicle or TBG at 10 or 20 µg/mouse three
times a week by intra peritoneal injection for two consecutive
weeks. The same concentration was administered to all mice
regardless of weight. The primary tumor and lung metastases were
isolated from the mammary fat pads and lung, which were separated
from surrounding tissue by surgical scissors. The primary tumor and
lung fixed with formalin and embedded in paraffin at 55°C for 3 h
and sectioned at 0.4 cm. The sections were placed in xylene for 10
min, then fresh xylene for another 10 min before rehydration in a
descending alcohol series (100% for 8 min, 100% for 8 min, 95% for
10 min, 80% for 5 min and 75% for 1 min). Antibody repair was
performed in a high-pressure vessel. After the pressure cooker
reached the maximum pressure (120 kPa) for 3 min, and the cold
water was cooled for 10 min. Immunohistochemistry was utilized to
detect the levels of E-cadherin (1:100; UM870076), vimentin (1:100;
UM870054) and Fibronectin (1:100; AM06754SU-N; all from OriGene
Technologies, Inc., Rockville, MD, USA) in the primary tumors by
incubating with the antibodies at 4°C overnight. The secondary
antibodies (sp-0022; were from an immunohistochemical kit purchased
from Shanghai Hao ran Biotechnologies Co., Ltd (Shanghai, China).
The sections were incubated with the second antibody reagent 1 at
37°C for 40 min and reagent 2 at 37°C for 40 min. The specimens
were imaged under afluorescence microscope (magnification, ×100).
To observe lung micro metastasis, sections were stained in
hematoxylin at room temperature for 5 min, and eosin at room
temperature for 30 sec.
Statistical analyses
Data and statistical graphs were analyzed using
GraphPad Prism (version 6.0; GraphPad Software, Inc., La Jolla, CA,
USA). The statistical significance among all groups was determined
using anone-way analysis of variance with Bonferroni's multiple
comparison test as a post hoc test. P<0.05 was considered to
indicate a statistically significant difference.
Results
TBG inhibits the migration and
invasion of breast cancer cells
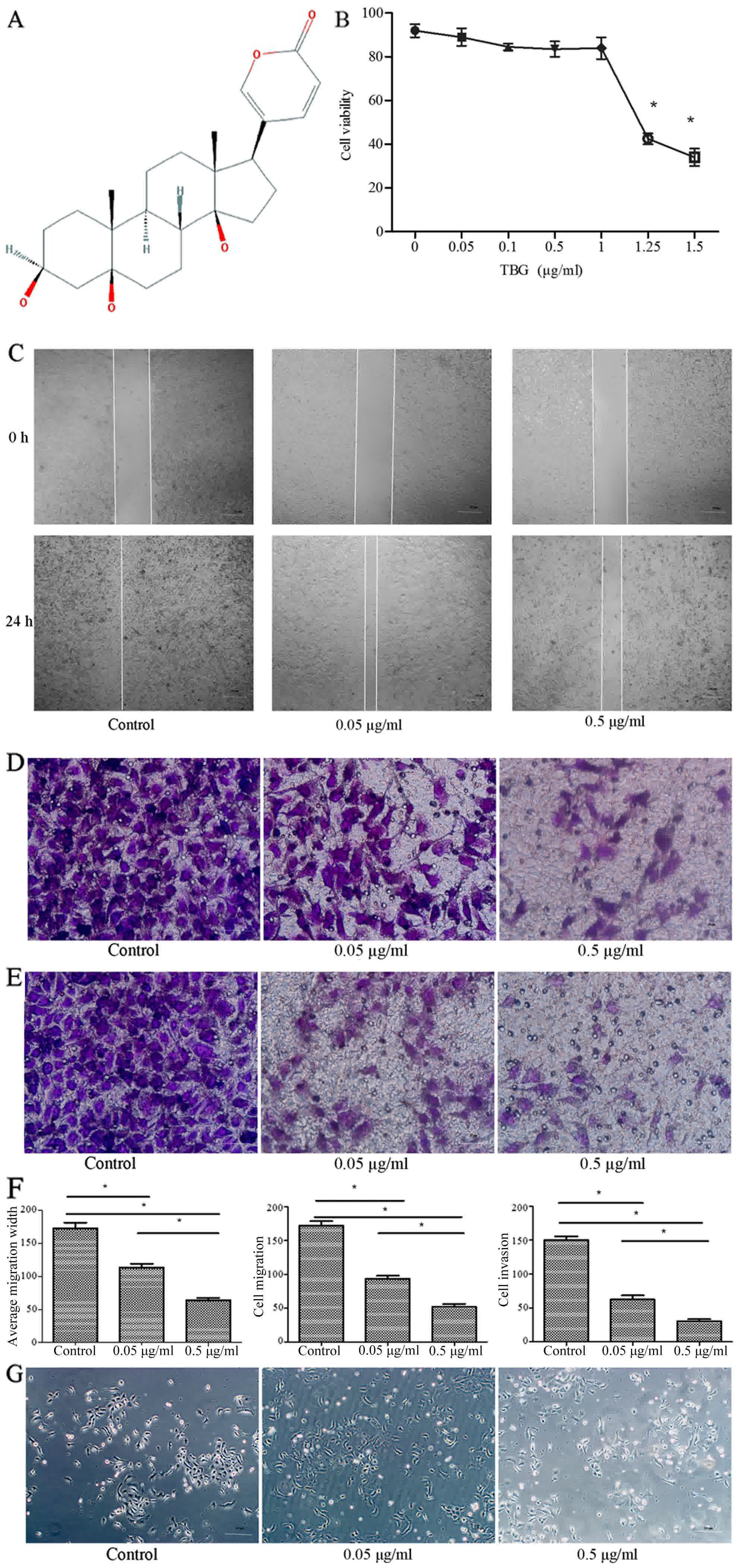
The molecular structure of TBG was obtained from the
product manual of Chan Su (V. bufonis) and is presented in
Fig. 1A. To determine the effect of
TBG treatment on cellular proliferation, 4T1 cells were treated
with varying concentrations of TBG. No significant proliferation
and inhibition activity was detected by an MTT assay following
administration of reasonable concentrations of the drug (0.05 and
0.5 µg/ml TBG). However, with an increase in the concentration of
TBG, the proliferation of cells was affected (Fig. 1B). A scratch assay was used to assess
the effects of TBG on cell migration. TBG was applied at 0.05 and
0.5 µg/ml. After 24 h, the migration abilities of 4T1 cells were
restrained in a concentration-dependent manner (Fig. 1C). The wounded area of the control
group was almost entirely occupied by the migrating cells after 24
h, which was in contrast to the relatively wider gap observed in
the TBG-treated groups.
The influence of TBG on migration and invasion was
assessed by conducting Transwell assays for 48 h. TBG was used at
0.05 and 0.5 µg/ml. TBG controlled the migration and invasion of
4T1 cells in a concentration-dependent manner. The number of
migrating cells in the high-concentration group was reduced
compared with that in the control group (Fig. 1D-F). No significant differences were
identified in the morphological characteristics of the cells
compared with the treatment groups (Fig.
1G).
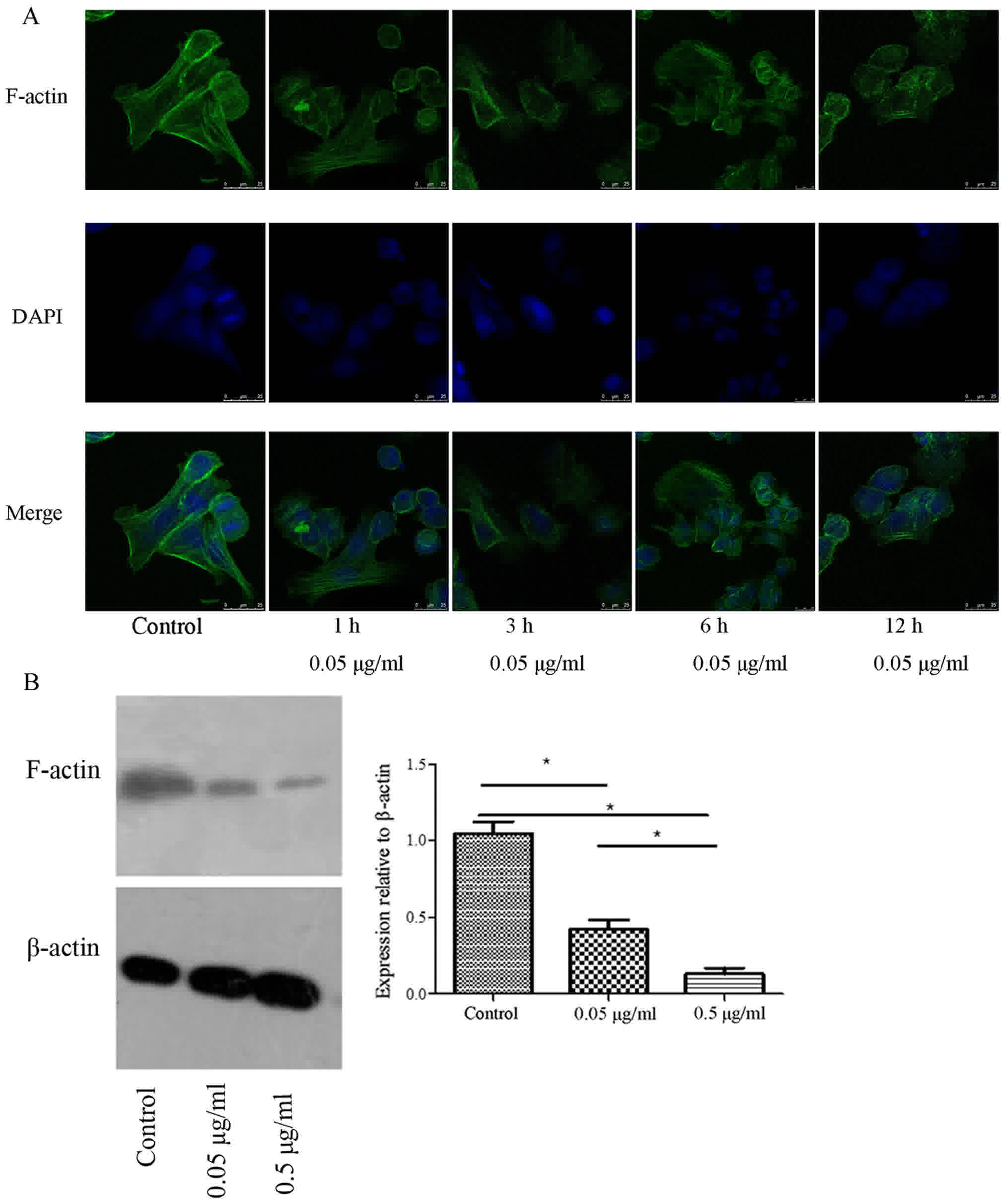
TBG induces the disintegration of
F-actin filament cytoskeleton in breast cancer cells
The cells were treated with TBG at 1, 3, 6 and 12 h
to observe the changes in the F-actin filaments. The F-actin
filaments were observed by staining with FITC-labeled phalloidin,
and the cell nuclei were labeled with DAPI. In the control group,
the F-actin filaments exhibited a regular arrangement and were
evenly distributed in the cytoplasm. In the cells treated with TBG,
the structure of the F-actin filaments changed at 3 h, and F-actin
filaments were destroyed at 12 h (Fig.
2A), suggesting that TBG triggered the collapse of the F-actin
filament cytoskeleton in vitro, as well as increasing the
contribution time (1, 3, 6 and 12 h). Furthermore, F-actin levels
were decreased relative to β-actin following TBG treatment
(Fig. 2B).
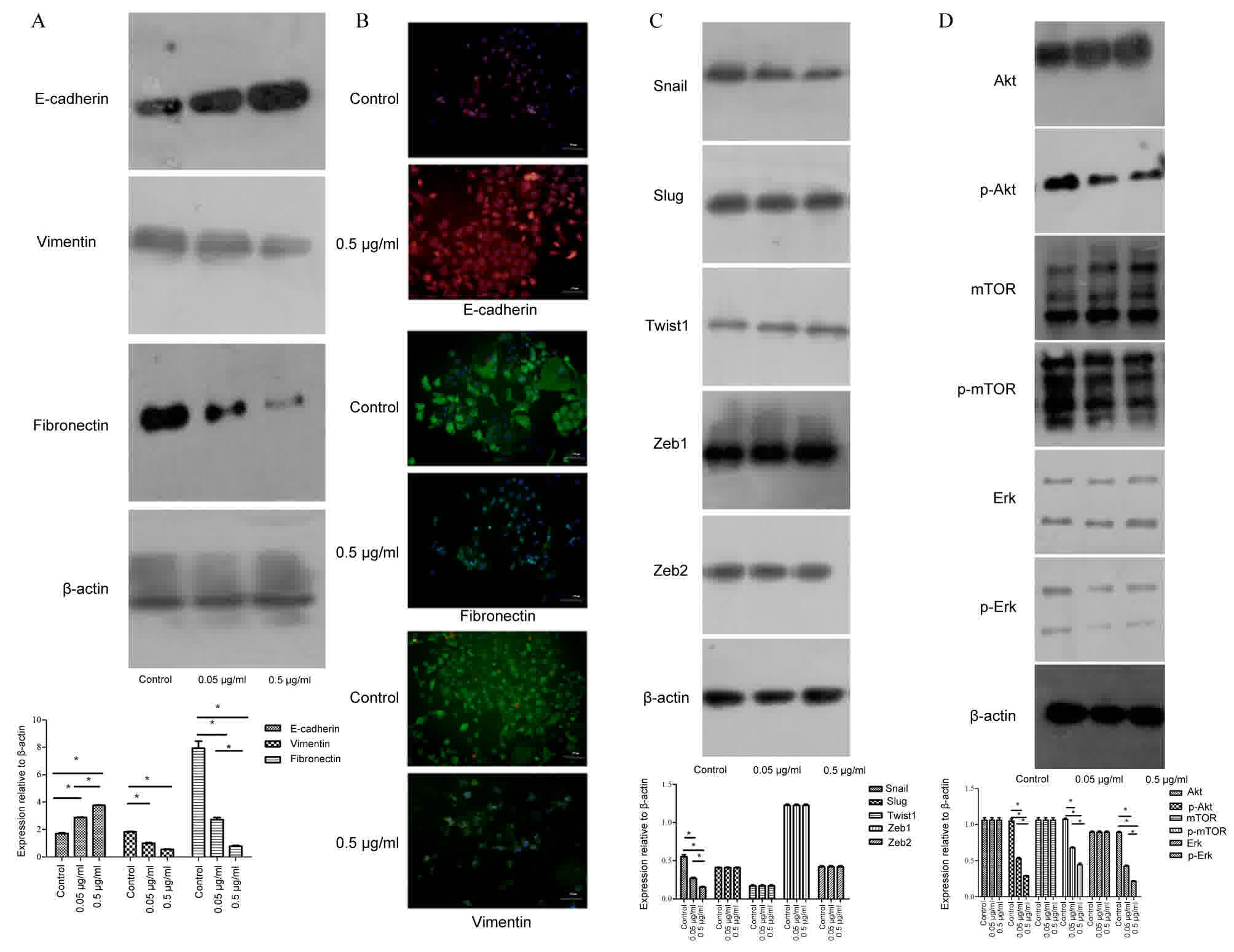
TBG regulates EMT markers
EMT has been positively associated with the
metastatic potential of tumor cells (17). To investigate whether TBG inhibits
breast cancer cell migration and invasion via EMT, epithelial and
mesenchymal markers, including E-cadherin, vimentin and fibronectin
were investigated. The results revealed that vimentin and
fibronectin were decreased in treated cells, but that E-cadherin
was increased following TBG treatment (Fig. 3A). Immunofluorescence analysis was
utilized to assess E-cadherin in the cell membrane and cytoplasm,
and vimentin and fibronectin in the cytoplasm, which revealed the
same results (Fig. 3B). Therefore,
these results suggested that TBG enhanced the epithelial traits and
inhibited the mesenchymal properties of breast cancer.
TBG downregulates the transcription
factor Snail via the Akt/ERK signaling pathway
Several crucial transcription factors were observed
in the 4T1 cells to determine which transcription factors,
modulated by TBG, further regulated and controlled EMT. Snail was
downregulated in 4T1 cells following TBG treatment (Fig. 3C). The other transcriptional factors,
including Slug, Twist1, Zeb1 and Zeb2 exhibited no notable
differences between the control group and the TBG treatment group
(Fig. 3C).
The PI3K/Akt and ERK/mitogen-activated protein
kinase (MAPK) signaling pathways are associated with EMT (22). Therefore, in order to reveal the
underlying molecular mechanisms associated with TBG-inhibited EMT
in 4T1 cells, markers including Akt, phosphorylated (p)-Akt,
mechanistic target of rapamycin (mTOR), P-mTOR, ERK and p-ERK. The
results identified that TBG inhibited p-AKT, P-mTOR and P-ERK in a
concentration-dependent manner. AKT, mTOR and ERK were unchanged
between the control group and the TBG treatment groups (Fig. 3D). Therefore, the data gathered
revealed that TBG inhibited EMT in vitro via the
Akt/ERK/Snail signaling pathway.
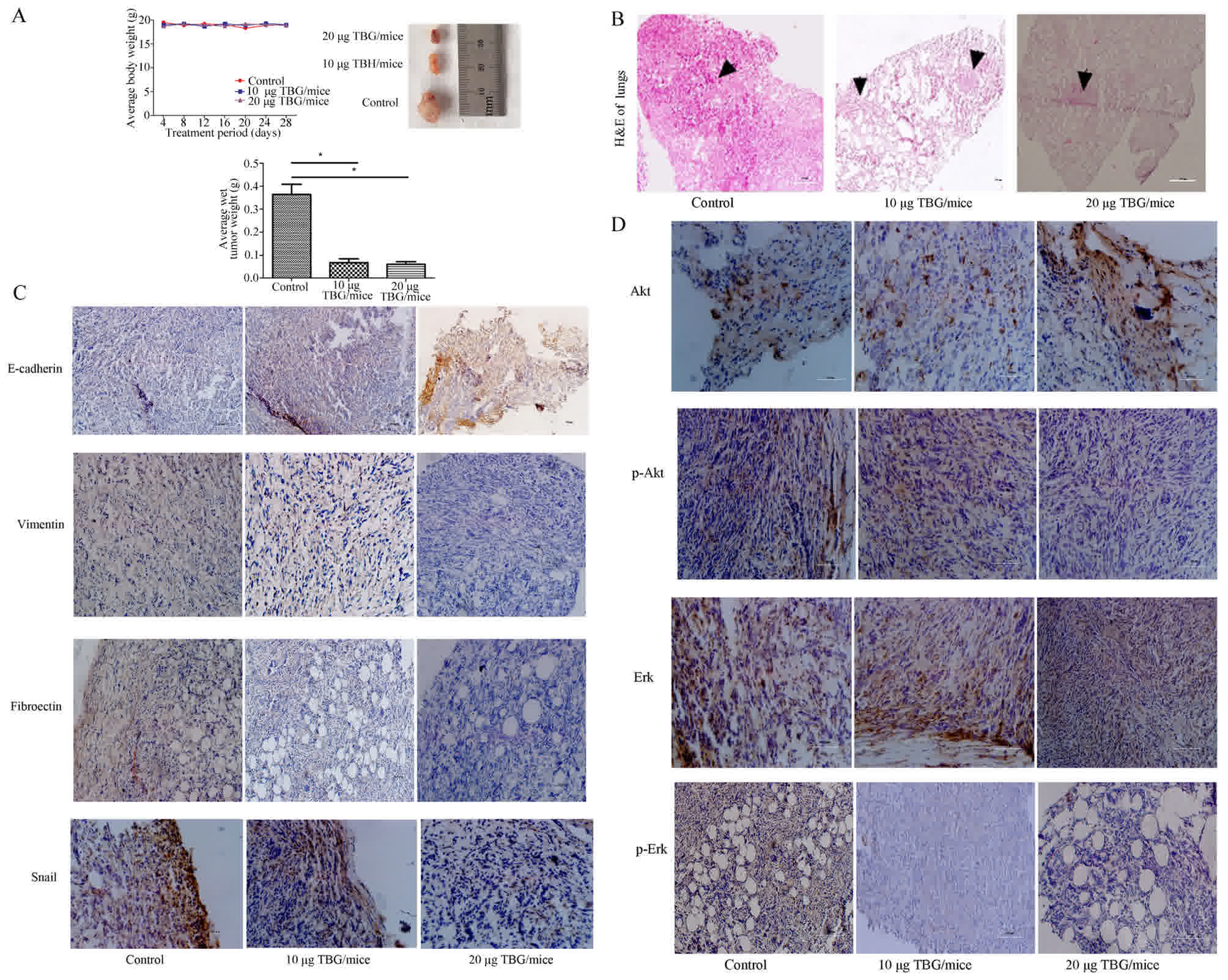
TBG inhibited tumor growth, metastasis
and EMT in the mouse model via the Akt/ERK/Snail signaling
pathway
An in vivo study was conducted to further
evaluate the anti-metastatic effect of TBG in highly metastatic 4T1
mouse models. TBG was considered non-toxic at the doses used, since
no body weight changes were observed in these mice (Fig. 4A). Notably, the single maximum tumor
volume of the vehicle-treated mice was increased compared with that
of the TBG-treated mice. Furthermore, the average tumor weight of
the TBG-treated mice was less than that of the vehicle-treated mice
(Fig. 4A). In the present study,
multiple tumors in mammary fat pads were not observed. H&E
staining was performed to confirm the pathological metastasis in
the lungs, and the metastatic tumors were observed via microscopy
(Fig. 4B). The mesenchymal biomarker
E-cadherin was decreased, whereas the epithelial biomarkers
vimentin and fibronectin were increased in vitro following
TBG treatment. To determine their expression in primary tumor
tissues immunohistochemical staining was conducted. The TBG
treatment downregulated Snail, vimentin, fibronectin, P-Akt and
P-ERK compared with the vehicle group (Fig. 4C and D). By contrast, E-cadherin was
upregulated (Fig. 4C). The survival
time of tumor-bearing mice was not measured or analyzed in the
present study. Taken together, the results gathered indicated that
TBG may be able to inhibit EMT by blocking the Akt/ERK/Snail
pathway in vivo.
Discussion
Chan Su is probably one of the most extensively used
TCMs owing to its pharmacological properties, including anticancer
and immuno regulatory effects (12).
TBG has potent anti-metastasis features (12) and the present study identified that
TBG inhibited EMT in breast cancer.
Breast cancer migration and invasion are associated
with EMT (23–25). Cell migration not only controls normal
cellular processes, including tissue development, chemotaxis and
wound healing (26,27), but is also involved in the invasion
and metastasis of neoplasms (28).
The results of the present study indicated that TBG significantly
suppressed cell migration and invasion in breast cancer. It was
identified that lower doses of TBG-treatment inhibited cell
migration and invasion, while higher doses of TBG inhibited
cellular proliferation in vitro. Furthermore, the results
revealed that TBG at 10 and 20 µg/mouse inhibited average tumor
volume, tumor weight and lung metastasis in these mice. In the
present study, the TBG-mediated inhibition of cellular
proliferation, migration and invasion was investigated, and the
potential underlying molecular mechanism of heTBG-mediated
anti-metastatic effect was evaluated using in vitro and
in vivo models. However, the use of a single cell line is a
limitation of the present study.
The polymerization and depolymerization of F-actin
filaments and cell migration are also associated with neoplasm
metastasis (29). The present study
detected whether the disintegration of the F-actin cytoskeleton was
associated with the anti-metastatic characteristics of TBG. It was
identified that TBG resulted in the collapse of the F-actin
cytoskeleton and further disrupted the polymerization and
depolymerization of F-actin filaments.
When cancer cells undergo EMT, the epithelial
phenotype is lost and a mesenchymal phenotype is obtained, further
increasing the invasive and migratory properties (30). E-cadherin has a significant effect on
the cell-to-cell adhesion that inhibits the invasion and metastasis
of tumors (31). Fibronectin and
vimentin are regarded as critical mesenchymal markers. The present
study investigated whether E-cadherin was upregulated, and
fibronectin and vimentin were downregulated following TBG treatment
in 4T1 cells. Therefore, these data identified that TBG inhibits
EMT in breast cancer cells.
The aberrant regulation of the transcription factor
Snail is associated with EMT in multiple types of cancer (32). Snail is a transcription factor that
serves a critical function in modulating genes, including
E-cadherin, fibronectin and vimentin, which may be regulated at
transcriptional and post-transcriptional levels via a complex
signaling pathway (33). The
experiments in the present study tested which transcription factors
were inhibited with TBG treatment, and identified that TBG
inhibited Snail, but that Slug, Twist1, Zeb1 and Zeb2 transcription
factors were not notably altered.
The PI3K/Akt and MAPK/ERK signaling pathway sserves
a critical function in cell polarization, apoptosis, invasion and
migration and are associated with the pathogenesis of multiple
types of cancer (34–38). Notably, Ras-MAPK activates the
relevant transcription factor Snail, which inhibits the
transcription of E-cadherin and accelerates EMT (39,40). ERK
cascades serve a function in a number of cellular processes,
including proliferation, differentiation, migration and invasion
(41). TBG repressed Snail via the
PI3K/Akt and MEK/ERK signaling pathway. Therefore, TBG promoted
E-cadherin expression, and suppressed vimentin and fibronectin
expression through the Akt/ERK/Snail signaling pathway.
In summary, TBG suppressed EMT, metastasis, invasion
and migration in vivo and in vitro. The present study
revealed that TBG suppressed EMT via the Akt/ERK/Snail signaling
pathway. The Akt/ERK/Snail signaling pathway was a promising target
of TBG. According to the results of the present study, TBG, a small
molecular agent, may have important implications for the effective
treatment of patients with breast cancer.
Acknowledgements
Not applicable.
Funding
The present study was supported by the funds from
the Scientific Foundation of Shandong Province (ZR2014HM086) and
the Scientific Foundation of Shandong Province (ZR2015HL119).
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
YG and LS wrote the paper and conducted the
experiments. ZC, XZ and FL conducted the western blot analysis. RW,
JX and JZ conducted the immunohistochemistry and H&E staining.
BZ and SL provided assistance with the experimental ideas and
technical side of the present study.
Ethics approval and consent to
participate
All animal experiments were implemented under the
guidelines approved by the Institutional Animal Care and Use
Committee (IACUC) of the company. Animal protocols were approved by
the guidelines built by the Animal Care Committee at Weifang
Medical University.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Downs-Holmes C and Silverman P: Breast
cancer: Overview & updates. Nurse Pract. 36:20–26. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2015. CA Cancer J Clin. 65:5–29. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Mehlen P and Puisieux A: Metastasis: A
question of life or death. Nat Rev Cancer. 6:449–458. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Naume B, Synnestvedt M, Falk RS, Wiedswang
G, Weyde K, Risberg T, Kersten C, Mjaaland I, Vindi L, Sommer HH,
et al: Clinical outcome with correlation to disseminated tumor cell
(DTC) status after DTC-guided secondary adjuvant treatment with
docetaxel in early breast cancer. J Clin Oncol. 32:3848–3857. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Early Breast Cancer Trialists'
Collaborative Group (EBCTCG): Aromatase inhibitors versus tamoxifen
in early breast cancer: Patient-level meta-analysis of the
randomised trials. Lancet. 386:1341–1352. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Lin H, Jie L and Ying Z: Developments in
cancer prevention and treatment using traditional Chinese medicine.
Front Med. 5:127–133. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Zhang DY, Wu J, Ye F, Xue L, Jiang S, Yi
J, Zhang W, Wei H, Sung M, Wang W and Li X: Inhibition of cancer
cell proliferation and prostaglandin E2 synthesis by Scutellaria
baicalensis. Cancer Res. 63:4037–4043. 2003.PubMed/NCBI
|
|
8
|
Chen Z and Li Y and Li Y: Review of study
on mechanism of traditional Chinese medicine in treating
autoimmunity disease. Zhong Yao Cai. 26:218–221. 2003.PubMed/NCBI
|
|
9
|
Wen MC, Wei CH, Hu ZQ, Srivastava K, Ko J,
Xi ST, Mu DZ, Du JB, Li GH, Wallenstein S, et al: Efficacy and
tolerability of anti-asthma herbal medicine intervention in adult
patients with moderate-severe allergic asthma. J Allergy Clin
Immunol. 116:517–524. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ernst E: Complementary AIDS therapies: The
good, the bad and the ugly. Int J STD AIDS. 8:281–285. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Chan WY, Ng TB and Yeung HW: Examination
for toxicity of a Chinese drug, the toad glandular secretory
product chan su, in pregnant mice and embryos. Biol Neonate.
67:376–380. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Wu SC, Fu BD, Shen HQ, Yi PF, Zhang LY, Lv
S, Guo X, Xia F, Wu YL and Wei XB: Telocinobufagin enhances the Th1
immune response and protects against Salmonella typhimurium
infection. Int Immunopharmacol. 25:353–362. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Cao Y, Song Y, An N, Zeng S, Wang D, Yu L,
Zhu T, Zhang T, Cui J, Zhou C and Deng X: The effects of
telocinobufagin isolated from Chan Su on the activation and
cytokine secretion of immunocytes in vitro. Fundam Clin Pharmacol.
23:457–464. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Qi F, Li A, Inagaki Y, Kokudo N, Tamura S,
Nakata M and Tang W: Antitumor activity of extracts and compounds
from the skin of the toad Bufo bufo gargarizans cantor. Int
Immunopharmacol. 11:342–349. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zhou LX and Guo JM: Research progress in
targeted therapy for hepatocellular carcinoma. J Oncol. 15:156–161.
2009.(In Chinese).
|
|
16
|
Baum B, Settleman J and Quinlan MP:
Transitions between epithelial and mesenchymal states in
development and disease. Semin Cell Dev Biol. 19:294–308. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Thiery JP, Acloque H, Huang RY and Nieto
MA: Epithelial-mesenchymal transitions in development and disease.
Cell. 139:871–890. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Tomaskovic-Crook E, Thompson EW and Thiery
JP: Epithelial to mesenchymal transition and breast cancer. Breast
Cancer Res. 11:2132009. View
Article : Google Scholar : PubMed/NCBI
|
|
19
|
Lamouille S, Xu J and Derynck R: Molecular
mechanisms of epithelial-mesenchymal transition. Nat Rev Mol Cell
Biol. 15:178–196. 2014. View
Article : Google Scholar : PubMed/NCBI
|
|
20
|
Mezencev R, Matyunina LV, Jabbari N and
McDonald JF: Snail-induced epithelial-to-mesenchymal transition of
MCF-7 breast cancer cells: Systems analysis of molecular changes
and their effect on radiation and drug sensitivity. BMC Cancer.
16:2362016. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Nieto MA: The snail superfamily of
zinc-finger transcription factors. Nat Rev Mol Cell Biol.
3:155–166. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
22
|
Zhang YQ, Wei XL, Liang YK, Chen WL, Zhang
F, Bai JW, Qiu SQ, Du CW, Huang WH and Zhang GJ: Over-expressed
twist associates with markers of epithelial mesenchymal transition
and predicts poor prognosis in breast cancers via ERK and Akt
activation. PLoS One. 10:e01358512015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Teschendorff AE, Journee M, Absil PA,
Sepulchre R and Caldas C: Elucidating the altered transcriptional
programs in breast cancer using independent component analysis.
PLoS Comput Biol. 3:e1612007. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Mironchik Y, Winnard PT Jr, Vesuna F, Kato
Y, Wildes F, Pathak AP, Kominsky S, Artemov D, Bhujwalla Z, Van
Diest P, et al: Twist overexpression induces in vivo angiogenesis
and correlates with chromosomal instability in breast cancer.
Cancer Res. 65:10801–10809. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Fedele M, Cerchia L and Chiappetta G: The
epithelial-to-mesenchymal transition in breast cancer: Focus on
basal-like carcinomas. Cancers (Basel). 9:pii: E134. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Lu S, Niu N, Guo H, Tang J, Guo W, Liu Z,
Shi L, Sun T, Zhou F, Li H, et al: ARK5 promotes glioma cell
invasion, and its elevated expression is correlated with poor
clinical outcome. Eur J Cancer. 49:752–763. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Pardo R, Andreolotti AG, Ramos B,
Picatoste F and Claro E: Opposed effects of lithium on the MEK-ERK
pathway in neural cells: Inhibition in astrocytes and stimulation
in neurons by GSK3 independent mechanisms. J Neurochem. 87:417–426.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Noritake J, Watanabe T, Sato K, Wang S and
Kaibuchi K: IQGAP1: A key regulator of adhesion and migration. J
Cell Sci. 118:2085–2092. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Jiang Y, Leung AW, Wang X, Zhang H and Xu
C: Effect of photodynamic therapy with hypocrellin B on apoptosis,
adhesion, and migration of cancer cells. Int J Radiat Biol.
90:575–579. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Yilmaz M and Christofori G: EMT, the
cytoskeleton, and cancer cell invasion. Cancer Metastasis Rev.
28:15–33. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Wu Y and Zhou BP: Epithelial-mesenchymal
transition in development and diseases. Springer; New York: pp.
187–211. 2010
|
|
32
|
de Herreros AG, Peiró S, Nassour M and
Savagner P: Snail family regulation and epithelial mesenchymal
transitions in breast cancer progression. J Mammary Gland Biol
Neoplasia. 15:135–147. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Singh A and Settleman J: EMT, cancer stem
cells and drug resistance: An emerging axis of evil in the war on
cancer. Oncogene. 29:4741–4751. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Xue G and Hemmings BA: PKB/Akt-dependent
regulation of cell motility. J Natl Cancer Inst. 105:393–404. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Krepischi AC, Maschietto M, Ferreira EN,
Silva AG, Costa SS, da Cunha IW, Barros BDF, Grundy PE, Rosenberg C
and Carraro DM: Genomic imbalances pinpoint potential oncogenes and
tumor suppressors in Wilms tumors. Mol Cytogenet. 9:202016.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Zhou Q, Chen J, Feng J, Xu Y, Zheng W and
Wang J: SOSTDC1 inhibits follicular thyroid cancer cell
proliferation, migration, and EMT via suppressing PI3K/Akt and
MAPK/Erk signaling pathways. Mol Cell Biochem. 435:87–95. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Martini M, De Santis MC, Braccini L,
Gulluni F and Hirsch E: PI3K/AKT signaling pathway and cancer: An
updated review. Ann Med. 46:372–383. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Shi L, Sun X, Zhang J, Zhao C, Li H, Liu
Z, Fang C, Wang X, Zhao C, Zhang X, et al: Gab2 expression in
glioma and its implications for tumor invasion. Acta Oncol.
52:1739–1750. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Batlle E, Sancho E, Francí C, Domínguez D,
Monfar M, Baulida J and De Herreros García A: The transcription
factor snail is a repressor of E-cadherin gene expression in
epithelial tumour cells. Nat Cell Biol. 2:84–89. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Cano A, Pérez-Moreno MA, Rodrigo I,
Locascio A, Blanco MJ, del Barrio MG, Portillo F and Nieto MA: The
transcription factor snail controls epithelial-mesenchymal
transitions by repressing E-cadherin expression. Nat Cell Biol.
2:76–83. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Roberts PJ and Der CJ: Targeting the
Raf-MEK-ERK mitogen-activated protein kinase cascade for the
treatment of cancer. Oncogene. 26:3291–3310. 2007. View Article : Google Scholar : PubMed/NCBI
|