Introduction
Gastric cancer is responsible for ~989,600 incident
diagnoses and ~738,000 cases of mortality annually worldwide
(1,2).
It is detected more commonly in certain regions, including Eastern
Asia, Europe and South America (3).
Continuous efforts are being made by chemists and clinicians
worldwide for improving the prognosis rate of gastric cancer.
However, despite the use of various chemotherapeutic agents and
surgery, the overall 5-year survival rate of gastric cancer
patients is <20% (4,5). Thus, the development of novel and
effective treatment strategies for gastric cancer is urgently
required.
Natural products isolated from plants, animals,
fungi and bacteria exhibit a diverse range of range of biological
activities (6–9). They have been used for the treatment of
numerous types of disease through the development of innovative
drugs (6–9). The main advantage of natural products
for the treatment of disease is that they have evolved to possess
functionalities that are well-suited as biomolecular frameworks
(10). Flavones are the natural
products with a wide range of biological activities due to the
presence of a benzoquinone pharmacophore (11). The molecule
3,3′,4′,7-tetrahydroxyflavone, commonly known as fisetin, is a
member of the flavonoid family (11).
Fisetin is present in fruits and vegetables (12) and its biological evaluation has
revealed promising anti-cancer activity. Treatment with fisetin led
to inhibition of proliferation and metastasis potential in bladder,
pancreatic and cervical carcinoma cells (12,13). In a
nude mouse model of prostate cancer, fisetin treatment caused a
marked reduction in tumor growth (14). Fisetin has been demonstrated to
activate extracellular signal-regulated kinase (ERK) 1/2 in other
cell line models (15,16). In the present study, the effect of
fisetin on proliferation of gastric carcinoma cells was
investigated. The results demonstrate that fisetin treatment
inhibits proliferation of gastric carcinoma cells through
suppression of ERK 1/2 activation.
Materials and methods
Cell lines and culture
A human gastric cancer cell line, SGC7901, and a
normal gastric cell line, GES-1, were obtained from the cell bank
of Xiangya Medical School, Central South University (Changsha,
China). The cells were cultured in Dulbecco's modified Eagle medium
(DMEM) containing 10% heat-inactivated fetal bovine serum (FBS;
HyClone; GE Healthcare, Logan, UT, USA), penicillin (100 U/ml) and
streptomycin (100 µg/ml). The cells were incubated at 37°C in a
humidified atmosphere of 5% CO2.
Reagents and chemicals
Fisetin and dimethyl sulfoxide (DMSO) were supplied
by Sigma-Aldrich (Merck KGaA, Darmstadt, Germany). The stock
solution of fisetin in DMSO was stored at −15°C. The inhibitor for
activation of ERK 1/2, PD98059, was obtained from Selleck Chemicals
LLC (Shanghai, China).
Analysis of cell proliferation by
CCK-8 assay
SGC7901 and GES-1 cell lines were distributed at a
density of 2×105 cells per well into 96-well culture
plates and incubated at 37°C overnight. Then, the medium was
replaced with fresh DMEM containing 0 (control), 1, 5, 10, 15 and
20 µM concentrations of fisetin. After 48 h of incubation under a
humidified atmosphere of 5% CO2 at 37°C, 200 µl CCK-8
solution (Dojindo Molecular Technologies, Inc., Kumamoto, Japan)
was added to each well. The plates were incubated at 37°C for a
further 4 h. Then, a microplate reader was used to measure the
absorbance at 450 nm. The experiments were performed independently
in triplicate.
Analysis of apoptosis using flow
cytometry
Apoptosis induction in gastric carcinoma cells
following treatment with fisetin was analyzed using an Annexin
V/Propidium Iodide (PI; BD Biosciences, Franklin Lakes, NJ, USA)
apoptosis kit, according to the manufacturer's protocol. SGC-7901
and GES-1 cells were treated with 0 (control), 5, 10 and 15 µM
concentrations of fisetin for 48 h under a humidified atmosphere of
5% CO2 at 37°C. The cells were harvested by
trypsinization, washed three times with phosphate-buffered saline
(PBS), and resuspended in binding buffer at a concentration of
2×107 cells/ml. The cells were then treated with Annexin
V-fluorescein isothiocyanate (5 µl) and PI (10 µl) at room
temperature in the dark for 10 min. The cells were analyzed using a
flow cytometer (FACSAria III; BD Biosciences, Franklin Lakes, NJ,
USA). CellQuest software version 3.3 (BD Biosciences) was used for
analysis of flow cytometry. The experiments were performed in
triplicate for each concentration.
Analysis of cell cycle arrest using
flow cytometry
SGC7901 cells at a density of 2.5×105
cells/well were distributed into 6-well plates and subjected to
incubation for 48 h. RPMI-1640 medium (Gibco; Thermo Fisher
Scientific, Inc., Waltham, MA, USA) supplemented with 10% FBS was
used for cell culture and incubation was performed at 37°C in an
atmosphere of 5% CO2. The medium was then replaced with
fresh medium containing fisetin (15 µM) in DMSO. Following 48 h
incubation at 37°C, the cells were subjected to trypsinization and
subsequent washing with cold PBS. The cells were then fixed with
70% ethyl alcohol at 4°C for at least 4 h, followed by addition of
20 µl RNase (Thermo Fisher Scientific,) and 20 µl PI
(Sigma-Aldrich; Merck KGaA). The cells were then incubated for 30
min at 37°C before analysis using a FACSCalibur flow cytometer (BD
Biosciences) and CellQuest software version 3.3 (BD
Biosciences).
Western blot analysis
The phosphorylation of ERK 1/2 and expression of
caspase-7, pro-caspase-7, B-cell lymphoma 2 (Bcl-2),
Bcl-2-associated X protein (Bcl-x), BH3 interacting domain death
agonist (Bid) and Bcl-2-like protein 11 (Bim) was analyzed using a
western blot assay. Effect of PD98059 (ERK 1/2 inhibitor) at 100 µM
on activation of ERK ½ was also analyzed using this assay. The
SGC7901 cells were treated with 15 µM fisetin for 48 h at 37°C
under a humidified atmosphere of 5% CO2. Following
incubation, the cells were treated with radioimmunoprecipitation
assay lysis buffer (Beyotime Institute of Biotechnology, Haimen,
China) under ice-cold conditions for 45 min. The cell lysates were
subjected to centrifugation at 12,000 × g for 15 min at 4°C. The
concentration of proteins in the cell lysates was determined using
a bicinchoninic acid assay. The proteins were separated using 10%
SDS-PAGE by loading 3 µl protein per lane and subsequently
transferred to polyvinylidene difluoride membranes. In the
membranes, non-specific sites were blocked with non-fat milk
containing Tris-buffered saline with Tween-20. The membranes were
incubated with rabbit primary monoclonal antibodies against ERK
(cat. no. 137F5; dilution 1:1,000) and p-ERK (cat. no. D13.14.4E;
dilution 1:1,000; both from Cell Signaling Technology, Inc.,
Danvers, MA, USA) at 4°C for overnight. The other antibodies used
were against Bcl-2 (cat. no. ab7973), Bcl-x (cat. no. ab77566), Bid
(cat. no. ab32060), Bim (cat. no. ab32158), β-actin (cat. no.
ab8226) and α-tubulin (cat. no. ab7291; all dilution 1:1,000,
Abcam, Cambridge, UK). The membranes were washed and incubated with
goat anti-rabbit HRP-conjugated polyclonal secondary antibodies
(cat. no. 12–348; dilution 1:2,000, Merck KGaA) for 1 h at room
temperature. The bands were visualized using an enhanced
chemiluminescence blotting detection system (FluorChem E;
ProteinSimple, San Jose, CA, USA).
Statistical analysis
Data are presented as the mean ± standard deviation
of ≥3 experiments performed independently. Statistical analysis was
performed with the SPSS 13.0 statistical software (SPSS, Inc.
Chicago, IL, USA). A one-way analysis of variance was used,
followed by Dunnett's test for multiple comparisons. P<0.05 was
considered to indicate a statistically significant difference.
Results
Fisetin inhibits proliferation of
gastric cancer cells
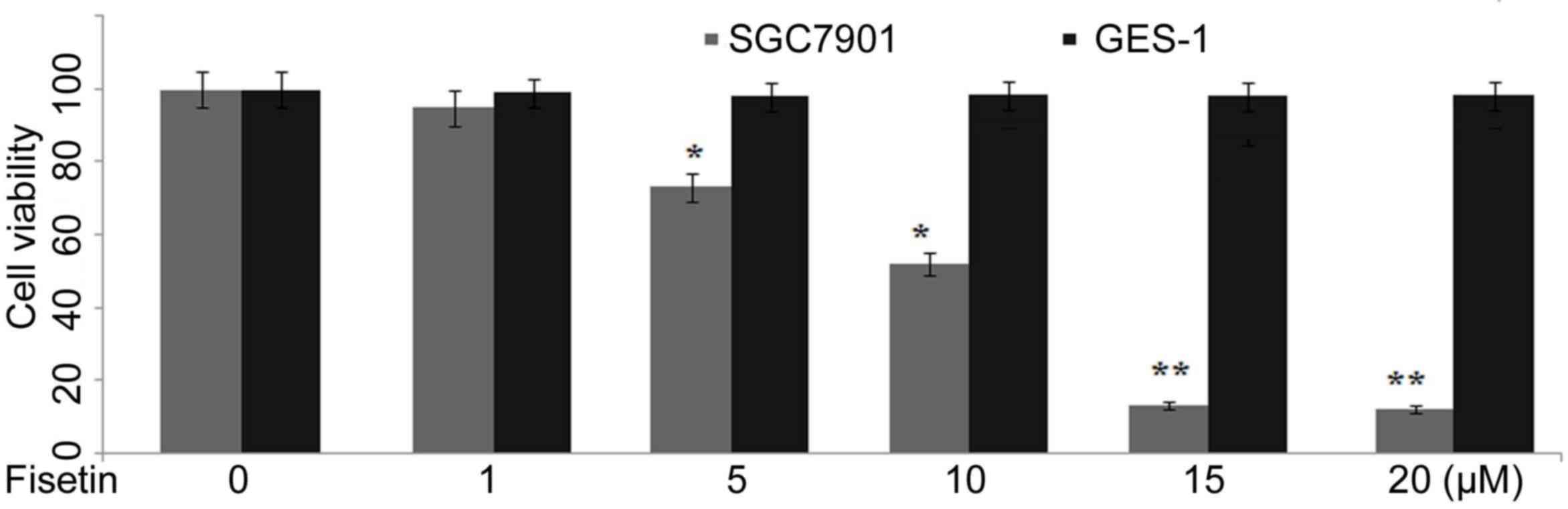
SGC7901 and GES-1 cells were incubated with various
concentrations (1, 5, 10, 15 and 20 µM) of fisetin for 48 h and
proliferation was examined. Fisetin treatment at 1, 5, 10, 15 and
20 µM concentration significantly reduced the proliferation rate of
SGC7901 cells to 98, 72, 51, 12 and 11%, respectively compared to
100% in control after 48 h (P<0.05; Fig. 1). The proliferation rate of GES-1
cells was found to be 100, 99, 99, 98 and 98% respectively at 1, 5,
10, 15 and 20 µM concentrations of fisetin compared with 100% in
untreated cultures (Fig. 1).
Fisetin induces apoptosis in gastric
cancer cells
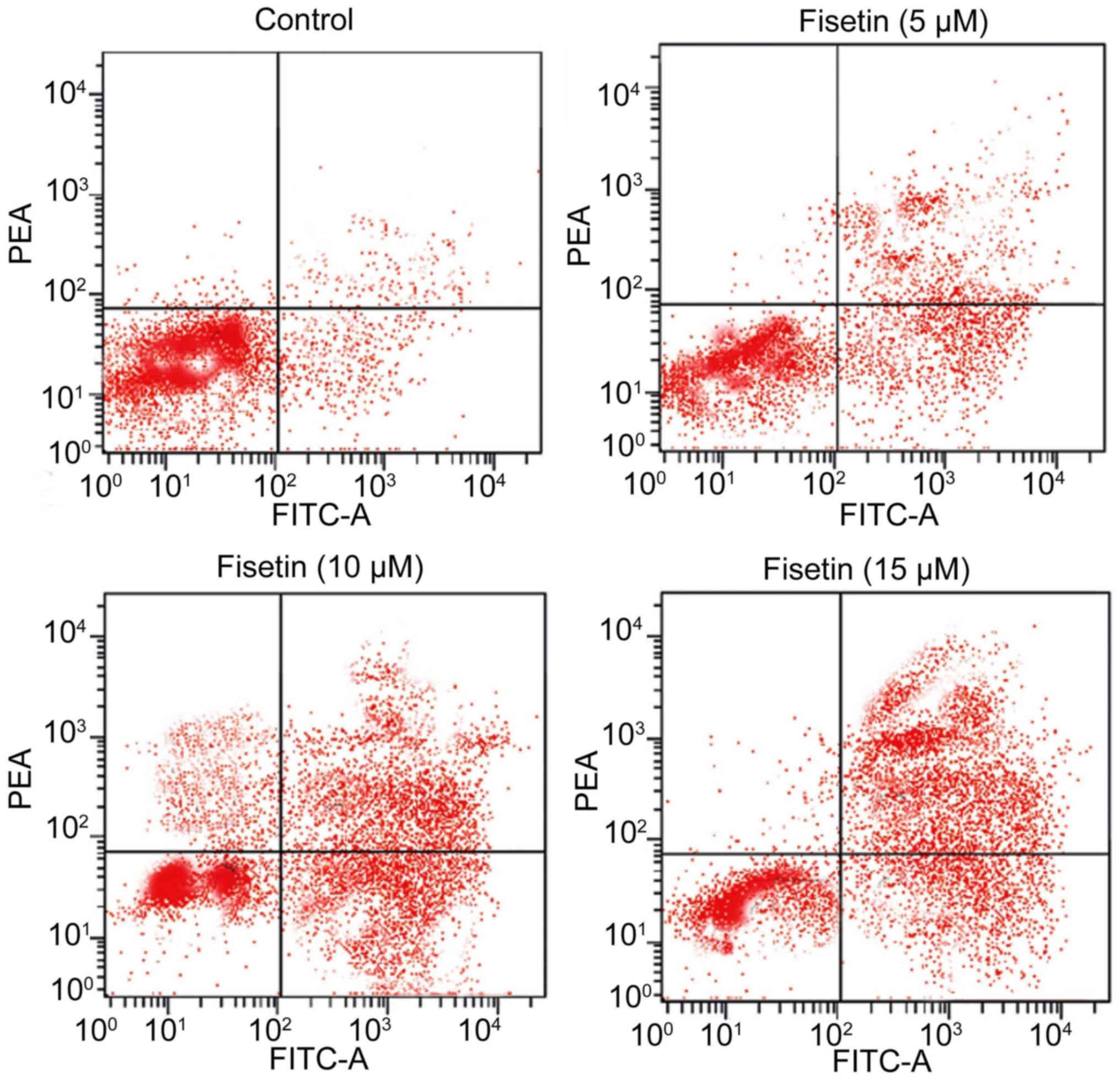
Treatment of SGC7901 cells with various
concentrations (5, 10 and 15 µM) of fisetin for 48 h induced cell
death in a dose-dependent manner (Fig.
2). Flow cytometry revealed a notable increase in the
proportion of apoptotic cells at 15 µM concentration of fisetin
after 48 h compared with the untreated control cells (Fig. 2). The percentage of apoptotic cells
increased to 87% following treatment with 15 µM fisetin compared
with 2% in the control for 48 h.
Cell cycle arrest analysis
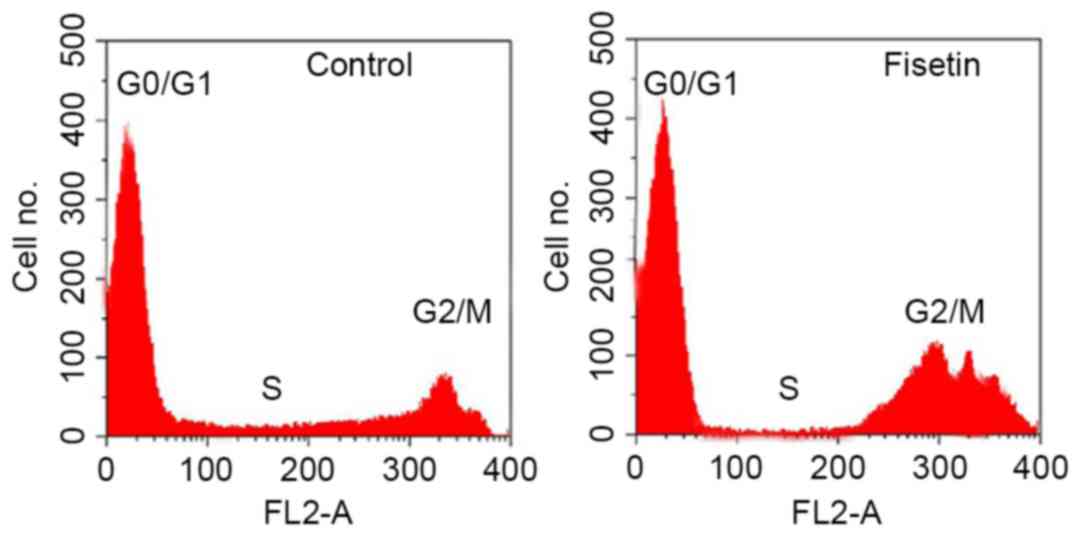
The effect of fisetin on SGC7901 cell cycle
progression was determined using flow cytometry. The results
indicated that the percentage of cells in the G2/M and S phases of
control cell cultures was 18.23 and 9.14%, respectively (Fig. 3). Following treatment of SGC7901 cells
with 15 µM fisetin for 48 h, the proportion of SGC7901 cells at the
G2/M and S phases was 23.72 and 8.65%, respectively (Fig. 3). Thus, fisetin treatment increased
the proportion of cells at G2/M phase with simultaneous reduction
of cells at S phase.
Analysis of caspase-7 and Bcl-2
protein expression
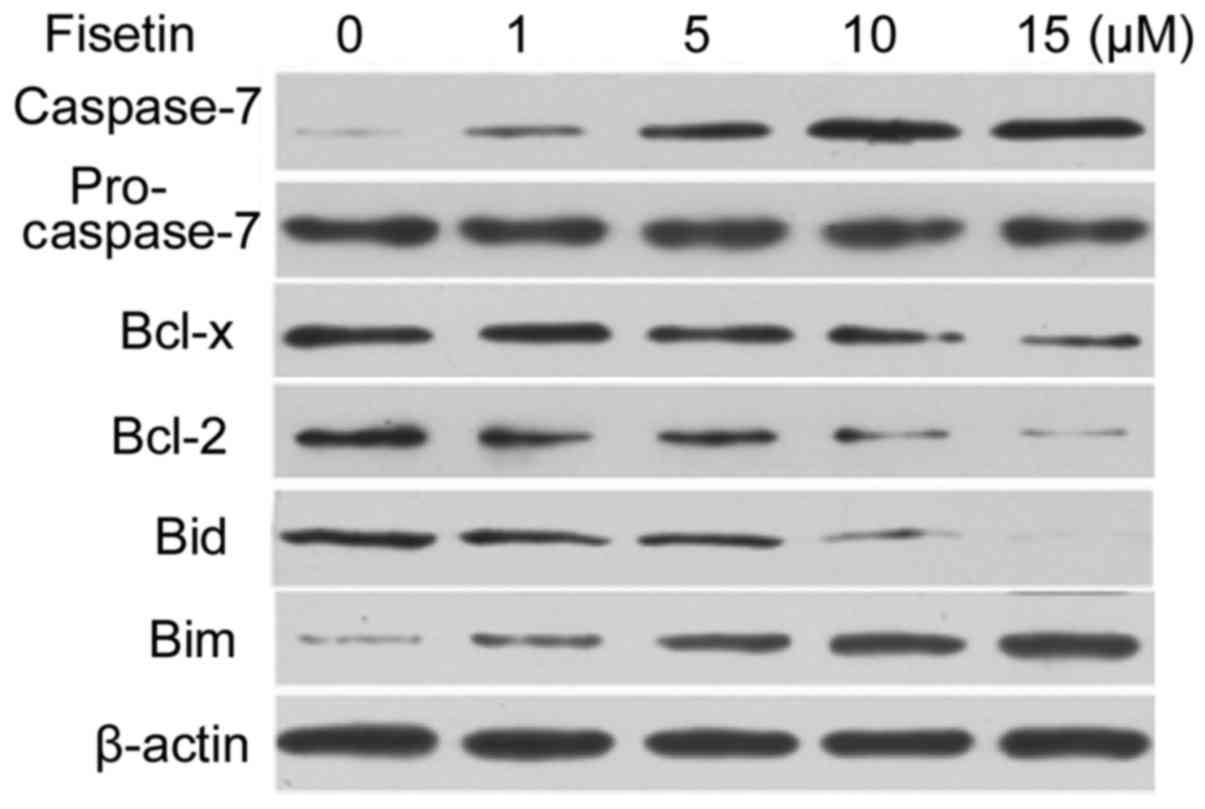
Western blot analysis indicated a marked increase in
the expression level of caspase-7 following treatment of SGC7901
cells with 1, 5, 10 and 15 µM fisetin for 48 h (Fig. 4). However, the procaspase-7 expression
level was slightly decreased by fisetin treatment (Fig. 4). In SGC7901 cells, fisetin treatment
led to a marked decrease in the expression level of anti-apoptotic
proteins Bcl-2 and Bcl-x. The expression of Bim was increased and
that of Bid decreased following treatment of SGC7901 cells with
fisetin for 48 h (Fig. 4).
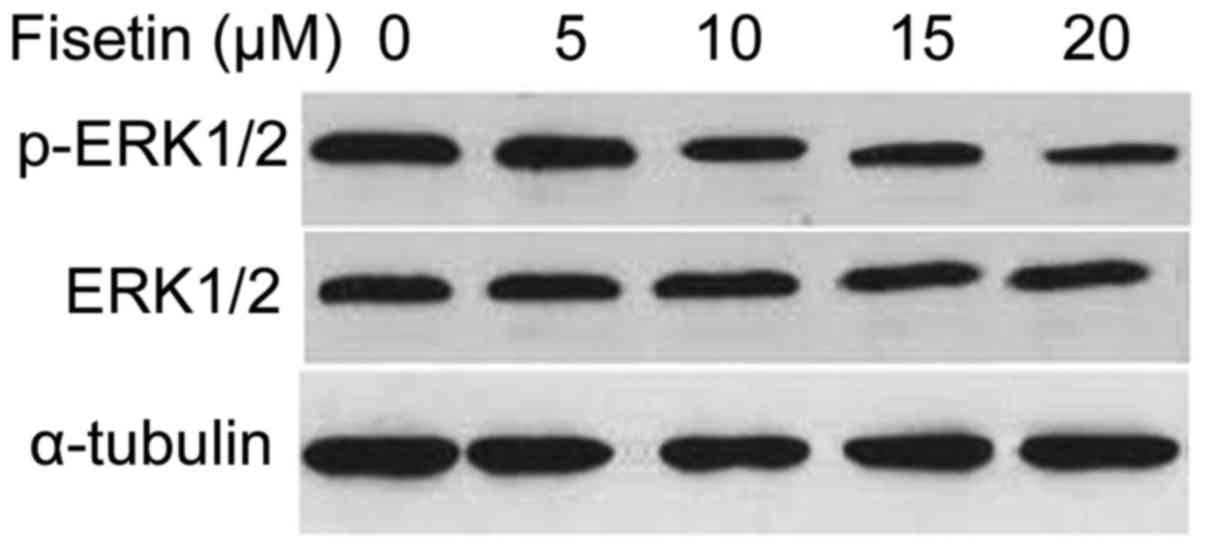
Inhibition of ERK 1/2 activation in
gastric cancer cells by fisetin treatment
Treatment of SGC7901 cells with fisetin for 48 h
resulted in marked reduction of the activation of ERK 1/2 in a
concentration-dependent manner (Fig.
5).
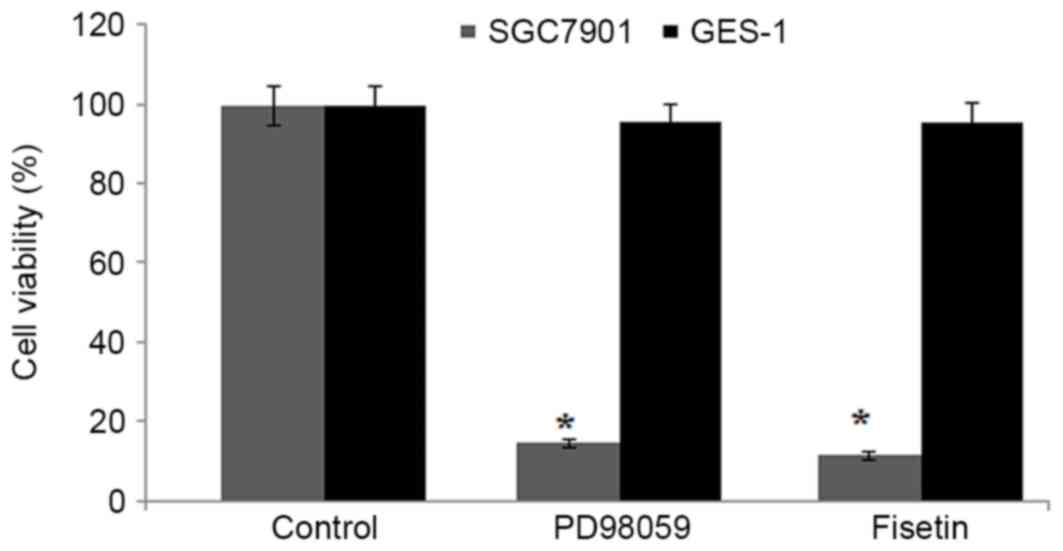
The inhibitory effect of fisetin on activation of
ERK 1/2 was further demonstrated using an ERK 1/2 inhibitor,
PD98059. The results indicated a marked decrease in the
proliferation of SGC7901 cells compared with control cells,
following treatment with 100 µM PD98059 (P<0.002). The reduction
by PD98059 administration was similar to that observed following
fisetin (15 µM) treatment for 48 h (Fig.
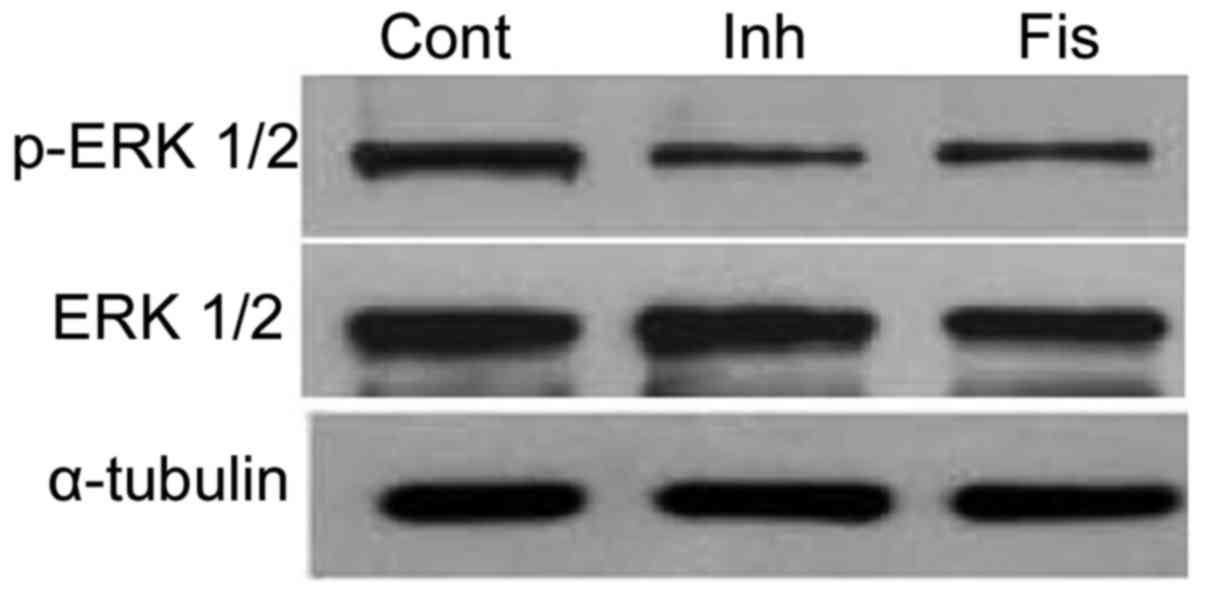
6). Furthermore, PD98059 was observed to markedly reduce the
activation of ERK 1/2 in SGC7901 cells (Fig. 7). Inhibition of ERK 1/2 activation by
PD98059 produced similar results to fisetin treatment (15 µM) for
48 h (Fig. 7).
Discussion
The current study demonstrates the effect of fisetin
on the proliferation of gastric cancer cells and provides insight
into its underlying mechanism. The results demonstrated that
fisetin treatment inhibited proliferation and induced apoptosis in
SGC7901 cells via inhibition of ERK 1/2 activation.
Fisetin treatment has been identified to inhibit the
proliferation and metastasis potential of numerous types of
carcinoma cell, including bladder, pancreas and cervical carcinoma
(11–13). In the current study, fisetin treatment
(10–20 µM of SGC7901 cells under acidic conditions led to a
significant due to the generation of acidic by-products during the
process of glycolysis (17,18). The rate of proliferation is inhibited
in gastric cancer cells through induction of apoptosis (19). The current study revealed that
treatment of SGC7901 cells with fisetin for 48 h induced apoptosis
in a dose-dependent manner. The percentage of apoptotic cells
increased to 87% following treatment with 15 µM fisetin for 48 h.
The inducers of apoptosis include caspase-3 and −7, since their
expression causes morphological changes in cells that are
characteristic of apoptosis (20).
The current results suggested that fisetin treatment of SGC7901
cells caused apoptosis induction by activating caspase-7 and
reducing the expression of anti-apoptotic proteins Bcl-2, Bcl-x and
Bid. The expression of pro-apoptotic protein Bim was increased
following treatment of cells with fisetin.
It has been reported that the rate of proliferation
is inhibited in gastric cancer cells through induction of apoptosis
via targeting the mitogen-activated protein kinase (MAPK) pathway
(21). It was reported that ERK1/2
phosphorylation can be selectively inhibited either by its
inhibitor (PD98059) or by using drugs, including matrine (22). The proliferation of cancer cells is
regulated by one of the important members of the MAPK family, ERK
1/2 (23). The current results
revealed that treatment of SGC7901 cells with fisetin for 48 h led
to a reduction in the activation of ERK 1/2 in a
concentration-dependent manner. The inhibitory effect of fisetin on
activation of ERK 1/2 was further demonstrated using ERK 1/2
inhibitor, PD98059. The results indicated a significant reduction
in the proliferation of SGC7901 cells following treatment with
PD98059. The reduction by PD98059 administration was comparable to
that observed following fisetin treatment for 48 h. PD98059
treatment was also observed to markedly reduce the activation of
ERK 1/2 in SGC7901 cells. This inhibition was similar to the result
observed for fisetin treatment (15 µM).
In conclusion, the current study demonstrates that
fisetin inhibits the proliferation of gastric cancer cells and
induces apoptosis through suppression of ERK 1/2 activation. Thus,
fisetin may have therapeutic applications in the treatment of
gastric cancer.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Torre LA, Bray F, Siegel RL, Ferlay J,
Lortet-Tieulent J and Jemal A: Global cancer statistics, 2012. CA
Cancer J Clin. 65:87–108. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Aguiar PN Jr, Tadokoro H, Forones NM and
de Mello RA: Treating operable patients with gastric cancer:
Macdonald's protocol versus adjuvant chemotherapy. Future Oncol.
11:2247–2249. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Hartgrink HH, Jansen EP, van Grieken NC
and van de Velde CJ: Gastric cancer. Lancet. 374:477–490. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Yamamoto M, Sakaguchi Y, Matsuyama A,
Yoshinaga K, Tsutsui S and Ishida T: Surgery after preoperative
chemotherapy for patients with unresectable advanced gastric
cancer. Oncology. 85:241–247. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Qiu HB, Zhang LY, Keshari RP, Wang GQ,
Zhou ZW, Xu DZ, Wang W, Zhan YQ and Li W: Relationship between H.
Pylori infection and clinicopathological features and prognosis of
gastric cancer. BMC Cancer. 10:3742010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Zhai YK, Pan YL, Niu YB, Li CR, Wu XL, Fan
WT, Lu TL, Mei QB and Xian CJ: The importance of the prenyl group
in the activities of osthole in enhancing bone formation and
inhibiting bone resorption in vitro. Int J Endocrinol.
2014:9219542014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Yogesh HS, Chandrashekhar VM, Katti HR,
Ganapaty S, Raghavendra HL, Gowda GK and Goplakhrishna B:
Anti-osteoporotic activity of aqueous-methanol extract of Berberis
aristata in ovariectomized rats. J Ethnopharmacol. 134:334–338.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Lee WS, Lee EG, Sung MS and Yoo WH:
Kaempferol inhibits IL-1β-stimulated, RANKL-mediated
osteoclastogenesis via downregulation of MAPKs, c-Fos, and NFATc1.
Inflammation. 37:1221–1230. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Tyagi AM, Srivastava K, Singh AK, Kumar A,
Changkija B, Pandey R, Lahiri S, Nagar GK, Yadav DK, Maurya R, et
al: Formononetin reverses established osteopenia in adult
ovariectomized rats. Menopause. 19:856–863. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Clardy J and Walsh C: Lessons from natural
molecules. Nature. 432:829–837. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Tannock IF and Rotin D: Acid pH in tumors
and its potential for therapeutic exploitation. Cancer Res.
49:4373–4384. 1989.PubMed/NCBI
|
|
12
|
Helmlinger G, Yuan F, Dellian M and Jain
RK: Interstitial pH and pO2 gradients in solid tumors in vivo:
High-resolution measurements reveal a lack of correlation. Nat Med.
3:177–182. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Yeo M, Kim DK, Kim YB, Oh TY, Lee JE, Cho
SW, Kim HC and Hahm KB: Selective induction of apoptosis with
proton pump inhibitor in gastric cancer cells. Clin Cancer Res.
10:8687–8696. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Ke Y, Ning T and Wang B: Establishment and
characterization of a SV40 transformed human fetal gastric
epithelial cell line-GES-1. Zhonghua Zhong Liu Za Zhi. 16:7–10.
1994.(In Chinese). PubMed/NCBI
|
|
15
|
Kim N, Lee SH, Son JH, Lee JM, Kang MJ,
Kim BH, Lee JS, Ryu JK and Kim YT: Fisetin reduces cell viability
through up-regulation of phosphorylation of ERK1/2 in
cholangiocarcinoma cells. Anticancer Res. 36:6109–6116. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Maher P, Dargusch R, Bodai L, Gerard PE,
Purcell JM and Marsh JL: ERK activation by the polyphenols fisetin
and resveratrol provides neuroprotection in multiple models of
Huntington's disease. Hum Mol Genet. 20:261–270. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Holm E, Hagmüller E, Staedt U,
Schlickeiser G, Günther HJ, Leweling H, Tokus M and Kollmar HB:
Substrate balances across colonic carcinomas in humans. Cancer Res.
55:1373–1378. 1995.PubMed/NCBI
|
|
18
|
Vaupel P, Kallinowski F and Okunieff P:
Blood flow, oxygen and nutrient supply, and metabolic
microenvironment of human tumors: A review. Cancer Res.
49:6449–6465. 1989.PubMed/NCBI
|
|
19
|
Fais S, De Milito A, You H and Qin W:
Targeting vacuolar H+-ATPases as a new strategy against cancer.
Cancer Res. 67:10627–10630. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Danial NN and Korsmeyer SJ: Cell death:
Critical control points. Cell. 116:205–219. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Kim EK and Choi EJ: Pathological roles of
MAPK signaling pathways in human diseases. Biochim Biophys Acta.
1802:396–405. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Zhu P, Chen JM, Guo HM, Fan XP, Zhang XS,
Fan RX, Zheng SY, Wu RB, Xiao XJ, Huang HL, et al: Matrine inhibits
disturbed flow-enhanced migration via downregulation of ERK1/2-MLCK
signaling vascular smooth muscle cells. Ann Vasc Surg. 26:268–275.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Ballif BA and Blenis J: Molecular
mechanisms mediating mammalian mitogen-activated protein kinase
(MAPK) kinase (MEK)-MAPK cell survival signals. Cell Growth Differ.
12:397–408. 2001.PubMed/NCBI
|