Introduction
Breast calcification lesions are notable imaging
features of early breast cancer (1).
In total, 20–30% of calcification lesions are pathologically
confirmed to be of malignant breast cancer (2–4). Breast
microcalcifications lesions are particularly significant for the
early diagnosis of breast cancer (5).
For example, the detection of 80–90% of ductal carcinoma in
situ (DCIS) is attributed to the diagnostic analysis of the
breast microcalcifications (6). It
has been reported that the breast microcalcification lesions may be
a unique positive feature of mammography X-ray imaging for patients
with non-palpable disease (7,8). Mammography X-ray imaging is highly
sensitive to breast microcalcifications lesions, and is therefore
considered to be the gold standard of breast microcalcifications
(9). However, breast
microcalcifications exist in malignant and benign lesions (for
example, hardening of breast disease, mammary dysplasia, hamartoma,
scarring following radiotherapy, fibrocystic disease and
fibroadenoma) (10). Thus, a breast
biopsy is always required to identify the microcalcifications
lesions.
However, breast microcalcifications lesions are
non-palpable. Thus, the surgical operation of the breast
microcalcifications lesions is different with that of the
clinically palpable lesions. Identifying the accurate location of
the microcalcification lesions is difficult, which can lead to
inaccurate and/or incomplete excision, potentially resulting in
misdiagnosis. Therefore, to localize the occult lesions clearly in
the surgical process and excise the lesions completely, it is
crucial to precisely localize the lesions prior to surgery.
However, it is difficult to excise non-palpable breast lesions by
established surgical biopsy. Thus, an easy, accurate, minimally
invasive biopsy method is required for the breast
microcalcifications lesions (11,12).
In 1965, Dodd et al (13) reported a method using wire to localize
the breast lesions prior to surgery. In 1976, Frank et al
(14) described the localizing of
breast lesions using mammography X-ray imaging as a guide prior to
the surgical excision of breast lesions for biopsy. Nowadays, the
wire-guided location (WGL) biopsy method has been widely applied
and is the gold standard for the biopsy of non-palpable breast
lesions (15–17). In recent years, the application of
vacuum-assisted breast biopsy (VABB) has reduced the percentage of
patients that undergo conventional surgical biopsy (18,19).
Barranger et al (20) claimed
that VABB could replace WGL to become the gold standard method of
biopsy of non-palpable breast lesions. However, VABB cannot excise
the breast microcalcifications lesions completely. Thus, a second
surgical procedure is required (21–23).
On the other hand, a major shortcoming of
conventional WGL biopsy is that the wire may shift in the processes
of lesion localization and removal (24), which usually leads to the incomplete
excision of the lesion. It was reported that a second surgical
procedure was required in ~50% patients who underwent the
conventional WGL biopsy method (25,26). The
other disadvantages involved in the conventional WGL biopsy include
the large volume and irregular shape of the specimen, the breakage
of breast structure, and the difficulty in localizing the lesion in
the specimen for further pathological identification (27,28).
To overcome the aforementioned shortcomings, the
present study reports on the development of a novel precision
biopsy method of breast microcalcifications based on the double
wire-guided localization and rotary cutting biopsy (DWGLB). Prior
to surgery, the precise localization of the lesions was assessed by
using two wires under the assistance of mammography X-ray and
ultrasound, followed by the complete excision of the lesions using
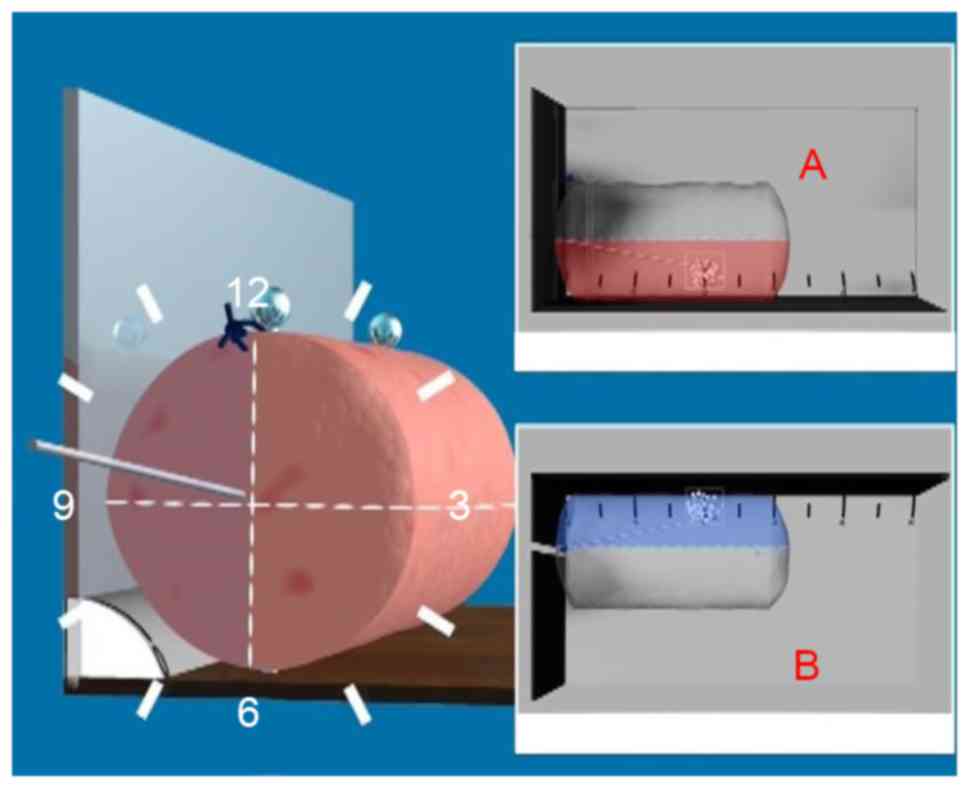
a novel rotary cutting tool (Fig. 1;
Chinese patent no., ZL 2009 1 0099174.7).
Materials and methods
Patients
A total of 108 mammographically detected
non-palpable breast lesions in 108 patients, comprising 62 lesions
on the left breast and 46 lesions on the right breast, were
attempted between May 2012 and March 2014 at the department of
Oncological Surgery, Hangzhou First People's Hospital (Hangzhou,
China) using DWGLB. The age range of the patients was 24–69 years,
with a mean age of 45.69 years. All the lesions were classified as
being of Breast Imaging Reporting and Data System (BI-RADS)
(29) category 4A (suspicious
abnormality).
Surgical procedure
The surgical procedure included five steps. i) The
3D localization of the microcalcification was assessed by
mammography using the Senographe DS Acquisition system (GE
Healthcare, Chicago, IL, USA). Subsequently, a BARD Dualok (C. R.
Bard, Inc., Murray Hill, NJ, USA) was inserted into the lesion and
fixed. Mammographic imaging was performed mediolaterally or
lateromedially to observe the position of the lesion and the BARD
Dualok. Ideally, the lesion would be localized at the bifurcation
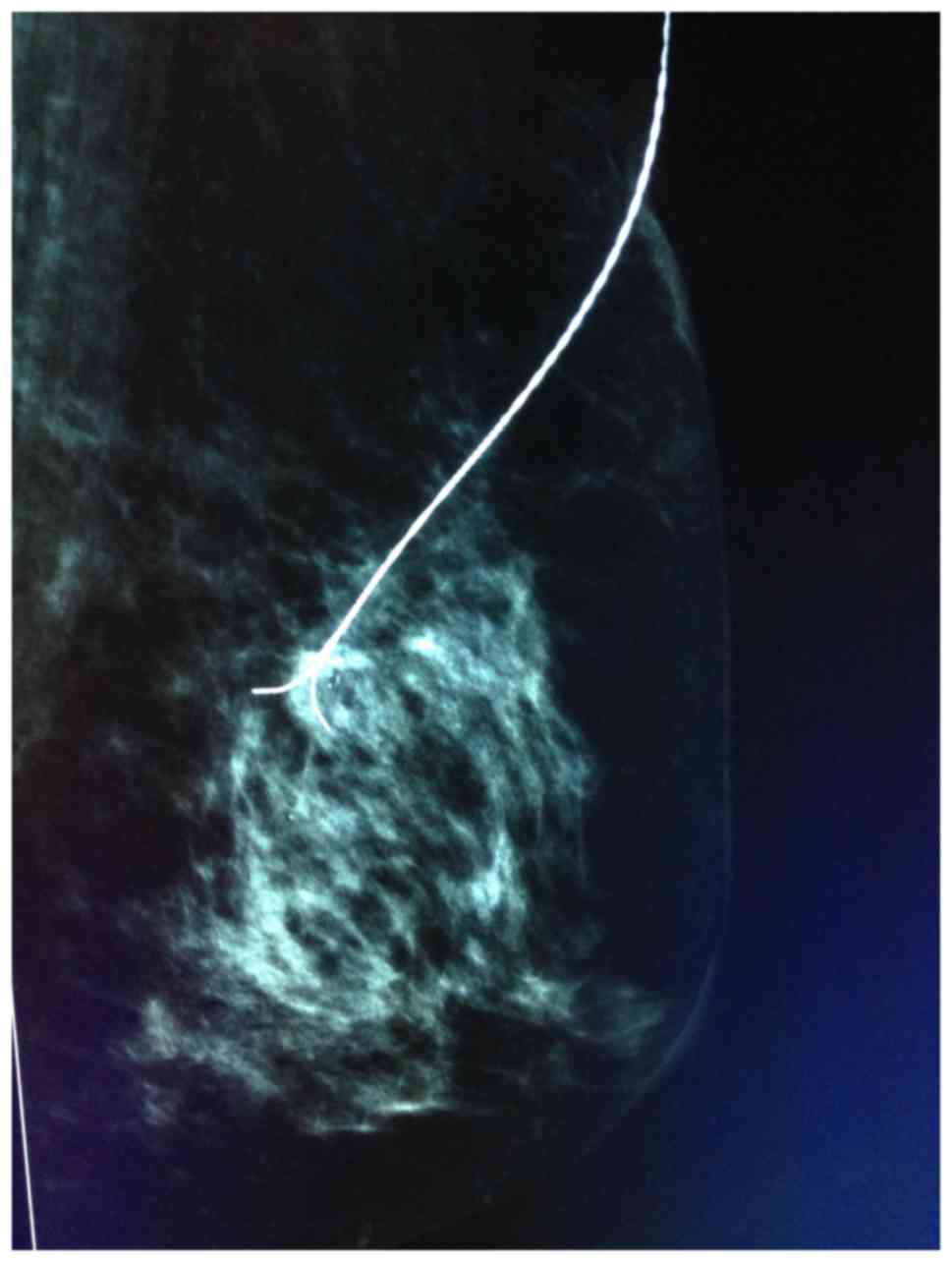
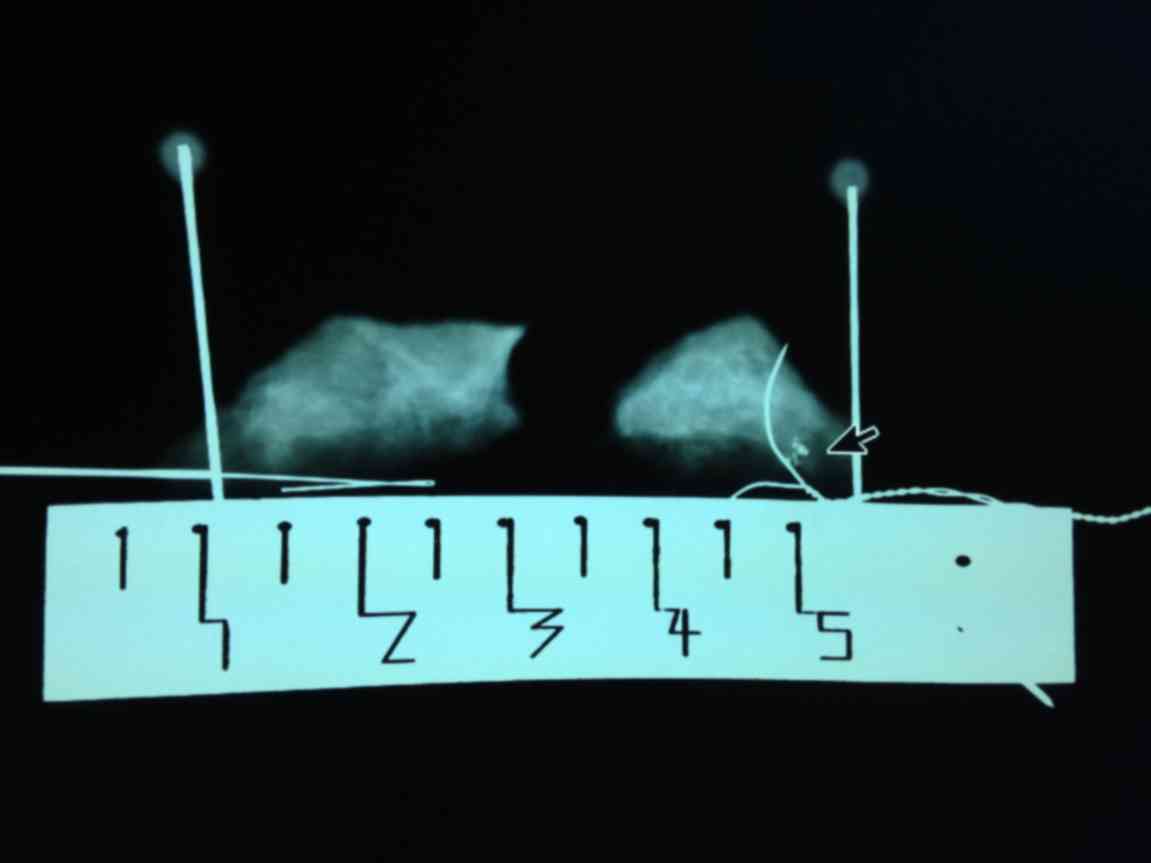
of the BARD Dualok (Fig. 2). ii)
Following transportation of the patients into an operating theatre,
the relative position of the lesion and the BARD Dualok was further
confirmed by an ultrasound scanner (MyLab Twice, Esaote SpA, Genoa,
Italy). Subsequently, a mark line (3 cm) vertical to the BARD
Dualok was made on the skin of the lesion, followed by insertion of
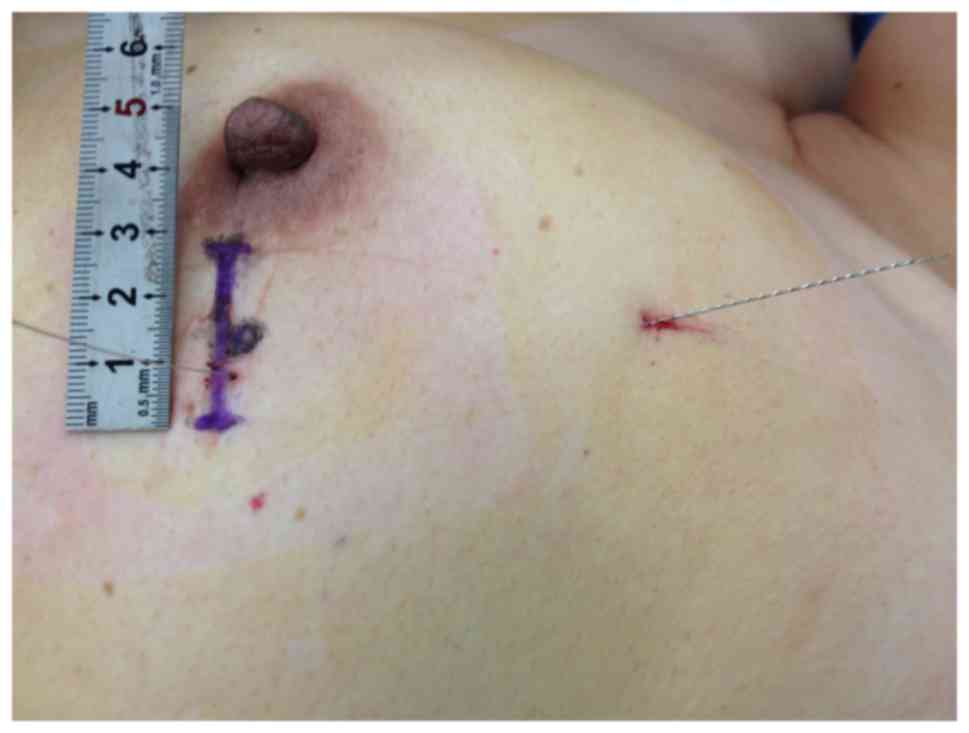
a single hook needle vertically into the lesion. The junction point
of two needles was the accurate position of the lesion (Fig. 3). iii) Local anesthesia (obtained with
buffered 1% lidocaine injected into the skin and superficial
tissues and with buffered 1% lidocaine containing epinephrine
within the deeper breast tissues). iv) A 3-cm skin incision was
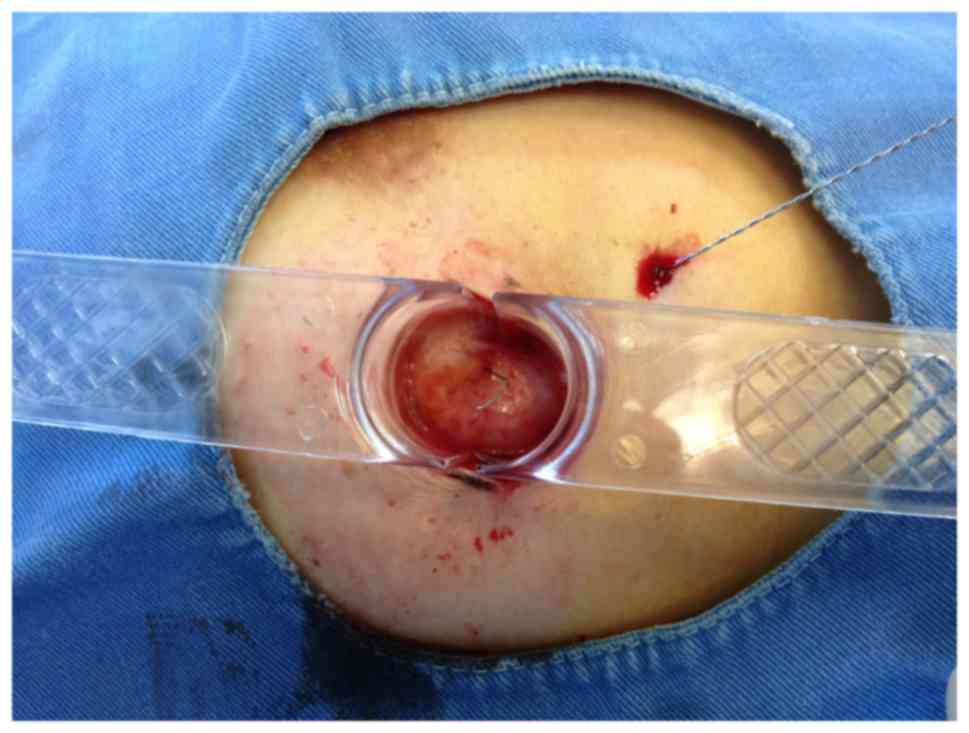
made according to the mark line. Double wire-guided lesion sampling
was performed using a rotary cutting device (Figs. 4 and 5).
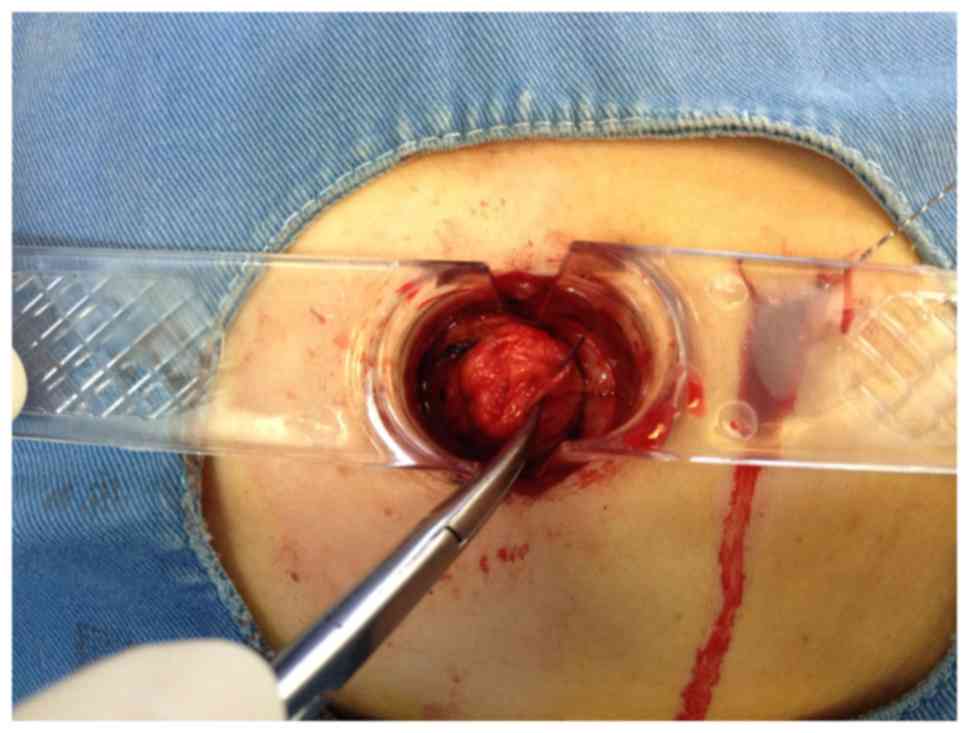
v) The lesion samples were reviewed by a pathologist (Figs. 6 and 7),
followed by compression and dressing of the wound if they were
benign calcifications, or proceeding to further surgical excision
if they were malignant lesions.
Statistical analysis
All statistical analyses were done using SPSS 19.0
(IBM Corp., Armonk, NY, USA). The association between two
categorical variables was evaluated using the χ2 test.
P<0.05 was considered to indicate a statistically significant
difference.
Results
Results of puncture localization and
specimen removal
Percutaneous localization of the lesions guided by
mammography and ultrasound were successful in all 108 lesions
(100%) with one puncture attempt, which is consistent with the
previous studies (98–100%) (30–32). No
evident complication was observed. All 108 lesions were excised
using DWGLB, with a mean distance between the needle and lesion of
4.1 mm (range, 0–20 mm) and mean specimen weight of 8.5 g (6–15 g).
The mean surgical time was <1 h per biopsy. The complete
excision rate of DWGLB was 100%. The comparison of DWGLB with the
conventional WGLB is depicted in Table
I (33–35).
 | Table I.Comparison of DWGLB with the
conventional WGLB. |
Table I.
Comparison of DWGLB with the
conventional WGLB.
| Study | Localization
method | Surgical
method | Distance between
needle and lesion, mm | Specimen weight,
g | Patients, n | Lesion
localization | (Refs.) |
|---|
| Mariscal Martinez
et al | WGL | Surgical
biopsy | <20 | 67.3 | 68 | Not assessed | (33) |
| Rampaul et
al | WGL | Surgical
biopsy | <20 | 31 | 47 | Not assessed | (34) |
| Rahusen et
al | WGL | Surgical
biopsy | Not assessed | 53 | 23 | Not assessed | (35) |
| Present study | DWGLB | Rotary cutting
biopsy | 4.1 | 8.5 | 108 | Submillimeter
accuracy | – |
Association of breast cancer detection
with the position and shape of lesions
A total of 108 mammographically detected
non-palpable breast lesions in 108 patients, comprising 62 lesions
on the left breast and 46 lesions on the right breast, included 62
lesions with cluster distribution, 38 lesions with regional
distribution/segment distribution and 8 lesions with linear
distribution. In total, 13 lesions (12.0%) were diagnosed as
malignant (DCIS of breast in 7 lesions, DCIS with focal invasive
carcinoma in 3 lesions and invasive ductal carcinoma in 3 lesions).
There were 88 negative lesions, including 75 cases of adenosis of
the breast, 10 of breast intracanalicular fibroma, 2 of intraductal
papilloma and 1 of papillary hyperplasia (Table II). Of the 62 lesions with cluster
distribution, breast cancer was diagnosed in 10 cases (16.1%) by
biopsy. Of the 46 lesions with non-cluster distribution, DCIS was
diagnosed by biopsy in 3 cases (6.5%) (Table III).
 | Table II.Clinicopathological features of
patients. |
Table II.
Clinicopathological features of
patients.
| Variable | Patients, n |
|---|
| Pathological
diagnosis (n=108) |
|
|
DCIS | 7 |
| DCIS
with invasive | 3 |
|
Invasive ductal carcinoma | 3 |
|
ADH | 7 |
|
Adenosis | 75 |
|
Fibroadenoma | 10 |
|
Intraductal papilloma | 2 |
|
Papillary hyperplasia | 1 |
| Selection of
operation (n=13) |
|
|
Modified radical
mastectomy | 7 |
|
BCS+SLNB | 2 |
|
Mastectomy+SLNB | 4 |
| Invasive carcinoma
TNM staging (n=6) |
|
| IA | 4 |
|
IIA | 2 |
| Molecular subtyping
(n=6) |
|
| Luminal
A | 3 |
| Luminal
B | 1 |
| Triple
negative | 1 |
| HER2
overexpression | 1 |
| Postoperative
adjuvant therapy (n=18) |
|
|
Endocrinotherapy | 10 |
|
Chemotherapy | 3 |
|
Radiotherapy | 3 |
|
Targeted therapy | 2 |
 | Table III.Association between breast cancer
incidence and the position and shape of the lesion. |
Table III.
Association between breast cancer
incidence and the position and shape of the lesion.
| Position and shape
of lesions | Breast cancer | Benign lesions |
χ2-value | P-value |
|---|
| Left side | 7 | 55 | 0.08 | >0.05 |
| Right side | 6 | 40 |
|
|
| Cluster
distribution | 10 | 52 | 2.30 | >0.05 |
| Non-cluster
distribution | 3 | 43 |
|
|
A total of 95 benign lesions underwent segmental
mastectomy (rotation biopsy). Hematoma was observed in 2 lesions
following the procedure, which disappeared following conservative
treatment. No cases of active bleeding were observed. All patients
with malignant lesions underwent surgery later, although none of
them exhibited residual tissue at the first follow-up with
mammography, including modified radical mastectomy for breast
cancer in 7 cases, the breast conservation surgery plus sentinel
lymph node biopsy in 2, and the unilateral mastectomy plus sentinel
lymph node biopsy in 4. In total, 10 of 13 malignant lesions
following surgical operation were given the endocrinotherapy. In
total, 3 patients were first given chemotherapy, followed by
radiotherapy in 1 patient with the lymphatic metastasis and the
targeted therapy using trastuzumab in 2 with human epidermal growth
factor receptor 2 (HER2) expression. The other 2 patients with BCS
underwent radiotherapy. In the follow-up period of 16–38 months, no
metastasis and recurrence were identified in the 13 malignant
lesions and no new tumor formation in 95 benign lesions was
observed, neither.
Discussion
Breast carcinoma is the most common malignant tumor
in female patients, with the second highest mortality rate
(36). The early diagnosis of breast
cancer significantly decreases the recurrence, metastasis and
mortality rate of the disease (37–39).
Breast microcalcification is a manifestation of early breast
cancer; it is therefore vital to localize the lesions precisely and
excise them completely for the following pathological examination
and the treatment.
The distance between the needle and lesion was
mostly 20 mm measured by the mammography (40), which further increased subsequent to
releasing the pressure plate of the molybdenum target. As a result,
there were usually certain residual lesions and the second surgery
was needed (15,25,41). To
avoid shifting the needle, the needle core was pushed out of the
needle sheath by ~1 cm after the puncture needle reached to the
lesion, which made the BARD Dualok insert into breast tissue from
two sides. Meanwhile, the needle core was kept under pressure to
cause it to continuously puncture into breast tissue whilst
withdrawing the needle sheath and releasing the plate, until the
breast restored itself to a natural state. It was observed that the
needle could puncture the breast several cm deep, varying with the
volume of the breast and the position of the lesion. In the series
of patients in the present study, the mean distance between the
needle and lesion was 4.1 mm, and the majority of lesions were
located at the bifurcation of the BARD Dualok. Thus, the precision
of localization under the mammography was significantly improved
compared with the previous reports, where the mean distance between
the needle and lesion was <20 mm (33,34).
Previous research demonstrates that detection rate
of breast microcalcifications using ultrasound alone is low, and it
is only 30–50% of detection rate of mammography X-ray (42,43). It
was reported that the combination of ultrasound and X ray
mammography was useful to detect the non-palpable lesions and
identify the breast lesions (benign or malignant) (44,45). To
overcome the disadvantages of conventional WGL, including
inaccurate localization, difficulty for localization of the lesions
(46), DWGLB utilized dual
localization using mammography and ultrasound. Following the
localization of microcalcifications by mammography, the patient lay
in the supine position in order to achieve the flattest state of
the breast. The ultrasound probe was used to detect the position of
the BARD Dualok. Subsequently, a mark 3 cm vertical to the BARD
Dualok was made on the skin of the lesion, followed by the
insertion of a single hook needle vertically into the lesion. The
junction point of the two needles was the accurate position of the
lesion (Fig. 3). Finally, a 3-cm skin
incision was made according to the mark and the lesions could be
found along with the single hook needle. Compared with the
conventional WGL, DWGLB could provide a more accurate localization
of the lesions and substantially decrease the surgical area.
In conventional WGL, the excision of breast tissues
is highly dependent on the experience of the surgeon. On the
contrary, the surgical approach and the size and shape of the
specimen are standardized following the dual localization using the
mammography and the ultrasound: A 3 cm incision was first made on
the skin according to the mark line, followed by scraping and
separating the subcutaneous adipose tissue to expose the mammary
gland and the single hook needle. In this way, the whole surgical
operation could be performed only in the breast tissue.
Subsequently, a 2-cm rotary cut tool was used to make a round cut
down to the breast tissue by the center of the single hook needle.
The rotary cut tool was withdrawn when it contacted the BARD Dualok
in the deep breast tissue. The lesion was then completely excised
using an electric knife by comparing of the position of lesion
shown in Fig. 2 and the relative
distance between the BARD Dualok and lesion shown in Fig. 5.
The specimen excised using conventional WGL is
usually irregular. Thus, it is difficult for the pathologists to
determine the position of the lesion in the specimen, resulting in
a potential missed diagnosis (24).
On the contrary, the specimen excised by using DWGLB was
cylindrical breast tissue, with the top side of the cylinder marked
by using a suture. The specimen was placed on a scaled specimen
holder (Fig. 6), enabling the
pathologists to find the lesion by pathological examination more
easily. It was observed that 12% (13/108) of the breast
microcalcifications of BI-RADS score 4A were diagnosed as
early-stage breast cancer in the present study.
There is no confirmed standard yet concerning the
weight of the surgically excised biopsy specimen. The guidelines of
British Association of Surgical Oncology state that the weight of
the specimen of at least 80.0% benign lesions excised by using
wire-localization techniques should be less than 20 g (47). However, this goal has become difficult
to achieve since the mammography screening was widely applied. The
mean weight of biopsy specimen of benign lesions was 28 g between
1997 and 1998, with 47.0% of benign lesion biopsy specimens
weighing <20 g (48). In the
present study, the mean weight of specimen was <8.5 g, which was
significantly decreased compared with that of the minimum group in
the previous studies (31 g) (34).
Thus, the shape of the breasts was retained.
In conclusion, compared with conventional WGL, DWGLB
has several advantages, including the precise localization of
calcifications, the small specimen volume, the complete excision of
the lesions and the increased accuracy of pathological examination.
Therefore, DWGLB should be recommended for the early diagnosis and
treatment of patients with breast cancer.
Acknowledgements
The authors would like to thank all other clinical
trials research unit staff, past and present, who contributed to
the DWGLB trial (including to trial coordination, statistical
analysis, data entry and administration, and database development
and support), the additional members of the trial management group,
the trial steering committee for their notable contributions.
Funding
The present study was funded by the Science and
Technology Department of Zhejiang Province (grant no. 2010 C33097)
and Technology Bureau of Hangzhou City (grant no. 20100633B02 and
20160533B12).
Availability of data and materials
All data or analyzed during this study are included
in this published article.
Author's contributions
ZYL contributed to trial design and protocol
development. YP performed statistical analysis, drafted the report
and approved the final version of the article. WHC, JiZ, JHF and PZ
contributed to mammography, ultrasound, pathological diagnosis and
data monitoring. JN was involved in the surgical biopsy and
postoperative data management and was involved in revising the
manuscript critically for important intellectual content. HDC and
BL collected and verified all data. AZX, JuZ and JWD analyzed and
interpreted the results.
Ethics approval and consent to
participate
This clinical trial was approved by Hangzhou First
People's Hospital Ethics Committee (approval number: 2010-004-01).
The trial was registered with the Chinese Clinical Trial Registry
(registration number: ChiCTR-DDT-14004231).
Consent for publication
All participants gave informed consent for
publication of any associated data and accompanying images were
obtained from all subjects.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Tabár L and Dean PB: Teaching atlas of
mammography. Fortschr Geb Rontgenstrahlen Nuklearmed Erganzungsbd.
116:1–222. 2001.
|
|
2
|
Craft M, Bicknell AM, Hazan GJ and Flegg
KM: Microcalcifications detected as an abnormality on screening
mammography: Outcomes and followup over a five-year period. Int J
Breast Cancer. 2013:4585402013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Bent CK, Bassett LW, D'Orsi CJ and Sayre
JW: The positive predictive value of BI-RADS microcalcification
descriptors and final assessment categories. AJR Am J Roentgenol.
194:1378–1383. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Lazarus E, Mainiero MB, Schepps B,
Koelliker SL and Livingston LS: BI-RADS lexicon for US and
mammography: Interobserver variability and positive predictive
value. Radiology. 239:385–391. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Naseem M, Murray J, Hilton JF,
Karamchandani J, Muradali D, Faragalla H, Polenz C, Han D, Bell DC
and Brezden-Masley C: Mammographic microcalcifications and breast
cancer tumorigenesis: A radiologic-pathologic analysis. BMC Cancer.
15:3072015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Evans A, Pinder S, Wilson R, Sibbering M,
Poller D, Elston C and Ellis I: Ductal carcinoma in situ of the
breast: Correlation between mammographic and pathologic findings.
AJR Am J Roentgenol. 162:1307–1311. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Bassett LW: Mammographic analysis of
calcifications. Radiol Clin North Am. 30:93–105. 1992.PubMed/NCBI
|
|
8
|
Fondrinier E, Lorimier G, Guerin-Boblet V,
Bertrand AF, Mayras C and Dauver N: Breast microcalcifications:
Multivariate analysis of radiologic and clinical factors for
carcinoma. World J Surg. 26:290–296. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Sick A: Mammographic detestability of
breast microcalcifications. AJR. 1399:231–236. 1982.
|
|
10
|
Barman I, Dingari NC, Saha A, McGee S,
Galindo LH, Liu W, Plecha D, Klein N, Dasari RR and Fitzmaurice M:
Application of Raman spectroscopy to indentify microcalcifications
and underlying breast lesions at stereotactic core needle biopsy.
Cancer Res. 73:3206–3215. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Ohsumi S, Taira N, Takabatake D, Takashima
S, Hara F, Takahashi M, Kiyoto S, Aogi K and Nishimura R: Breast
biopsy for mammographically detected nonpalpable lesions using a
vacuum-assisted biopsy device (Mammotome) and upright-type
stereotactic mammography unit without a digital imaging system:
Experience of 500 biopsies. Breast Cancer. 21:123–127. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Fusco R, Petrillo A, Catalano O, Sansone
M, Granata V, Filice S, D'Aiuto M, Pankhurst Q and Douek M:
Procedures for location of non-palpable breast lesions: A
systematic review for the radiologist. Breast Cancer. 21:522–531.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Dodd G, Fry K and Delany W: Pre-operative
localization of occult carcinoma of the breast. Management of the
patient with cancer. Nealon TF: Saunders; Philadelphia, PA; pp.
88–113. 1965
|
|
14
|
Frank HA, Hall FM and Steer ML:
Preoperative localization of nonpalpable breast lesions
demonstrated by mammography. N Engl J Med. 295:259–260. 1976.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Burkholder HC, Witherspoon LE, Burns RP,
Horn JS and Biderman MD: Breast surgery techniques: Preoperative
bracketing wire localization by surgeons. Am Surg. 73:574–578.
2007.PubMed/NCBI
|
|
16
|
Kouskos E, Gui GP, Mantas D, Revenas K,
Rallis N, Antonopoulou Z, Lampadariou E, Gogas H and Markopoulos C:
Wire localisation biopsy of non-palpable breast lesions: Reasons
for unsuccessful excision. Eur J Gynaecol Oncol. 27:262–266.
2006.PubMed/NCBI
|
|
17
|
Jackman RJ and Marzoni FA Jr:
Needle-localized breast biopsy: Why do we fail? Radiology.
204:677–684. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kettritz U, Rotter K, Schreer I, Murauer
M, Schulz-Wendtland R, Peter D and Heywang-Köbrunner SH:
Stereotactic vacuum-assisted breast biopsy in 2874 patients: A
multicenter study. Cancer. 100:245–251. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Agacayak F, Ozturk A, Bozdogan A,
Selamoglu D, Alco G, Ordu C, Pilanci KN, Killi R and Ozmen V:
Stereotactic vacuum-assisted core biopsy results for non-palpable
breast lesions. Asian Pac J Cancer Prev. 15:5171–5174. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Barranger E, Marpeau O, Chopier J, Antoine
M and Uzan S: Site-select procedure for non-palpable breast
lesions: Feasibility study with a 15-mm cannula. J Surg Oncol.
90:14–19. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Park HL and Kim LS: The current role of
vacuum assisted breast biopsy system in breast disease. J Breast
Cancer. 14:1–7. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Abbate F, Cassano E, Menna S and Viale G:
Ultrasound-guided vacuum-assisted breast biopsy: Use at the
European institute of oncology in 2010. J Ultrasound. 14:177–181.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Youn I, Kim MJ, Moon HJ and Kim EK:
Absence of residual microcalcifications in atypical ductal
hyperplasia diagnosed via stereotactic vacuum-assisted breast
biopsy: Is surgical excision obviated? J Breast Cancer. 17:265–269.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Ocal K, Dag A, Turkmenoglu O, Gunay EC,
Yucel E and Duce MN: Radioguided occult lesion localization versus
wire-guided localization for non-palpable breast lesions:
Randomized controlled trial. Clinics (Sao Paulo). 66:1003–1007.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Fleming FJ, Hill AD, Mc Dermott EW,
O'Doherty A, O'Higgins NJ and Quinn CM: Intraoperative margin
assessment and re-excision rate in breast conserving surgery. Eur J
Surg Oncol. 30:233–237. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Postma EL, Witkamp AJ, van den Bosch MA,
Verkooijen HM and van Hillegersberg R: Localization of nonpalpable
breast lesions. Expert Rev Anticancer Ther. 11:1295–1302. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Staradub VL, Rademaker AW and Morrow M:
Factors influencing outcomes for breast conservation therapy of
mammographically detected malignancies. J Am Coll Surg.
196:518–524. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Medina-Franco H, Abarca-Pérez L,
García-Alvarez MN, Ulloa-Gómez JL, Romero-Trejo C and
Sepúlveda-Méndez J: Radioguided occult lesion localization (ROLL)
versus wire-guided lumpectomy for non-palpable breast lesions: A
randomized prospective evaluation. J Surg Oncol. 97:108–111. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Liberman L and Menell JH: Breast imaging
reporting and data system (BI-RADS). Radiol Clin North Am.
40:409–430. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Lovrics PJ, Goldsmith CH, Hodgson N,
McCready D, Gohla G, Boylan C, Cornacchi S and Reedijk M: A
multicentered, randomized, controlled trial comparing radioguided
seed localization to standard wire localization for nonpalpable,
invasive and in situ breast carcinomas. Ann Surg Oncol.
18:3407–3414. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Rissanen TJ, Mäkäräinen HP, Mattila SI,
Karttunen AI, Kiviniemi HO, Kallioinen MJ and Kaarela OI: Wire
localized biopsy of breast lesions: A review of 425 cases found in
screening or clinical mammography. Clin Radiol. 47:14–22. 1993.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Vrieling C, Collette L, Fourquet A,
Hoogenraad WJ, Horiot JH, Jager JJ, Pierart M, Poortmans PM,
Struikmans H, Maat B, et al: The influence of patient, tumor and
treatment factors on the cosmetic results after breast-conserving
therapy in the EORTC ‘boost vs. no boost’ trial. EORTC radiotherapy
and breast cancer cooperative groups. Radiother Oncol. 55:219–232.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Mariscal Martinez A, Solà M, de Tudela AP,
Julián JF, Fraile M, Vizcaya S and Fernández J: Radioguided
localization of nonpalpable breast cancer lesions: Randomized
comparison with wire localization in patients undergoing
conservative surgery and sentinel node biopsy. AJR Am J Roentgenol.
193:1001–1009. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Rampaul RS, Bagnall M, Burrell H, Pinder
SE, Evans AJ and Macmillan RD: Randomized clinical trial comparing
radioisotope occult lesion localization and wire-guided excision
for biopsy of occult breast lesions. Br J Surg. 91:1575–1577. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Rahusen FD, Bremers AJ, Fabry HF, van
Amerongen AH, Boom RP and Meijer S: Ultrasound-guided lumpectomy of
nonpalpable breast cancer versus wireguided resection: A randomized
clinical trial. Ann Surg Oncol. 9:994–998. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Siegel R, Ma J, Zou Z and Jemal A: Cancer
statistics, 2014. CA Cancer J Clin. 64:9–29. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Youlden DR, Cramb SM, Dunn NA, Muller JM,
Pyke CM and Baade PD: The descriptive epidemiology of female breast
cancer: An international comparison of screening, incidence,
survival and mortality. Cancer Epidemiol. 36:237–248. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Paap E, Holland R, den Heeten GJ, van
Schoor G, Botterweck AA, Verbeek AL and Broeders MJ: A remarkable
reduction of breast cancer deaths in screened versus unscreened
women: A case-referent study. Cancer Causes Control. 21:1569–1573.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Tabár L, Vitak B, Chen TH, Yen AM, Cohen
A, Tot T, Chiu SY, Chen SL, Fann JC, Rosell J, et al: Swedish
two-county trial: Impact of mammographic screening on breast cancer
mortality during 3 decades. Radiology. 260:658–663. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Tinnemans JG, Wobbes T, Hendriks JH, van
der Sluis RF, Lubbers EJ and de Boer HH: Localization and excision
of nonpalpable breast lesions. A surgical evaluation of three
methods. Arch Surg. 122:802–806. 1987.
|
|
41
|
Lovrics PJ, Cornacchi SD, Farrokhyar F,
Garnett A, Chen V, Franic S and Simunovic M: The relationship
between surgical factors and margin status after breast
conservation surgery for early stage breast cancer. Am J Surg.
197:740–746. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Moon WK, Im JG, Koh YH, Noh DY and Park
IA: US of mammographically detected clustered microcaleifications.
Radiology. 217:849–854. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Yu PC, Lee YW, Chou FF, Wu SC, Huang CC,
Ng SH, Sheen-Chen SM and Ko SF: Clustered microcalcifications of
intermediate concern detected on digital mammography: Ultrasound
assessment. Breast. 20:495–500. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Boonlikit S: Comparison of mammography in
combination with breast ultrasonography versus mammography alone
for breast cancer screening in asymptomatic women. Asian Pac J
Cancer Prev. 14:7731–7736. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Köhler J, Krause B, Grunwald S, Thomas A,
Köhler G, Schwesinger G, Schimming A, Jäger B, Paepke S and
Ohlinger R: Ultrasound and mammography guided wire marking of
non-palpable breast lesions: analysis of 741 cases. Ultraschall
Med. 28:283–290. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Saarela AO, Rissanen TJ, Lähteenmäki KM,
Soini Y, Haukipuro K, Kaarela O and Kiviniemi HO: Wire-guided
excision of non-palpable breast cancer: Determinants and
correlations between radiologic and histologic margins and residual
disease in re-excisions. Breast. 10:28–34. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Sauven P, Bishop H, Patnick J, Walton J,
Wheeler E and Lawrence G; National Health Service Breast Screening
Programme; British Association of Surgical Oncology: The national
health service breast screening programme and British association
of surgical oncology audit of quality assurance in breast screening
1996–2001. Br J Surg. 90:82–87. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Blamey RW: The British association of
surgical oncology guidelines for surgeons in the management of
symptomatic breast disease in the UK (1998 revision). BASO Breast
Specialty Group. Eur J Surg Oncol. 24:464–476. 1998. View Article : Google Scholar : PubMed/NCBI
|