The cDNA of CCR9 was submitted to Genbank (Genbank
no. HSU45982) by Lautens in 1996 as G-protein-coupled-receptor-9-6
(GPR-9-6). The expression and function of GPR-9-6 was investigated
by Zaballos et al (1) in 1999.
GPR-9-6 was renamed as CCR9, due to its similarity in structure and
sequence to chemokine receptor 6 (CCR6), chemokine receptor 7
(CCR7) and STRL33/Bonzo (1).
Furthermore, in vitro stimulation of CCR9 with
thymus-expressing chemokine (TECK) induced intra-cytoplasmic
calcium mobilization and migration of 293 cells (1). TECK, also known as CCL25, was discovered
in thymic dendritic cells in 1997 (2), and was subsequently confirmed to be the
sole ligand of CCR9 (3). CCR9
expression was revealed to be low in human and murine spleen and
lymph nodes but high in thymic tissues (1).
Further studies demonstrated that CCR9 is highly
expressed in the colon, small intestine and several other tissues
involved in the development and maturation of T cells, macrophages
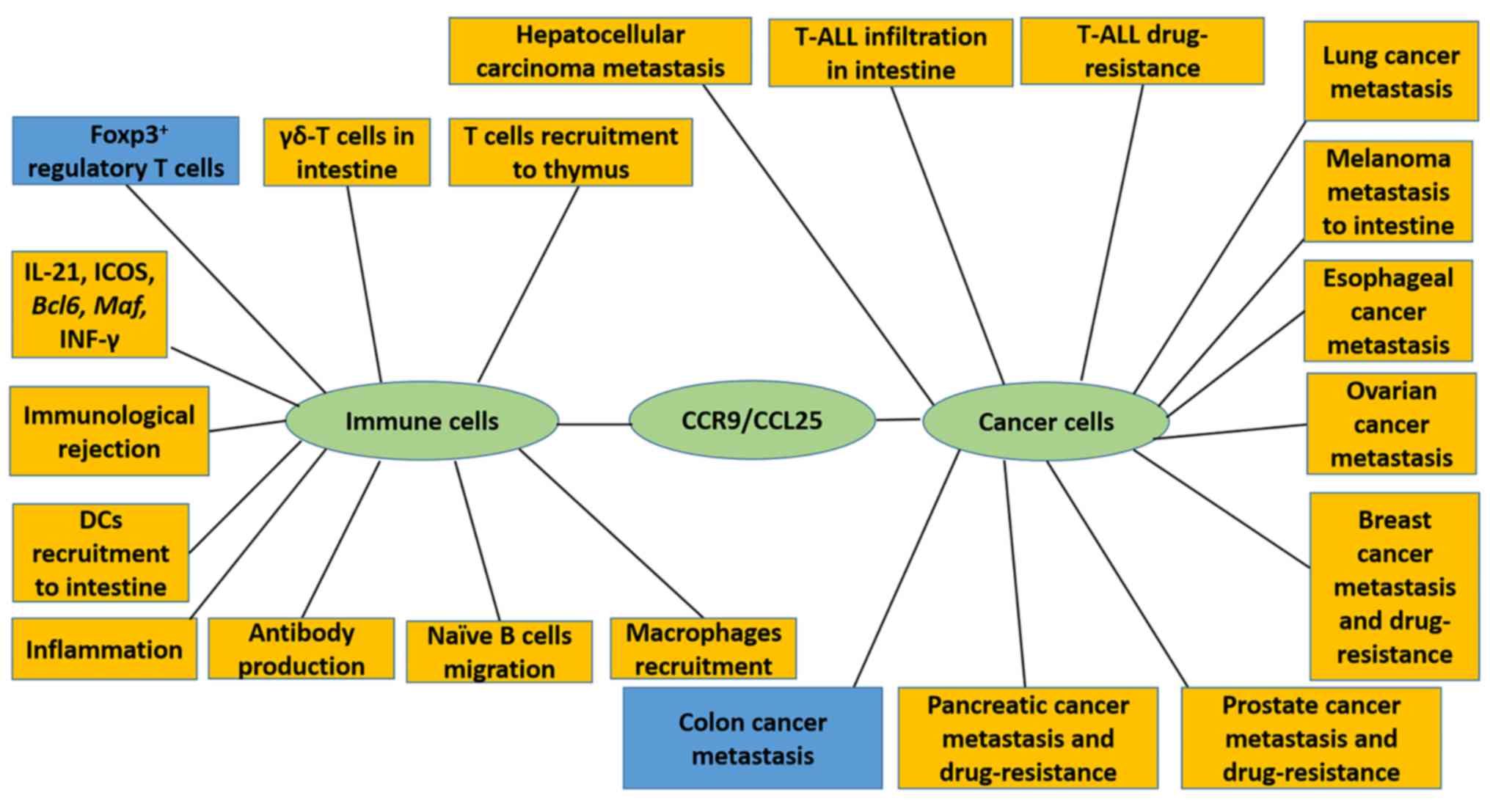
and dendritic cells (DCs) (4–9). CCR9 signaling may therefore have an
influence on processes, including inflammatory responses and
transplantation rejection (10–12).
Furthermore, CCR9 has been demonstrated to influence cancer cell
migration, proliferation and drug resistance (13–15). This
is a review of the current literature on the function of CCR9 in
leukocytes and cancer cells.
Retinoic acid (RA) has been demonstrated to serve a
role in DC-T cell interactions. Several studies revealed that CCR9
expression fluctuated at different stages of DC development
(9,24). Furthermore, several other studies
demonstrated that DCs produced RA, which mediated CCR9 expression
in T cells (25) and induced
interleukin 10 (IL-10) expression in α4β7+
CCR9+ T cells (26). T
cells were also mediated by RA directly via the RA receptor, which
upregulated CCR9 expression (27,28).
Therefore, decreased expression of the RA receptor was associated
with a reduction in CCR9 expression and an attenuation of
graft-versus-host disease (29).
In summary, CCR9 serves a role in the migration,
maturation and function of leukocytes.
Following the discovery of CCR9/CCL25 signaling
pathways in leukocytes, oncology researchers revealed that CCR9
promotes invasion, migration, anti-apoptosis and drug-resistance in
several types of tumors (Fig. 1).
CCR9/CCL25 signaling has been rigorously studied in
hematological malignancies. In T cell lineage acute lymphocytic
leukemia (T-ALL) CD4+ cells, CCR9 was highly expressed,
while T cell chronic lymphocytic leukemia (T-CLL) CD4+
cells moderately expressed CCR9, and a low CCR9 expression was
demonstrated in normal CD4+ T cells (36). The CCR9+ T-ALL Jurkat,
MOLT-4, CEM, SupT1, TALL-1 and DND41 cell lines were revealed to be
chemotactic to CCL25 (37). CCL25
stimulation resulted in pseudopodium formation (38), invasion and migration (39). It has been observed that T cells from
adult patients with leukemia, that had infiltrated the
gastrointestinal tract, were frequently positive for CCR9 (40), which was in line with conclusions from
a study that reported that CCR9 expression was associated with gut
relapse in pediatric T-ALL (41).
Furthermore, CCR9/CCL25 signaling induced P-gp co-localization
within the actin cytoskeleton (15).
This mechanism led to drug resistance to doxorubicin in the MOLT-4
cell line (15). TNF-α mediated
apoptosis was inhibited in CD4+ T-ALL, T-CLL and MOLT-4
cells by CCR9/CCL25 signaling (42).
Transformation of gastric mucosa-associated lymphoid tissue to
gastric extra-nodal diffuse large B-cell lymphoma was mediated by
several chemokines, including CCR9 (43). However, CCR9+ multiple
myeloma cells only migrated toward CXC chemokine ligand 12
(CXCL12), indicating a limited function of CCR9 in multiple myeloma
(44).
The regulation of melanoma cell metastasis by
CCR9/CCL25 signaling remains controversial. Certain studies have
demonstrated that CCR9 expression is associated with organ-specific
metastasis of melanoma cells (45,46).
Melanoma cells that had metastasized to the intestine expressed
CCR9, whereas cells that had metastasized to other organs did not
(47). Besides the intestine, lymph
nodes and skin were the sites where melanoma metastasis often
occurred; however, CCR9+ melanoma cells were not
observed in these locations (47–49).
CCR9+ T cells in the melanoma microenvironment have been
demonstrated to inhibit metastasis (50). However, certain studies have
demonstrated that the function of CCR9 in melanoma was only
moderate; the proportion of patients with intestine metastases was
low (51), indicating limited
organ-specific metastasis (49),
which corresponds with the low proportion of CCR9+ cells
reported in circulating tumor cells (52). Clinical studies demonstrated that CCR7
and chemokine receptor 10 (CCR10), but not CCR9, were associated
with a poorer prognosis (53).
Several types of ovarian carcinoma, including serous
adenocarcinoma, serous papillary cystadenoma and endometrioid
adenocarcinoma, revealed an increased CCR9 expression compared with
normal ovarian tissues (54), and
demonstrated migration and invasion potential toward chemotactic
gradients of CCL25 (55). Intestinal
cells, a source of CCL25, were also a frequent metastatic site for
ovarian carcinoma (55).
CCR9 expression of two breast cancer cell lines,
MDA-MB-231 and MCF7, and benign tissue, were high, medium and low,
respectively, which corresponded with their respective
aggressiveness (56). Lymph nodes,
bone marrow, lung, liver and brain were frequent sites of breast
cancer metastasis, and this process might be regulated by
CCR9/CCL25 (56–58). CCR9/CCL25 signaling was also revealed
to provide a survival advantage to breast cancer cells and
inhibited cisplatin-induced apoptosis (59).
In prostate cancer, CCR9 was highly, moderately and
lowly expressed in highly invasive LNCaP, moderately invasive PC3
and non-invasive prostatic epithelial cells, respectively (60). CCR9/CCL25 also regulated metastasis
(60) and drug-resistance against
etoposide (61) in prostate
cancer.
In pancreatic cancer, pancreatic intraepithelial
neoplasia and pancreatic cancer cells were demonstrated to be
CCR9+ (62). The
pancreatic cancer PANC-1 cell line was CCR9+, and
enhanced invasion was observed following CCL25 treatment (63). Drug-resistance of gemcitabine was
stimulated by CCL25 signaling in pancreatic cancer PANC-1,
MIAPaCa-2 and AsPC-1 cell lines (14).
In the hepatocellular carcinoma cell lines HepG2 and
HUH7, CCR9 promoted invasion and migration (65), and might be a marker to predict the
prognosis of patients (66).
Similarly, CCR9 could be beneficial in predicting
lymph node metastasis and prognosis in lung adenocarcinoma
(67). Adenocarcinoma cells revealed
a higher migratory and invasive potential in response to CCL25,
compared with squamous cell carcinoma cells, which had lower
expression of CCR9 and CCL25 (68).
Esophageal cancer cells also highly expressed CCR9 and potentially
achieved metastasis via CCR9 signaling (69).
Based on these findings, researchers attempted to
design CCR9-specific therapies, including CCR9 antagonists,
monoclonal antibodies against CCR9, and RNAi of CCR9. Computational
modeling of CCR9 antagonists revealed several compounds, one of
which inhibited proliferation and invasion of pancreatic cancer
cell lines and interacted synergistically with gemcitabine
(14). In vivo models revealed
that a CCR9 monoclonal antibody increased apoptosis, necrosis of
tumor tissue, complement-dependent cytotoxicity and
antibody-dependent cytotoxicity by natural killer cells. The
antibody was also demonstrated to decrease proliferation and tumor
vascularization in MOLT-4 cell lines (70). CCR9 inhibition by RNAi facilitated T
cell-associated immunotherapy of breast and pancreatic cancer cell
lines (71). Another trial used CCL25
fused with Pseudomonas exotoxin 38 (PE38) toxin, a truncated
derivative of Pseudomonas exotoxin A, which induced apoptosis in
MOLT-4 cell lines (72).
In summary, CCR9/CCL25 signaling is an important
mediator of malignant behaviors in a number of cancer cells.
Blocking of CCR9/CCL25 signaling appears to be a potential novel
strategy for cancer therapy.
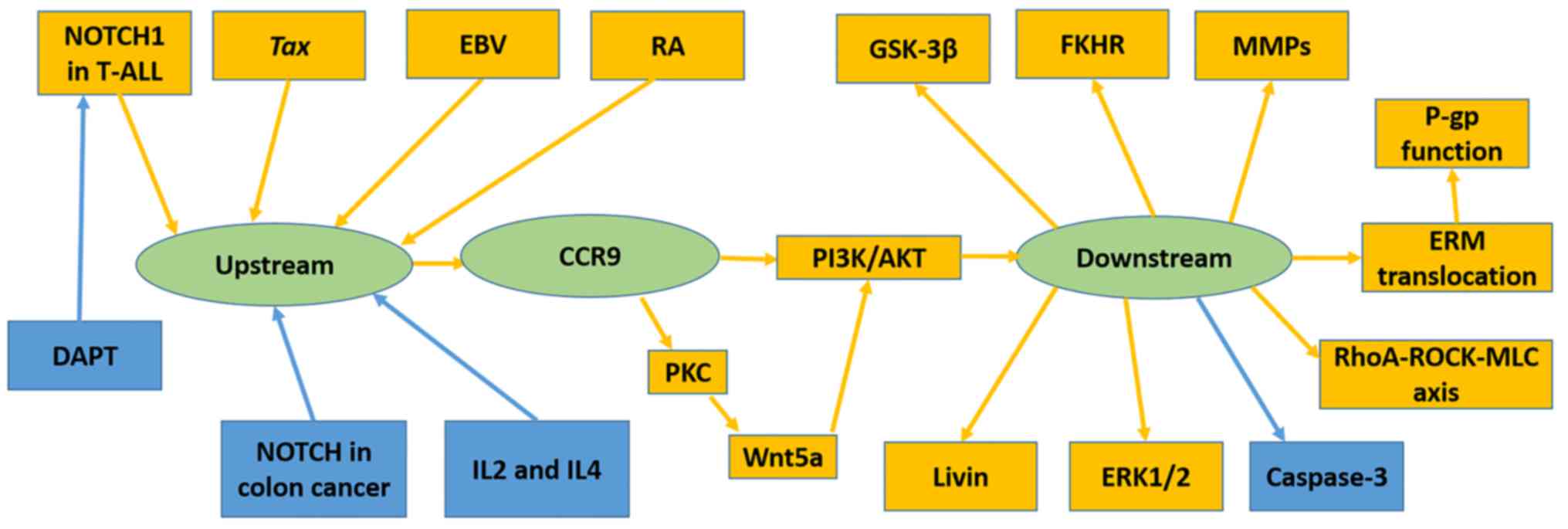
Several immunological factors have been demonstrated
to upregulate CCR9, including human T-lymphotropic virus type 1
(HTLV-1)-encoded transcriptional activator Tax (40), RA (25)
and Epstein-Barr virus (32). Others,
including DAPT (37) and
co-stimulation of IL-2 with IL-4 (36), have been revealed to downregulate
CCR9. The NOTCH pathway has been revealed to mediate CCR9
expression; however, its function differs in T-ALL and colon cancer
(Fig. 2).
NOTCH1 mutations were frequently identified in
T-ALL; certain mutations resulted in δ1 ligand-independent NOTCH
signaling or lengthened the half-life period of NOTCH1 signaling.
Suppression of NOTCH1 by DAPT or RNAi, downregulated CCR9 in the
T-ALL cell lines Jurkat, MOLT-4, CEM, SupT1, TALL-1 and DND41
(37).
Mediation of cell invasion and migration by
CCR9/CCL25 signaling may involve matrix metalloproteinases (MMPs)
(55,56,60), Ras
homologue (RhoA)Rho kinase (ROCK)-myosin light chain (MLC) signal
(39), Wnt5a (74) and ERM protein family (38). In the ovarian cancer OVCAR-3 and
CAOV-3 cell lines, CCR9/CCL25 selectively regulated certain MMPs,
which degraded the cell matrix to promote invasion (55). Different patterns of MMP regulation
were observed in each cell line; for example, in the OVCAR-3 cell
line, expression of MMP-2, 3, 8, 9, 10, 11 and 13 was increased,
while MMP-1, 2, 3, 8 and 10 expression levels were elevated in the
CAOV-3 cell line (55). A similar
mechanism was observed in prostate cancer cell lines (60). CCL25 treatment increased MMP-1 and 2
expression in LNCaP, and MMP-2, 11 and 13 in PC3 cells (60). RhoA-ROCK-MLC signaling, which is
associated with cell motility and morphology was stimulated by
CCL25 in the MOLT-4 cell line (39).
Another pathway has been demonstrated to promote the invasion and
migration of MOLT-4 cells; CCL25 induced Wnt5a activation by
promoting protein kinase C expression and activation in MOLT-4
cells (74). Activated Wnt5a further
induced PI3K/Akt signaling and enhanced cell migration and invasion
(74). Invasion-related ERM protein
was revealed to be translocated from the cytoplasm to the cell
membrane following CCL25 treatment, which contributed to metastasis
(38).
NOTCH and several other signaling proteins regulate
CCR9, and CCR9/CCL25 signaling mediates certain downstream proteins
to promote metastasis and drug-resistance in cancer cells. Based on
the findings that healthy intestine and colon cells physiologically
produce CCL25 (2,6,7,75), we hypothesized that CCL25 induces
chemotaxis and cell survival signaling in leukocytes and cancer
cells. For extra-intestinal cancer, the CCL25 concentration in
healthy intestinal micro-environment is higher than that in the
primary tumor micro-environment; thus, CCR9+ cancer
cells tend to metastasize to the intestine. However, the
micro-environment of colon cancer is rich in CCL25 and therefore,
CCR9 may be downregulated by NOTCH to reduce chemotaxis and promote
metastasis. Investigation of CCL25 levels in the micro-environment
of primary and metastatic lesions would further test this
hypothesis. Another possible mechanism is based on the seed-earth
hypothesis, which states that CCL25 promotes the survival of
circulating tumor cells that have metastasized to the
intestine.
NOTCH signaling has an opposite function in T-ALL
and colon cancer. This contradiction might be explained by the
diversity in ligands and downstream signaling. In T-ALL, δ1 is a
ligand of NOTCH1 (37), while
delta-like 4 (DLL4), Jagged 1 (JAG1) and delta-like proteins 1
(DLK1) are the most probable ligands of NOTCH in colon cancer
(13).
In summary, CCR9/CCL25 signaling mediated the
migration, invasion and drug resistance of cancer cells. Further
studies should focus on elucidating the mechanisms of associated
upstream and downstream signaling of CCR9.
Not applicable.
No funding was received.
Not applicable.
CW and ZL were major contributors in writing the
manuscript. ZX, XW, and DZ retrieved, selected the articles, and
collected useful information from these articles. ZZ and JW
proposed writing this review, made the outline, submitted and
revised this manuscript. All authors read and approved the final
version of the manuscript.
Not applicable.
Not applicable.
The authors declare that they have no competing
interests.
|
1
|
Zaballos A, Gutiérrez J, Varona R, Ardavín
C and Márquez G: Cutting edge: Identification of the orphan
chemokine receptor GPR-9-6 as CCR9, the receptor for the chemokine
TECK. J Immunol. 162:5671–5675. 1999.PubMed/NCBI
|
|
2
|
Vicari AP, Figueroa DJ, Hedrick JA, Foster
JS, Singh KP, Menon S, Copeland NG, Gilbert DJ, Jenkins NA, Bacon
KB and Zlotnik A: TECK: A novel CC chemokine specifically expressed
by thymic dendritic cells and potentially involved in T cell
development. Immunity. 7:291–301. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Yu CR, Peden KW, Zaitseva MB, Golding H
and Farber JM: CCR9A and CCR9B: Two receptors for the chemokine
CCL25/TECK/Ck beta-15 that differ in their sensitivities to ligand.
J Immunol. 164:1293–1305. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Wendland M, Czeloth N, Mach N, Malissen B,
Kremmer E, Pabst O and Förster R: CCR9 is a homing receptor for
plasmacytoid dendritic cells to the small intestine. Proc Natl Acad
Sci USA. 104:6347–6352. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Mizuno S, Kanai T, Mikami Y, Sujino T, Ono
Y, Hayashi A, Handa T, Matsumoto A, Nakamoto N, Matsuoka K, et al:
CCR9+ plasmacytoid dendritic cells in the small intestine suppress
development of intestinal inflammation in mice. Immunol Lett.
146:64–69. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Wurbel MA, McIntire MG, Dwyer P and
Fiebiger E: CCL25/CCR9 interactions regulate large intestinal
inflammation in a murine model of acute colitis. PLoS One.
6:e164422011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Wurbel MA, Philippe JM, Nguyen C,
Victorero G, Freeman T, Wooding P, Miazek A, Mattei MG, Malissen M,
Jordan BR, et al: The chemokine TECK is expressed by thymic and
intestinal epithelial cells and attracts double- and
single-positive thymocytes expressing the TECK receptor CCR9. Eur J
Immunol. 30:262–271. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Schmutz C, Cartwright A, Williams H,
Haworth O, Williams JH, Filer A, Salmon M, Buckley CD and Middleton
J: Monocytes/macrophages express chemokine receptor CCR9 in
rheumatoid arthritis and CCL25 stimulates their differentiation.
Arthritis Res Ther. 12:R1612010. View
Article : Google Scholar : PubMed/NCBI
|
|
9
|
Dursun E, Endele M, Musumeci A, Failmezger
H, Wang SH, Tresch A, Schroeder T and Krug AB: Continuous single
cell imaging reveals sequential steps of plasmacytoid dendritic
cell development from common dendritic cell progenitors. Sci Rep.
6:374622016. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Eberhardson M, Marits P, Jones M, Jones P,
Karlen P, Karlsson M, Cotton G, Woznica K, Maltman B, Glise H and
Winqvist O: Treatment of inflammatory bowel disease by chemokine
receptor-targeted leukapheresis. Clin Immunol. 149:73–82. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Alvarez C, Benítez A, Rojas L, Pujol M,
Carvajal P, Díaz-Zúñiga J and Vernal R: Differential expression of
CC chemokines (CCLs) and receptors (CCRs) by human T lymphocytes in
response to different Aggregatibacter actinomycetemcomitans
serotypes. J Appl Oral Sci. 23:536–546. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Li J, Xiong T, Xiao R, Xiong A, Chen J,
Altaf E, Zheng Y, Zhu G, He Y and Tan J: Anti-CCL25 antibody
prolongs skin allograft survival by blocking CCR9 expression and
impairing splenic T-cell function. Arch Immunol Ther Exp (Warsz).
61:237–244. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Chen HJ, Edwards R, Tucci S, Bu P, Milsom
J, Lee S, Edelmann W, Gümüs ZH, Shen X and Lipkin S: Chemokine
25-induced signaling suppresses colon cancer invasion and
metastasis. J Clin Invest. 122:3184–3196. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Lee S, Heinrich EL, Li L, Lu J, Choi AH,
Levy RA, Wagner JE, Yip ML, Vaidehi N and Kim J: CCR9-mediated
signaling through β-catenin and identification of a novel CCR9
antagonist. Mol Oncol. 9:1599–1611. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zhang L, Xiao R, Xiong J, Leng J, Ehtisham
A, Hu Y, Ding Q, Xu H, Liu S, Wang J, et al: Activated ERM protein
plays a critical role in drug resistance of MOLT4 cells induced by
CCL25. PLoS One. 8:e523842013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Krueger A, Willenzon S, Lyszkiewicz M,
Kremmer E and Forster R: CC chemokine receptor 7 and 9
double-deficient hematopoietic progenitors are severely impaired in
seeding the adult thymus. Blood. 115:1906–1912. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Zlotoff DA, Sambandam A, Logan TD, Bell
JJ, Schwarz BA and Bhandoola A: CCR7 and CCR9 together recruit
hematopoietic progenitors to the adult thymus. Blood.
115:1897–1905. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Liu C, Saito F, Liu Z, Lei Y, Uehara S,
Love P, Lipp M, Kondo S, Manley N and Takahama Y: Coordination
between CCR7- and CCR9-mediated chemokine signals in prevascular
fetal thymus colonization. Blood. 108:2531–2539. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Uehara S, Grinberg A, Farber JM and Love
PE: A role for CCR9 in T lymphocyte development and migration. J
Immunol. 168:2811–2819. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Evans-Marin HL, Cao AT, Yao S, Chen F, He
C, Liu H, Wu W, Gonzalez MG, Dann SM and Cong Y: Unexpected
regulatory role of CCR9 in regulatory T cell development. PLoS One.
10:e01341002015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
McGuire HM, Vogelzang A, Ma CS, Hughes WE,
Silveira PA, Tangye SG, Christ D, Fulcher D, Falcone M and King C:
A subset of interleukin-21+ chemokine receptor CCR9+ T helper cells
target accessory organs of the digestive system in autoimmunity.
Immunity. 34:602–615. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Tubo NJ, Wurbel MA, Charvat TT, Schall TJ,
Walters MJ and Campbell JJ: A systemically-administered small
molecule antagonist of CCR9 acts as a tissue-selective inhibitor of
lymphocyte trafficking. PLoS One. 7:e504982012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Greis C, Rasuly Z, Janosi RA, Kordelas L,
Beelen DW and Liebregts T: Intestinal T lymphocyte homing is
associated with gastric emptying and epithelial barrier function in
critically ill: A prospective observational study. Crit Care.
21:702017. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Drakes ML, Stiff PJ and Blanchard TG:
Inverse relationship between dendritic cell CCR9 expression and
maturation state. Immunology. 127:466–476. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Stock A, Booth S and Cerundolo V:
Prostaglandin E2 suppresses the differentiation of retinoic
acid-producing dendritic cells in mice and humans. J Exp Med.
208:761–773. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Bakdash G, Vogelpoel LT, van Capel TM,
Kapsenberg ML and de Jong EC: Retinoic acid primes human dendritic
cells to induce gut-homing, IL-10-producing regulatory T cells.
Mucosal Immunol. 8:265–278. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Chen X, Dodge J, Komorowski R and Drobyski
WR: A critical role for the retinoic acid signaling pathway in the
pathophysiology of gastrointestinal graft-versus-host disease.
Blood. 121:3970–3980. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Duurland CL, Brown CC, O'Shaughnessy RF
and Wedderburn LR: CD161+Tconv and CD161+Treg
share a transcriptional and functional phenotype despite limited
overlap in TCRβ repertoire. Front Immunol. 8:1032017. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Aoyama K, Saha A, Tolar J, Riddle MJ,
Veenstra RG, Taylor PA, Blomhoff R, Panoskaltsis-Mortari A,
Klebanoff CA, Socié G, et al: Inhibiting retinoic acid signaling
ameliorates graft-versus-host disease by modifying T-cell
differentiation and intestinal migration. Blood. 122:2125–2134.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Wurbel MA, Malissen M, Guy-Grand D, Meffre
E, Nussenzweig MC, Richelme M, Carrier A and Malissen B: Mice
lacking the CCR9 CC-chemokine receptor show a mild impairment of
early T- and B-cell development and a reduction in T-cell receptor
gammadelta(+) gut intraepithelial lymphocytes. Blood. 98:2626–2632.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Demberg T, Mohanram V, Venzon D and
Robert-Guroff M: Phenotypes and distribution of mucosal memory
B-cell populations in the SIV/SHIV rhesus macaque model. Clin
Immunol. 153:264–276. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Ehlin-Henriksson B, Liang W, Cagigi A,
Mowafi F, Klein G and Nilsson A: Changes in chemokines and
chemokine receptor expression on tonsillar B cells upon
Epstein-Barr virus infection. Immunology. 127:549–557. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Mizukami T, Kanai T, Mikami Y, Hayashi A,
Doi T, Handa T, Matsumoto A, Jun L, Matsuoka K, Sato T, et al:
CCR9+ macrophages are required for eradication of peritoneal
bacterial infections and prevention of polymicrobial sepsis.
Immunol Lett. 147:75–79. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Chu PS, Nakamoto N, Ebinuma H, Usui S,
Saeki K, Matsumoto A, Mikami Y, Sugiyama K, Tomita K, Kanai T, et
al: C-C motif chemokine receptor 9 positive macrophages activate
hepatic stellate cells and promote liver fibrosis in mice.
Hepatology. 58:337–350. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Amiya T, Nakamoto N, Chu PS, Teratani T,
Nakajima H, Fukuchi Y, Taniki N, Yamaguchi A, Shiba S, Miyake R, et
al: Bone marrow-derived macrophages distinct from tissue-resident
macrophages play a pivotal role in Concanavalin A-induced murine
liver injury via CCR9 axis. Sci Rep. 6:351462016. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Qiuping Z, Qun L, Chunsong H, Xiaolian Z,
Baojun H, Mingzhen Y, Chengming L, Jinshen H, Qingping G, Kejian Z,
et al: Selectively increased expression and functions of chemokine
receptor CCR9 on CD4+ T cells from patients with T-cell lineage
acute lymphocytic leukemia. Cancer Res. 63:6469–6477.
2003.PubMed/NCBI
|
|
37
|
Mirandola L, Chiriva-Internati M, Montagna
D, Locatelli F, Zecca M, Ranzani M, Basile A, Locati M, Cobos E,
Kast WM, et al: Notch1 regulates chemotaxis and proliferation by
controlling the CC-chemokine receptors 5 and 9 in T cell acute
lymphoblastic leukaemia. J Pathol. 226:713–722. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Zhou B, Leng J, Hu M, Zhang L, Wang Z, Liu
D, Tong X, Yu B, Hu Y, Deng C, et al: Ezrin is a key molecule in
the metastasis of MOLT4 cells induced by CCL25/CCR9. Leuk Res.
34:769–776. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Zhang L, Yu B, Hu M, Wang Z, Liu D, Tong
X, Leng J, Zhou B, Hu Y, Wu R, et al: Role of Rho-ROCK signaling in
MOLT4 cells metastasis induced by CCL25. Leuk Res. 35:103–109.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Nagakubo D, Jin Z, Hieshima K, Nakayama T,
Shirakawa AK, Tanaka Y, Hasegawa H, Hayashi T, Tsukasaki K, Yamada
Y and Yoshie O: Expression of CCR9 in HTLV-1+ T cells and ATL cells
expressing Tax. Int J Cancer. 120:1591–1597. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Annels NE, Willemze AJ, van der Velden VH,
Faaij CM, van Wering E, Sie-Go DM, Egeler RM, van Tol MJ and Révész
T: Possible link between unique chemokine and homing receptor
expression at diagnosis and relapse location in a patient with
childhood T-ALL. Blood. 103:2806–2808. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Qiuping Z, Jei X, Youxin J, Wei J, Chun L,
Jin W, Qun W, Yan L, Chunsong H, Mingzhen Y, et al: CC chemokine
ligand 25 enhances resistance to apoptosis in CD4+ T cells from
patients with T-cell lineage acute and chronic lymphocytic leukemia
by means of livin activation. Cancer Res. 64:7579–7587. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Deutsch AJ, Steinbauer E, Hofmann NA,
Strunk D, Gerlza T, Beham-Schmid C, Schaider H and Neumeister P:
Chemokine receptors in gastric MALT lymphoma: Loss of CXCR4 and
upregulation of CXCR7 is associated with progression to diffuse
large B-cell lymphoma. Mod Pathol. 26:182–194. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Badr G, Lefevre EA and Mohany M:
Thymoquinone inhibits the CXCL12-induced chemotaxis of multiple
myeloma cells and increases their susceptibility to Fas-mediated
apoptosis. PLoS One. 6:e237412011. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Letsch A, Keilholz U, Schadendorf D,
Assfalg G, Asemissen AM, Thiel E and Scheibenbogen C: Functional
CCR9 expression is associated with small intestinal metastasis. J
Invest Dermatol. 122:685–690. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Seidl H, Richtig E, Tilz H, Stefan M,
Schmidbauer U, Asslaber M, Zatloukal K, Herlyn M and Schaider H:
Profiles of chemokine receptors in melanocytic lesions: de novo
expression of CXCR6 in melanoma. Hum Pathol. 38:768–780. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Amersi FF, Terando AM, Goto Y, Scolyer RA,
Thompson JF, Tran AN, Faries MB, Morton DL and Hoon DS: Activation
of CCR9/CCL25 in cutaneous melanoma mediates preferential
metastasis to the small intestine. Clin Cancer Res. 14:638–645.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Richmond A: CCR9 homes metastatic melanoma
cells to the small bowel. Clin Cancer Res. 14:621–623. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Salerno EP, Olson WC, McSkimming C, Shea S
and Slingluff CL Jr: T cells in the human metastatic melanoma
microenvironment express site-specific homing receptors and
retention integrins. Int J Cancer. 134:563–574. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Jacquelot N, Enot DP, Flament C, Vimond N,
Blattner C, Pitt JM, Yamazaki T, Roberti MP, Daillère R, Vétizou M,
et al: Chemokine receptor patterns in lymphocytes mirror metastatic
spreading in melanoma. J Clin Invest. 126:921–937. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Park J, Ostrowitz MB, Cohen MS and
Al-Kasspooles M: A patient with metastatic melanoma of the small
bowel. Oncology (Williston Park). 23:98–102. 2009.PubMed/NCBI
|
|
52
|
Fusi A, Liu Z, Kümmerlen V, Nonnemacher A,
Jeske J and Keilholz U: Expression of chemokine receptors on
circulating tumor cells in patients with solid tumors. J Transl
Med. 10:522012. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Kühnelt-Leddihn L, Müller H, Eisendle K,
Zelger B and Weinlich G: Overexpression of the chemokine receptors
CXCR4, CCR7, CCR9, and CCR10 in human primary cutaneous melanoma: A
potential prognostic value for CCR7 and CCR10? Arch Dermatol Res.
304:185–193. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Singh R, Stockard CR, Grizzle WE, Lillard
JW Jr and Singh S: Expression and histopathological correlation of
CCR9 and CCL25 in ovarian cancer. Int J Oncol. 39:373–381.
2011.PubMed/NCBI
|
|
55
|
Johnson EL, Singh R, Singh S,
Johnson-Holiday CM, Grizzle WE, Partridge EE and Lillard JW Jr:
CCL25-CCR9 interaction modulates ovarian cancer cell migration,
metalloproteinase expression, and invasion. World J Surg Oncol.
8:622010. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Johnson-Holiday C, Singh R, Johnson E,
Singh S, Stockard CR, Grizzle WE and Lillard JW Jr: CCL25 mediates
migration, invasion and matrix metalloproteinase expression by
breast cancer cells in a CCR9-dependent fashion. Int J Oncol.
38:1279–1285. 2011.PubMed/NCBI
|
|
57
|
Feng LY, Ou ZL, Wu FY, Shen ZZ and Shao
ZM: Involvement of a novel chemokine decoy receptor CCX-CKR in
breast cancer growth, metastasis and patient survival. Clin Cancer
Res. 15:2962–2970. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Müller A, Homey B, Soto H, Ge N, Catron D,
Buchanan ME, McClanahan T, Murphy E, Yuan W, Wagner SN, et al:
Involvement of chemokine receptors in breast cancer metastasis.
Nature. 410:50–56. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Johnson-Holiday C, Singh R, Johnson EL,
Grizzle WE, Lillard JW Jr..Singh S: CCR9-CCL25 interactions promote
cisplatin resistance in breast cancer cell through Akt activation
in a PI3K-dependent and FAK-independent fashion. World J Surg
Oncol. 9:462011. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Singh S, Singh UP, Stiles JK, Grizzle WE
and Lillard JW Jr: Expression and functional role of CCR9 in
prostate cancer cell migration and invasion. Clin Cancer Res.
10:8743–8750. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Sharma PK, Singh R, Novakovic KR, Eaton
JW, Grizzle WE and Singh S: CCR9 mediates PI3K/AKT-dependent
antiapoptotic signals in prostate cancer cells and inhibition of
CCR9-CCL25 interaction enhances the cytotoxic effects of etoposide.
Int J Cancer. 127:2020–2030. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Shen X, Mailey B, Ellenhorn JD, Chu PG,
Lowy AM and Kim J: CC chemokine receptor 9 enhances proliferation
in pancreatic intraepithelial neoplasia and pancreatic cancer
cells. J Gastrointest Surg. 13:1955–1962. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Heinrich EL, Arrington AK, Ko ME, Luu C,
Lee W, Lu J and Kim J: paracrine activation of chemokine receptor
CCR9 enhances the invasiveness of pancreatic cancer cells. Cancer
Microenviron. 6:241–245. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Chen HJ, Sun J, Huang Z, Hou H Jr, Arcilla
M, Rakhilin N, Joe DJ, Choi J, Gadamsetty P, Milsom J, et al:
Comprehensive models of human primary and metastatic colorectal
tumors in immunodeficient and immunocompetent mice by chemokine
targeting. Nat Biotechnol. 33:656–660. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Zhang Z, Sun T, Chen Y, Gong S, Sun X, Zou
F and Peng R: CCL25/CCR9 Signal promotes migration and invasion in
hepatocellular and breast cancer cell lines. DNA Cell Biol.
35:348–357. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Zhang Z, Qin C, Wu Y, Su Z, Xian G and Hu
B: CCR9 as a prognostic marker and therapeutic target in
hepatocellular carcinoma. Oncol Rep. 31:1629–1636. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Zhong Y, Jiang L, Lin H, Li B, Lan J,
Liang S, Shen B, Lei Z and Zheng W: Expression of CC chemokine
receptor 9 predicts poor prognosis in patients with lung
adenocarcinoma. Diagn Pathol. 10:1012015. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Gupta P, Sharma PK, Mir H, Singh R, Singh
N, Kloecker GH, Lillard JW Jr and Singh S: CCR9/CCL25 expression in
non-small cell lung cancer correlates with aggressive disease and
mediates key steps of metastasis. Oncotarget. 5:10170–10179. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Mishan MA, Heirani-Tabasi A, Mokhberian N,
Hassanzade M, Kalalian Moghaddam H, Bahrami AR and Ahmadiankia N:
Analysis of chemokine receptor gene expression in esophageal cancer
cells compared with breast cancer with insights into metastasis.
Iran J Public Health. 44:1353–1358. 2015.PubMed/NCBI
|
|
70
|
Chamorro S, Vela M, Franco-Villanueva A,
Carramolino L, Gutiérrez J, Gómez L, Lozano M, Salvador B,
García-Gallo M, Martínez-A C and Kremer L: Antitumor effects of a
monoclonal antibody to human CCR9 in leukemia cell xenografts.
MAbs. 6:1000–1012. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Khandelwal N, Breinig M, Speck T, Michels
T, Kreutzer C, Sorrentino A, Sharma AK, Umansky L, Conrad H,
Poschke I, et al: A high-throughput RNAi screen for detection of
immune-checkpoint molecules that mediate tumor resistance to
cytotoxic T lymphocytes. EMBO Mol Med. 7:450–463. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Hu Y, Zhang L, Wu R, Han R, Jia Y, Jiang
Z, Cheng M, Gan J, Tao X and Zhang Q: Specific killing of CCR9
high-expressing acute T lymphocytic leukemia cells by CCL25 fused
with PE38 toxin. Leuk Res. 35:1254–1260. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Youn BS, Kim YJ, Mantel C, Yu KY and
Broxmeyer HE: Blocking of c-FLIP(L)-independent
cycloheximide-induced apoptosis or Fas-mediated apoptosis by the CC
chemokine receptor 9/TECK interaction. Blood. 98:925–933. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Deng X, Tu Z, Xiong M, Tembo K, Zhou L,
Liu P, Pan S, Xiong J, Yang X, Leng J, et al: Wnt5a and CCL25
promote adult T-cell acute lymphoblastic leukemia cell migration,
invasion and metastasis. Oncotarget. 8:39033–39047. 2017.PubMed/NCBI
|
|
75
|
Shang L, Thirunarayanan N, Viejo-Borbolla
A, Martin AP, Bogunovic M, Marchesi F, Unkeless JC, Ho Y, Furtado
GC, Alcami A, et al: Expression of the chemokine binding protein M3
promotes marked changes in the accumulation of specific leukocytes
subsets within the intestine. Gastroenterology. 137:1006–1018,
1018.e1-3. 2009. View Article : Google Scholar : PubMed/NCBI
|