Introduction
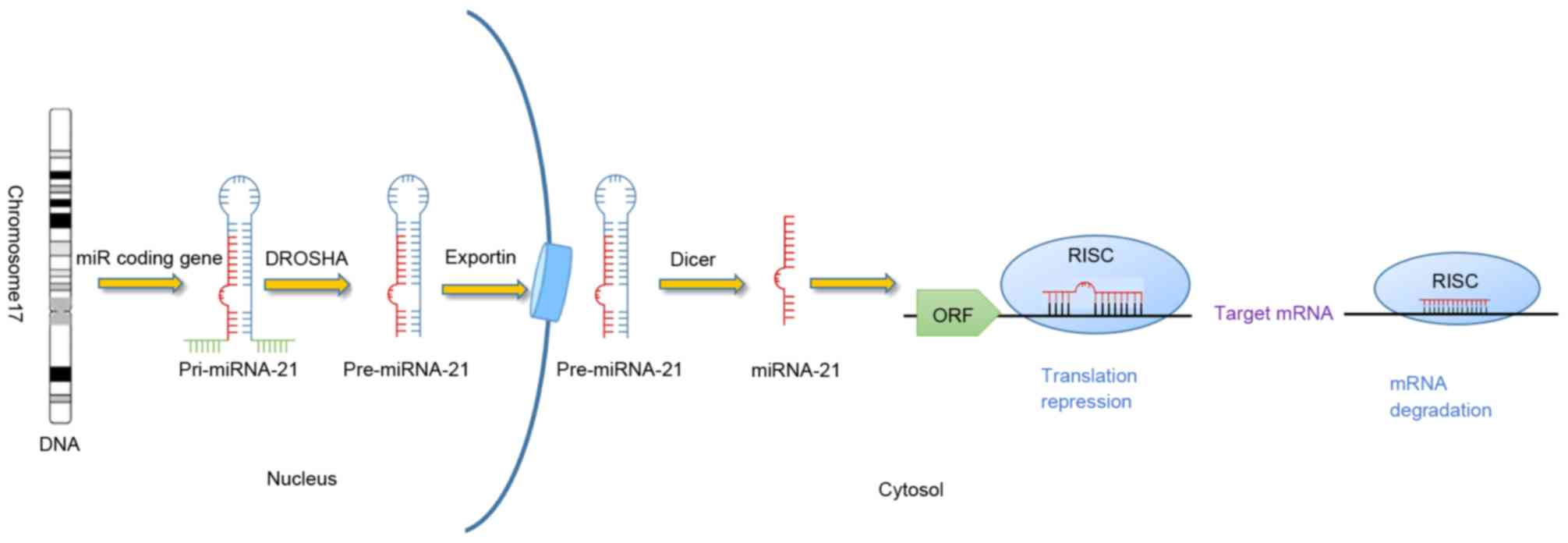
MicroRNA-21 (miRNA-21/miR-21), a member of the miRNA
family, is encoded by the MIR21 gene located on chromosome
17q23.2 in humans (1). The mature
miR-21, which is formed from endogenous non-coding RNA molecules of
~22 nucleotides, is incorporated into an RNA-induced silencing
complex, which binds to the 3′-untranslated region of various
target mRNAs through imperfect base pairing with the miRNA
(Fig. 1). The expression of miR-21 is
significantly increased in a number of solid tumors, including
lung, breast, colon, gastric and pancreatic cancer (2–9). Moreover,
in previous studies, miR-21 was also upregulated in immune cells,
promoted immune-related inflammatory diseases and played important
roles in the pathogenesis of autoimmune diseases, including
systemic lupus erythematosus, multiple sclerosis and type 1
diabetes (10–13). Emerging studies have shown that
miR-21, as an oncogenic miRNA (oncomiR), is upregulated in
non-small cell lung cancer (NSCLC) and can regulate the growth,
metastasis and apoptosis, as well as the genetic instability, of
cancer cells through altering the expression of various target
molecules, such as phosphatase and tensin homolog, programmed cell
death 4, Purinergic Receptor P2× 7 and phosphoinositide 3-kinase
(14–18). Importantly, even though the underlying
mechanism of the expression of miR-21 remains largely unknown, a
large number of studies have investigated the potential value of
miR-21 expression in the prognosis prediction and diagnosis of
NSCLC, indicating its potential use as a novel biomarker for cancer
diagnosis, recurrence and prognosis prediction, which may harbor
relevant clinical implications.
miR-21 and the prognosis of NSCLC
Tissue miR-21 expression and the
prognosis prediction of NSCLC
In 2006, Yanaihara et al (19) was the first study to examine miRNA
expression in frozen lung adenocarcinoma tissues and normal lung
tissues using the microarray technique. The results showed that the
expression level of 5 microRNAs, miR-155, miR-17, miR-21, miR-145
and let-7a, differed significantly among the various tissues from
NSCLC patients, which may be associated with patient mortality. In
particular, the expression of the 3 oncomiRs, miR-21, miR-17 and
miR-155, was significantly elevated in the adenocarcinoma tissues.
Further analysis (20) suggested that
high miR-21 expression was closely associated with the prognosis
and progression of early NSCLC [Tumor-Node-Metastasis (TMN) stage
I] (21), supporting the hypothesis
that abnormal miR-21 expression may be critical for the prognosis
prediction of NSCLC (22,23). Furthermore, a recent case-control
study performed by Cinegaglia et al (24) measured the expression of miR-21 in 24
fresh frozen tissues (17 lung adenocarcinoma and 7 normal tissues)
by TaqMan quantitative polymerase chain reaction (qPCR). The data
were subsequently compared with the published database of Mirbase
(http://www.mirbase.org) and it was found that
high miR-21 expression was closely correlated with a worse
prognosis in NSCLC patients, further confirming the potential value
of miR-21 expression as a prognostic biomarker in NSCLC.
A study on miRNA-21 expression in formalin-fixed
paraffin-embedded tissues (FFPETs) also yielded notable findings.
Tian et al (25) determined
the level of miRNA-21 expression in 204 pairs of FFPET samples
(including cancer tissue samples and their corresponding adjacent
normal tissues), using TaqMan qPCR, and found that miR-21
expression was significantly increased in the NSCLC tissues
compared with the adjacent normal tissues. The miR-21 expression in
late NSCLC (stage IIb-IIIa) tissues [mean ± standard deviation
(SD), 7.9±2.1] was much higher than that in early NSCLC (stage
Ia-IIa) tissues (mean ± SD, 5.1±1.6). Patients with high miR-21
expression also had higher rates of lymph node metastasis, while no
correlation was found between miR-21 expression and tumor size. A
Kaplan-Meier survival analysis further confirmed that the overall
survival time (OS) and the progression-free survival time (PFS)
were significantly reduced in the NSCLC patients with high miR-21
expression compared with those in patients with low miR-21
expression. Finally, multiple logistic regression analysis
indicated that high miR-21 expression was a novel independent
prognostic factor for the reduced OS of NSCLC patients. Given the
reliability of FFPETs in clinical pathology, it may be concluded
that these results indicate that the regular measurement of
miRNA-21 expression may represent an appropriate approach to the
prognosis prediction of NSCLC.
Serum miR-21 expression and the
prognosis prediction of NSCLC
Recent studies have suggested that the level of
serum-distinct miRNA molecules may be a useful biomarker for the
prognosis prediction of various cancer types (26–29). In
particular, the association between the level of serum miR-21 and
the prognosis prediction of NSCLC has become the focus of a number
of studies. The study by Wang et al (30) found that the serum miR-21 level in
NSCLC patients were markedly higher than those in healthy
individuals. The 3-year survival rate of the NSCLC patients with
high serum miR-21 expression was significantly decreased compared
with that of the patients with low serum miR-21 expression.
Moreover, the study also noted a positive association between high
levels of serum miR-21 and TNM staging and lymph node metastases in
NSCLC patients. Based on a multiple logistic regression analysis of
the OS in NSCLC patients, the serum miR-21 level is an independent
prognostic factor for the disease (relative risk, 2.01).
Additionally, Liu et al (31)
reported that serum level of miR-21 was consistent with the cancer
tissue level of miR-21 in the prognosis of NSCLC patients.
Notably, in a recent study, using a receiver
operating characteristic curve to evaluate the prognostic value of
serum miR-21 expression in NSCLC patients, Zhao et al
(32) demonstrated that the
sensitivity and specificity of using the relative expression of
miR-21 in the prognosis of NSCLC patients were up to 73.8 and
71.1%, respectively, when the cut-off value was set at 1.22. When
the NSCLC patients were separated based on this cut-off value, a
Kaplan-Meier analysis indicated that the survival time of 23.1
months in the high miR-21 expression group (≥1.22) was
significantly reduced compared with the 34.3 months recorded in the
low miR-21 expression group (<1.22). In addition, Pearson's
correlation analysis demonstrated that serum miR-21 levels were
negatively correlated with the survival of NSCLC patients
(r=−0.508). Since blood collection is an easy procedure to perform,
these findings suggested that combining serum miR-21 detection with
the evaluation of associated clinical information may become an
important direction in predicting the prognosis of NSCLC.
miR-21 expression and cancer recurrence and
metastasis in NSCLC patients
In 2013, Yang et al (33) detected 141 miRNAs in the cancer tissue
from NSCLC patients and found significantly abnormal expression of
4 of these, namely miR-155, miR-21, miR-34 and let-7. Subsequent
hazard ratio (HR) analyses further indicated that miR-21 expression
was not only negatively correlated with the OS (HR, 2.32), but that
it was also negatively correlated with recurrence-free survival
(HR, 2.43), indicating that miR-21 also has a clinical value in
predicting cancer recurrence in NSCLC patients (34).
Accumulating evidence has suggested a potential
value of cancer cell-derived exosomes in reflecting the development
of various types of cancer. Consistent with this research, a recent
study by Munagala et al (35)
found that miR-21 levels in cancer exosomes could also be a
biomarker of the recurrence of lung cancer. The study cultured a
normal lung cell line (Beas-2b) and a lung cancer cell line (H1299)
in vitro, and analyzed the miRNA expression profiles between
cells and their exosomes. Data showed that there were significant
changes in the expression of 77 miRNAs (including miR-21) in the
H1299 cells compared with that in the Beas-2b cells. Notably, the
miR-21 level in the exosomes from the H1299 cells was also
significantly increased. Primary and recurrent xenograft lung
cancer nude mouse models were then established and the
significantly upregulated expression of miR-21 in the recurrent
tumor tissues compared with the primary tumor tissues was observed.
Moreover, miRNA expression, including miR-21, in the serum exosomes
was also detected and a consistent result was obtained. This study
suggested that miR-21 expression in serum exosomes could also be
employed as a potential biomarker for recurrent lung cancer.
Previous studies have found that miR-21 expression
may also be a potential prognostic marker in NSCLC with lymph node
metastases. Stenvold et al (36) collected 335 tumor tissues from
patients with NSCLC stage I to IIIa and measured the level of
miR-21 in the tumor cells and basal cells using in situ
hybridization. The study demonstrated that high miR-21 expression
in the tumor cells was a positive prognostic indicator in the
patients with lymph node metastases. By contrast, high miR-21
expression in the basal cells was an adverse prognostic indicator
in the patients without lymph node metastases. A multiple logistic
regression analysis further demonstrated that a low miR-21 level in
the cancer cells was an independent adverse prognostic indicator in
the patients with lymph node metastases (HR, 2.03). However, it is
important to note that the findings from this study were
inconsistent with those from other studies, in which it had been
reported that miR-21 plays a role as an oncogene in regulating lung
cancer metastases. Therefore, further studies are required to
elucidate the reasons for such disparities. We hypothesize that the
mechanism of differential miR-21 expression in the cancer and basal
cells, which was associated with the abnormal activity of multiple
cancer-related regulators, such as the signal transduction and
activation of signal transducer and activator of transcription 3,
activator protein 1, transforming growth factor β, and epidermal
growth factor receptor (25), may
have contributed to this unexpected difference.
miR-21 expression and an early diagnosis of
NSCLC
Plasma miR-21 level and an early
diagnosis of NSCLC
Although the detection of the plasma miR-21 level
alone is not useful for an early diagnosis in NSCLC patients
(37,38), recent studies have suggested that the
combined detection of miR-21 and other associated molecules could
improve the accuracy and specificity of an early NSCLC diagnosis.
Tang et al (39) examined the
abnormal plasma expression of 3 miRNAs (miR-21, miR-145 and
miR-155) in 62 lung cancer patients and 60 smokers by qPCR. The
area under the curve (AUC) analysis found that the combination of
these 3 miRNAs had a high diagnostic rate for lung cancer (AUC,
0.874), with a sensitivity and specificity of up to 69.4 and 78.3%,
respectively. The study then validated the diagnostic effect of
these 3 miRNAs in 34 lung cancer patients, 30 patients with benign
pulmonary nodules and 32 healthy smokers, and found that the
combination of these miRNAs not only distinguished the lung cancer
patients from the healthy smokers (AUC, 0.872; sensitivity, 76.5%;
specificity, 81.3%), but also distinguished the patients with lung
cancer from those with benign pulmonary nodules (AUC, 0.841;
sensitivity, 76.5%; specificity, 80.0%). This study suggested that
the detection of a combination of plasma miR-21, miR-145 and
miR-155 could be a novel strategy for the early non-invasive
screening of lung cancer.
Furthermore, researchers have also examined the
potential diagnostic values of different combinations of miR-21 and
other molecules in NSCLC. Geng et al (40) tested the expression of 5 miRNAs
(miR-20a, miR-223, miR-21, miR-221 and miR-145) in plasma samples
from 126 patients with early lung cancer, 42 patients with
non-tumor lung diseases and 60 healthy volunteers, and analyzed the
value of this combination in the early diagnosis of NSCLC. The
results indicated that the AUC when using these 5 miRNAs
independently in the diagnosis of early NSCLC were 0.89, 0.94,
0.77, 0.92 and 0.77, respectively. Furthermore, the plasma levels
of miR-20a, miR-223, miR-21 and miR-145 could improve the
prediction of NSCLC in smokers. Further analysis also revealed that
the combination of these 5 miRNAs could more accurately distinguish
the clinical stages and pathological types of NSCLC, in particular
the subtypes of squamous cell carcinoma (40). In summary, these studies indicate that
the measurement of the plasma miR-21 level could be an important
complement to the early diagnosis of NSCLC patients.
Bronchoalveolar lavage fluid (BAL)
miR-21 level in sputum and the early diagnosis of NSCLC
A recent study revealed that the combined detection
of miR-21 and other associated molecules in the sputum and BAL can
be used in the diagnosis of NSCLC. Kim et al (41) examined sputum samples from 27 NSCLC
patients and 11 healthy individuals, and a clustering analysis
indicated that the sensitivity and specificity of using a
combination of 5 miRNAs (miR-21, miR-143, miR-155, miR-210 and
miR-372) in the early diagnosis of NSCLC were up to 67.8 and 90%,
respectively. Meanwhile, the sensitivity and specificity of this
miRNA combination in the BAL group were up to 86.7 and 100%,
respectively, indicating that the miRNA expression profiles in
sputum and BAL are potential biomarkers for the early diagnosis of
NSCLC.
Based on the aforementioned study, a recent study by
Razzak et al (42) examined
the potential value of using 3 of the aforementioned miRNAs
(miR-21, miR-210 and miR-372) in the early diagnosis of NSCLC.
Sputum samples were collected from 21 early NSCLC patients (stages
I and II), 22 late NSCLC patients (stage III and above) and 10
healthy controls. Clustering analysis further revealed that the
sensitivity and specificity of using a combination of these 3
miRNAs in the diagnosis of early NSCLC were up to 67 and 90%,
respectively, and that for late NSCLC, the sensitivity and
specificity were up to 64 and 100%, respectively. Therefore, this
study raised a notable point that, although the sensitivity of
using the detection of 3 miRNAs in the diagnosis of late NSCLC was
less than that of 5 miRNAs, their sensitivity and specificity in
the diagnosis of early NSCLC remained relatively high. In clinical
testing, detecting fewer miRNAs reduces the difficulty of
performing the test, and thus, this study suggested that optimizing
the combination of specific miRNAs in sputum samples for the early
diagnosis of NSCLC will be an important focus in future
studies.
Serum miR-21 level and an early
diagnosis of NSCLC
It is worth mentioning that a recent studies have
revealed that serum miR-21 level also have a potential value in the
early diagnosis of NSCLC. Yang et al (43) examined the abnormal expression of 4
miRNAs (miR-21, miR-148a, miR-148b and miR-152) in 152 NSCLC
patients and 300 healthy individuals using qPCR, and AUC analysis
showed that the combination of these miRNAs (AUC, 0.98) had a
higher accuracy in the early diagnosis of NSCLC than a single miRNA
(AUC, 0.81, 0.86, 0.90 and 0.82, respectively). In particular, the
sensitivity and specificity of this combination in the diagnosis of
early NSCLC were up to 96 and 91%, respectively, which was
significantly higher than the miR-21 combinations from the sputum
and the BALs used in the aforementioned studies. These data
indicate that, in the future, the detection of an miR-21
combination in serum will be a useful method for the early
diagnosis of NSCLC.
Conclusion
Recent studies have demonstrated that miR-21 is
closely associated with the prognosis, recurrence, metastases and
diagnosis of NSCLC, and may be applied as a potential biomarker in
the disease (Table I). However, there
remain a number of problems that are yet to be elucidated; for
example, whether or not miR-21 is associated with drug resistance
in NSCLC, the regulatory mechanism of miR-21 in NSCLC, and how to
develop miR-21-based clinical and biological therapeutic strategies
for treating NSCLC. Further investigation of these questions will
not only aid in elucidating the mechanism of NSCLC occurrence, but
will also have significant implications for the development of
associated clinical diagnostic methods, and novel therapeutic
targets and strategies.
 | Table I.miR-21 expression in early diagnosis,
prognosis and recurrence, as well as metastases, in non-small cell
lung cancer patients. |
Table I.
miR-21 expression in early diagnosis,
prognosis and recurrence, as well as metastases, in non-small cell
lung cancer patients.
| Type of samples | Early diagnosis | Prognosis | Recurrence | Metastases |
|---|
| Plasma | miR-21/145/155
(38) miR-21/221/223/145/20a
(39) | – | miR-21 (32,33) | – |
| BAL |
miR-21/143/155/210/372 (40) | – | – | – |
| Sputum | miR-21/210/372
(40,41) | – | – | – |
| Serum |
miR-21/148a/148b/152 (42) | miR-21 (29,31) | miR-21 (32–34) | miR-21 (29) |
| Frozen tumor
tissues | – | miR-21 (19,20) | miR-21 (32,33) | – |
| Quick-frozen tumor
tissues | – | miR-21 (23) | miR-21 (32,33) | – |
| FFPETs | – | miR-21 (24) | miR-21 (32,33) | miR-21 (24,35) |
Acknowledgements
The authors would like to thank Professor Wei Xu
(Soochow University, Suzhou, Jiangsu, China) for providing valuable
suggestions on the writing of the original manuscript.
Funding
This study was supported by Program for Program for
High level innovative talents in Guizhou Province
(QKH-RC-2016-4031), New Century Excellent Talents in University,
Ministry of Education of China (NCET-12-0661), National Natural
Science foundation of China (31760258), Program for Excellent Young
Talents of Zunyi Medical University (15ZY-001) and Project of
Guizhou Provincial Department of Science and Technology
(2009C491).
Availability of data and materials
Not applicable.
Authors' contributions
WZ wrote the initial draft and designed the outline
of the manuscript. JJZ, YT and MG designed the outline and revised
the manuscript. YZ, CC, NQ, JZ and JL revised and expanded the
manuscript. LX designed the outline of manuscript and wrote the
manuscript. All authors read and approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Lagos-Quintana M, Rauhut R, Lendeckel W
and Tuschl T: Identification of novel genes coding for small
expressed RNAs. Science. 294:853–858. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Chen Z, Liu H, Jin W, Ding Z, Zheng S and
Yu Y: Tissue microRNA-21 expression predicted recurrence and poor
survival in patients with colorectal cancer-a meta-analysis. Onco
Targets Ther. 9:2615–2624. 2016.PubMed/NCBI
|
|
3
|
Gao Y, Cai Q, Huang Y, Li S, Yang H, Sun
L, Chen K and Wang Y: MicroRNA-21 as a potential diagnostic
biomarker for breast cancer patients: A pooled analysis of
individual studies. Oncotarget. 7:34498–34506. 2016.PubMed/NCBI
|
|
4
|
Wu ZH, Tao ZH, Zhang J, Li T, Ni C, Xie J,
Zhang JF and Hu XC: MiRNA-21 induces epithelial to mesenchymal
transition and gemcitabine resistance via the PTEN/AKT pathway in
breast cancer. Tumour Biol. 37:7245–7254. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Hu GY, Tao F, Wang W and Ji KW: Prognostic
value of microRNA-21 in pancreatic ductal adenocarcinoma: A
meta-analysis. World J Surg Oncol. 14:822016. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Jiang S, Wang R, Yan H, Jin L, Dou X and
Chen D: MicroRNA-21 modulates radiation resistance through
upregulation of hypoxia-inducible factor-1α-promoted glycolysis in
non-small cell lung cancer cells. Mol Med Rep. 13:4101–4107. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Yan J, Liu T, Zhou X, Dang Y, Yin C and
Zhang G: FZD6, targeted by miR-21, represses gastric cancer cell
proliferation and migration via activating non-canonical wnt
pathway. Am J Transl Res. 8:2354–2364. 2016.PubMed/NCBI
|
|
8
|
Tao YJ, Li YJ, Zheng W, Zhao JJ, Guo MM,
Zhou Y, Qin NL, Zheng J and Xu L: Antisense oligonucleotides
against microRNA-21 reduced the proliferation and migration of
human colon carcinoma cells. Cancer Cell Int. 15:772015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Sha M, Ye J, Luan ZY, Guo T, Wang B and
Huang JX: Celastrol induces cell cycle arrest by
MicroRNA-21-mTOR-mediated inhibition p27 protein degradation in
gastric cancer. Cancer Cell Int. 15:1012015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Husakova M: MicroRNAs in the key events of
systemic lupus erythematosus pathogenesis. Biomed Pap Med Fac Univ
Palacky Olomouc Czech Repub. 160:327–342. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Sanders KA, Benton MC, Lea RA, Maltby VE,
Agland S, Griffin N, Scott RJ, Tajouri L and Lechner-Scott J:
Next-generation sequencing reveals broad down-regulation of
microRNAs in secondary progressive multiple sclerosis CD4+ T cells.
Clin Epigenetics. 8:872016. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zhao XY and Shao K: Roles of MicroRNA-21
in the pathogenesis of insulin resistance and diabetic
mellitus-induced non-alcoholic fatty liver disease. Zhongguo Yi Xue
Ke Xue Yuan Xue Bao. 38:144–149. 2016.(In Chinese). PubMed/NCBI
|
|
13
|
Sekar D, Venugopal B, Sekar P and
Ramalingam K: Role of microRNA 21 in diabetes and
associated/related diseases. Gene. 582:14–18. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Guo Q, Zhang H, Zhang L, He Y, Weng S,
Dong Z, Wang J, Zhang P and Nao R: MicroRNA-21 regulates non-small
cell lung cancer cell proliferation by affecting cell apoptosis via
COX-19. Int J Clin Exp Med. 8:8835–8841. 2015.PubMed/NCBI
|
|
15
|
Li X, Zang A, Jia Y, Zhang J, Fan W, Feng
J, Duan M, Zhang L, Huo R, Jiao J and Zhu X: Triptolide reduces
proliferation and enhances apoptosis of human non-small cell lung
cancer cells through PTEN by targeting miR-21. Mol Med Rep.
13:2763–2768. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Yang Y, Meng H, Peng Q, Yang X, Gan R,
Zhao L, Chen Z, Lu J and Meng QH: Downregulation of microRNA-21
expression restrains non-small cell lung cancer cell proliferation
and migration through upregulation of programmed cell death 4.
Cancer Gene Ther. 22:23–29. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Boldrini L, Giordano M, Ali G, Melfi F,
Romano G, Lucchi M and Fontanini G: P2X7 mRNA expression in
non-small cell lung cancer: MicroRNA regulation and prognostic
value. Oncol Lett. 9:449–453. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Yan LX, Liu YH, Xiang JW, Wu QN, Xu LB,
Luo XL, Zhu XL, Liu C, Xu FP, Luo DL, et al: PIK3R1 targeting by
miR-21 suppresses tumor cell migration and invasion by reducing
PI3K/AKT signaling and reversing EMT, and predicts clinical outcome
of breast cancer. Int J Oncol. 48:471–484. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Yanaihara N, Caplen N, Bowman E, Seike M,
Kumamoto K, Yi M, Stephens RM, Okamoto A, Yokota J, Tanaka T, et
al: Unique microRNA molecular profiles in lung cancer diagnosis and
prognosis. Cancer Cell. 9:189–198. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Saito M, Schetter AJ, Mollerup S, Kohno T,
Skaug V, Bowman ED, Mathé EA, Takenoshita S, Yokota J, Haugen A and
Harris CC: The association of microRNA expression with prognosis
and progression in early-stage, non-small cell lung adenocarcinoma:
A retrospective analysis of three cohorts. Clin Cancer Res.
17:1875–1882. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Rami-Porta R, Crowley JJ and Goldstraw P:
The revised TNM staging system for lung cancer. Ann Thorac
Cardiovasc Surg. 15:4–9. 2009.PubMed/NCBI
|
|
22
|
Zhu W and Xu B: MicroRNA-21 identified as
predictor of cancer outcome: A meta-analysis. PLoS One.
9:e1033732014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Ma XL, Liu L, Liu XX, Li Y, Deng L, Xiao
ZL, Liu YT, Shi HS and Wei YQ: Prognostic role of microRNA-21 in
non-small cell lung cancer: A meta-analysis. Asian Pac J Cancer
Prev. 13:2329–2334. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Cinegaglia NC, Andrade SC, Tokar T,
Pinheiro M, Severino FE, Oliveira RA, Hasimoto EN, Cataneo DC,
Cataneo AJ, Defaveri J, et al: Integrative transcriptome analysis
identifies deregulated microRNA-transcription factor networks in
lung adenocarcinoma. Oncotarget. 7:28920–28934. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Tian L, Shan W, Zhang Y, Lv X, Li X and
Wei C: Up-regulation of miR-21 expression predicate advanced
clinicopathological features and poor prognosis in patients with
non-small cell lung cancer. Pathol Oncol Res. 22:161–167. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Vychytilova-Faltejskova P, Radova L,
Sachlova M, Kosarova Z, Slaba K, Fabian P, Grolich T, Prochazka V,
Kala Z, Svoboda M, et al: Serum-based microRNA signatures in early
diagnosis and prognosis prediction of colon cancer. Carcinogenesis.
37:941–950. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Sun L, Jiang R, Li J, Wang B, Ma C, Lv Y
and Mu N: MicoRNA-425-5p is a potential prognostic biomarker for
cervical cancer. Ann Clin Biochem. 54:127–133. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Jiang X, Du L, Duan W, Wang R, Yan K, Wang
L, Li J, Zheng G, Zhang X, Yang Y and Wang C: Serum microRNA
expression signatures as novel noninvasive biomarkers for
prediction and prognosis of muscle-invasive bladder cancer.
Oncotarget. 7:36733–36742. 2016.PubMed/NCBI
|
|
29
|
Zhu HT, Hasan AM, Liu RB, Zhang ZC, Zhang
X, Wang J, Wang HY, Wang F and Shao JY: Serum microRNA profiles as
prognostic biomarkers for HBV-positive hepatocellular carcinoma.
Oncotarget. 7:45637–45648. 2016.PubMed/NCBI
|
|
30
|
Wang ZX, Bian HB, Wang JR, Cheng ZX, Wang
KM and De W: Prognostic significance of serum miRNA-21 expression
in human non-small cell lung cancer. J Surg Oncol. 104:847–851.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Liu XG, Zhu WY, Huang YY, Ma LN, Zhou SQ,
Wang YK, Zeng F, Zhou JH and Zhang YK: High expression of serum
miR-21 and tumor miR-200c associated with poor prognosis in
patients with lung cancer. Med Oncol. 29:618–626. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Zhao W, Zhao JJ, Zhang L, Xu QF, Zhao YM,
Shi XY and Xu AG: Serum miR-21 level: A potential diagnostic and
prognostic biomarker for non-small cell lung cancer. Int J Clin Exp
Med. 8:14759–14763. 2015.PubMed/NCBI
|
|
33
|
Yang M, Shen H, Qiu C, Ni Y, Wang L, Dong
W, Liao Y and Du J: High expression of miR-21 and miR-155 predicts
recurrence and unfavourable survival in non-small cell lung cancer.
Eur J Cancer. 49:604–615. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Wang Y, Li J, Tong L, Zhang J, Zhai A, Xu
K, Wei L and Chu M: The prognostic value of miR-21 and miR-155 in
non-small-cell lung cancer: A meta-analysis. Jpn J Clin Oncol.
43:813–820. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Munagala R, Aqil F and Gupta RC: Exosomal
miRNAs as biomarkers of recurrent lung cancer. Tumour Biol.
37:10703–10714. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Stenvold H, Donnem T, Andersen S, Al-Saad
S, Valkov A, Pedersen MI, Busund LT and Bremnes RM: High tumor cell
expression of microRNA-21 in node positive non-small cell lung
cancer predicts a favorable clinical outcome. BMC Clin Pathol.
14:92014. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Wu R, Jiang Y, Wu Q, Li Q, Cheng D, Xu L,
Zhang C, Zhang M and Ye L: Diagnostic value of microRNA-21 in the
diagnosis of lung cancer: Evidence from a meta-analysis involving
11 studies. Tumour Biol. 35:8829–8836. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Meng X, Xiao C, Zhao Y, Jia L, Tang Y and
Li D: Meta-analysis of microarrays: Diagnostic value of microRNA-21
as a biomarker for lung cancer. Int J Biol Markers. 30:e282–e285.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Tang D, Shen Y, Wang M, Yang R, Wang Z,
Sui A, Jiao W and Wang Y: Identification of plasma microRNAs as
novel noninvasive biomarkers for early detection of lung cancer.
Eur J Cancer Prev. 22:540–548. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Geng Q, Fan T, Zhang B, Wang W, Xu Y and
Hu H: Five microRNAs in plasma as novel biomarkers for screening of
early-stage non-small cell lung cancer. Respir Res. 15:1492014.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Kim JO, Gazala S, Razzak R, Guo L, Ghosh
S, Roa WH and Bedard ELR: Non-small cell lung cancer detection
using microRNA expression profiling of bronchoalveolar lavage fluid
and sputum. Anticancer Res. 35:1873–1880. 2015.PubMed/NCBI
|
|
42
|
Razzak R, Bédard EL, Kim JO, Gazala S, Guo
L, Ghosh S, Joy A, Nijjar T, Wong E and Roa WH: MicroRNA expression
profiling of sputum for the detection of early and locally advanced
non-small-cell lung cancer: A prospective case-control study. Curr
Oncol. 23:e86–e94. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Yang JS, Li BJ, Lu HW, Chen Y, Lu C, Zhu
RX, Liu SH, Yi QT, Li J and Song CH: Serum miR-152, miR-148a,
miR-148b, and miR-21 as novel biomarkers in non-small cell lung
cancer screening. Tumour Biol. 36:3035–3042. 2015. View Article : Google Scholar : PubMed/NCBI
|