Introduction
Liver receptor homologue 1 (LRH-1), also known as
nuclear receptor subfamily 5 group A member 2 (NR5A2), is a member
of the NR5A subfamily of NRs that was originally found in the liver
of mice, and then later in rats, chickens, horses, zebrafish, frogs
and the human body (1). Since then,
it has been shown to be involved in the development of a variety of
malignant tumors, including breast, liver, gastric, colon and
pancreatic cancer (2–4). LRH-1 is involved in regulating numerous
functions, with studies showing that it participates in the
metabolism, differentiation and development of organisms (4–6). LRH-1
also regulates a variety of biological processes, including bile
acid metabolism (7), reverse
cholesterol transport (8) and glucose
balance in the human body (9).
Recently, LRH-1 was found to serve a decisive role in controlling
the development of neural stem cells (10). Therefore, LRH-1 has been identified as
a specific tumor marker for the evaluation of disease in patients
with cancer.
The association between the expression and prognosis
of LRH-1 in malignant tumors has been investigated by certain
studies. Although, it has been shown that LRH-1 is highly expressed
in colon cancer (11), there is no
research showing the association between its positive expression
and prognosis. The following study was undertaken in order to
investigate the expression of LRH-1 in colon cancer and its
association with the prognosis of affected patients.
Materials and methods
Patients and tissue samples
A total of 128 colon cancer tissue samples of
different stages obtained between April 2011 and December 2011 were
randomly collected from the Department of Gastrointestinal Surgery
in The First People's Hospital of Changzhou, The Third Affiliated
Hospital of Soochow University (Changzhou, Jiangsu, China). The
selected tissue samples were routinely preserved in 10% buffered
neutral formalin at 24°C for 12 h. The normal tissues were removed
from at least 5 cm away from the edge of these tumors. All cases of
colon cancer were clinically and pathologically proven, and all of
the patients recruited in this study had not received neoadjuvant
chemotherapy or preoperative radiotherapy. All patients provided
written informed consent prior to the collection and the research
program used in the study was approved by the Ethical Committee of
The Third Affiliated Hospital of Soochow University. Tumor, Node,
Metastasis (TNM) stages and clinicopathological classification were
defined based on the Union for International Cancer Control
classification (12). Patients
received telephone follow-up or outpatient review until December
2016 or mortality.
Immunohistochemical analysis
Immunohistochemical staining was performed using the
Elivsion two-step method (13). The
following primary antibody was used: LRH-1 (1:100 dilution; catalog
no. NBP1-90094; Novus Biologicals, LLC, Littleton, CO, USA). The
secondary antibody (1:100 dilution; catalog no. kit-0028) and DAB
solution were provided by Maxim Biomedical, Inc. (Fuzhou, China).
All samples were fixed in 10% formalin at 24°C for 12 h solution
and embedded in paraffin. Sections (3–4 mm) were dewaxed in xylene,
dehydrated in ethanol (75, 95 and 100%), and incubated in 3%
H2O2 for 15 min to destroy the activity of
endogenous peroxidase. Following incubation in 10% bovine serum
(dissolved in PBS at 24°C; Novus Biologicals, LLC, Littleton, CO,
USA) for 10 min, each slide was incubated with the primary antibody
at 4°C overnight. The aforementioned biotin-labeled mouse-rabbit
immunoglobulin was selected as the secondary antibody. Staining
using the DAB Detection kit (Polymer) (cat. no. kit-0014; Maxim
Biomedical, Inc.) was performed according to the manufacturer's
protocol, and sections positive for colon cancer were selected as
positive controls, with non-immune animal serum IgG replacing the
antibody as a negative control.
All tissue specimens were evaluated separately by
two pathologists who were unaware of the clinicopathological status
of the patients. Five high magnification fields of view were
selected randomly from each section. The main expression of LRH-1
was found in the cytoplasm and shown as a tan or brown color. The
scoring of the positive cell count fraction was determined as
follows: Score 0, ≤5; score 1, 6–25; score 2, 26–50; score 3,
51–75; and score 4, >75%. The scoring of the staining intensity
and the dyeing depth was determined as follows: Score 0, no
staining (colorless); score 1, weak staining (yellow); score 2,
moderate staining (pale brown); and score 3, strong staining
(sepia). The total score was calculated by multiplying the staining
intensity fraction with the positive cell count fraction. A total
score of <5 was considered as negative expression of LRH-1, and
a score of ≥5 was considered as positive expression of LRH-1.
Statistical analysis
All statistical analyses were accomplished with the
SPSS 17.0 statistical software (SPSS, Inc. Chicago, IL, USA). The
association between LRH-1 expression and the clinicopathological
characteristics was tested using the non-parametric χ2
test. The overall survival (OS) curves were plotted using the
Kaplan-Meier method, and the positive and negative expression of
LRH-1 samples was compared using the log-rank test. P<0.05 was
considered to indicate a statistically significant difference.
Results
Expression of LRH-1 in colon cancer
tissues and adjacent normal tissues
The clinicopathological characteristics of the 128
patients who were recruited in the present study are shown in
Table I. Among the patients, 42 were
female and 86 were male, with a mean age of 58.38±8.3 years (range,
38–82 years). A total of 63 cases were tubular adenocarcinoma, 25
were mucinous adenocarcinoma, 28 were papillary adenocarcinoma and
12 were squamous cell carcinoma; 65 cases were located in the
rectum and sigmoid colon, 37 in the right colon and 26 in the left
colon. In terms of differentiation degree, 55 cases highly
differentiated, 33 were moderately differentiated and 40 were
poorly differentiated. The number of cases at stages I, II, III and
IV was 40, 21, 60 and 7, respectively. The expression of LRH-1 in
the 128 pairs of resected specimens (including tumor tissue samples
and matched adjacent normal tissue samples) from patients who were
diagnosed with colon cancer was determined by immunohistochemistry.
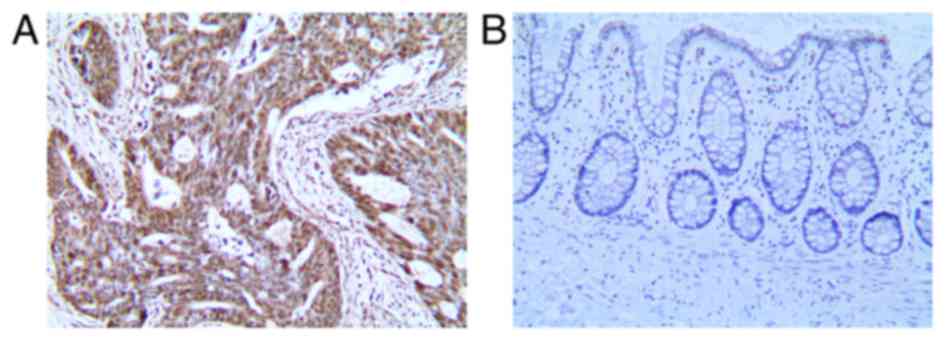
It was found that 108/128 colon cancer tissues exhibited
significantly LRH-1 expression higher compared with 17/128 adjacent
non-cancerous tissue (Table II). The
expression of LRH-1 in the colon cancer tissues was significantly
higher than that in the adjacent tissues (Fig. 1).
 | Table I.Demographic and pathological
parameters of colon cancer patients. |
Table I.
Demographic and pathological
parameters of colon cancer patients.
| Parameter | Value, n (%) |
|---|
| Sex |
|
| Male | 86 (67.2) |
|
Female | 42 (32.8) |
| Age, years |
|
| ≥60 | 83 (64.8) |
|
<60 | 45 (35.2) |
| Pathological
type |
|
| Tubular
adenocarcinoma | 63 (49.2) |
| Mucinous
adenocarcinoma | 25 (19.5) |
| Papillary
adenocarcinoma | 28 (21.9) |
| Squamous
cell carcinoma | 12 (9.4) |
| Tumor location |
|
| Rectum
and sigmoid colon | 65 (50.8) |
| Right
colon | 37 (28.9) |
| Left
colon | 26 (20.3) |
| Distant
metastasis |
|
|
Absent | 121 (94.5) |
|
Present | 7 (5.5) |
| pT stage |
|
| T1 | 35 (27.3) |
| T2 | 20 (15.6) |
| T3 | 66 (51.6) |
| T4 | 7 (5.5) |
| pN stage |
|
| N0 | 61 (47.7) |
|
N1+N2 | 67 (52.3) |
| TNM stage |
|
| I | 40 (31.2) |
| II | 21 (16.4) |
| III | 60 (46.9) |
| IV | 7 (5.5) |
| Differentiation |
|
| High | 55 (43.0) |
|
Moderate | 33 (25.8) |
| Poor | 40 (31.2) |
 | Table II.Expression of LRH-1 in colon cancer
tissue and adjacent non-cancerous tissue (ANCTs). |
Table II.
Expression of LRH-1 in colon cancer
tissue and adjacent non-cancerous tissue (ANCTs).
|
| LRH-1 expression |
|
|
|---|
|
|
|
|
|
|---|
| Tissues | Low | High | χ2 | P-value |
|---|
| Colon cancer tissues,
n | 20 | 108 | 129.462 | <0.001 |
| ANCTs, n | 111 | 17 |
|
|
Association of LRH-1 with different
clinicopathological characteristics
The association between the expression level of LRH1
and the common clinicopathological parameters of colon cancer,
including sex, age, TNM stage, pathological type, tumor location,
degree of differentiation, depth of tumor invasion (pT), lymph node
metastasis (pN) and distant metastasis, were analyzed (Table III). The level of LRH-1 expression
was not found to be associated with the sex, age, tumor location,
distant metastasis, degree of differentiation or pathological type
of the colon cancer patients, but was significantly associated with
the clinicopathological stage, depth of tumor invasion and lymph
node metastasis.
 | Table III.Association between LRH-1 expression
and clinicopathological features of colon cancer patients. |
Table III.
Association between LRH-1 expression
and clinicopathological features of colon cancer patients.
|
| LRH-1 expression,
n |
|
|
|---|
|
|
|
|
|
|---|
| Clinicopathological
feature | Low | High | χ2 | P-value |
|---|
| Sex |
|
|
|
|
| Male | 12 | 74 | 0.555 | 0.605 |
|
Female | 8 | 34 |
|
|
| Age, years |
|
|
|
|
| ≥60 | 16 | 67 | 2.389 | 0.136 |
|
<60 | 4 | 41 |
|
|
| Pathological
type |
|
|
|
|
| Tubular
adenocarcinoma | 9 | 54 | 0.204 | 0.982 |
|
Mucinous adenocarcinoma | 4 | 21 |
|
|
|
Papillary adenocarcinoma | 5 | 23 |
|
|
|
Squamous cell carcinoma | 2 | 10 |
|
|
| Tumor location |
|
|
|
|
| Rectum
and sigmoid colon | 14 | 51 | 4.585 | 0.102 |
| Right
colon | 5 | 32 |
|
|
| Left
colon | 1 | 25 |
|
|
| Distant
metastasis |
|
|
|
|
|
Absent | 20 | 101 | 1.371 | 0.371 |
|
Present | 0 | 7 |
|
|
| pT |
|
|
|
|
| T1 | 8 | 27 | 4.878a | 0.030a |
| T2 | 4 | 16 |
|
|
| T3 | 8 | 58 |
|
|
| T4 | 0 | 7 |
|
|
| pN |
|
|
|
|
| N0 | 17 | 44 | 13.252 | <0.001 |
|
N1+N2 | 3 | 64 |
|
|
| TNM stage |
|
|
|
|
| I | 12 | 28 | 13.252b |
<0.001b |
| II | 5 | 16 |
|
|
|
III | 3 | 57 |
|
|
| IV | 0 | 7 |
|
|
|
Differentiation |
|
|
|
|
|
High | 10 | 45 | 5.677 | 0.055 |
|
Moderate | 1 | 32 |
|
|
|
Poor | 9 | 31 |
|
|
Association between LRH-1 expression
levels and the prognosis of patients
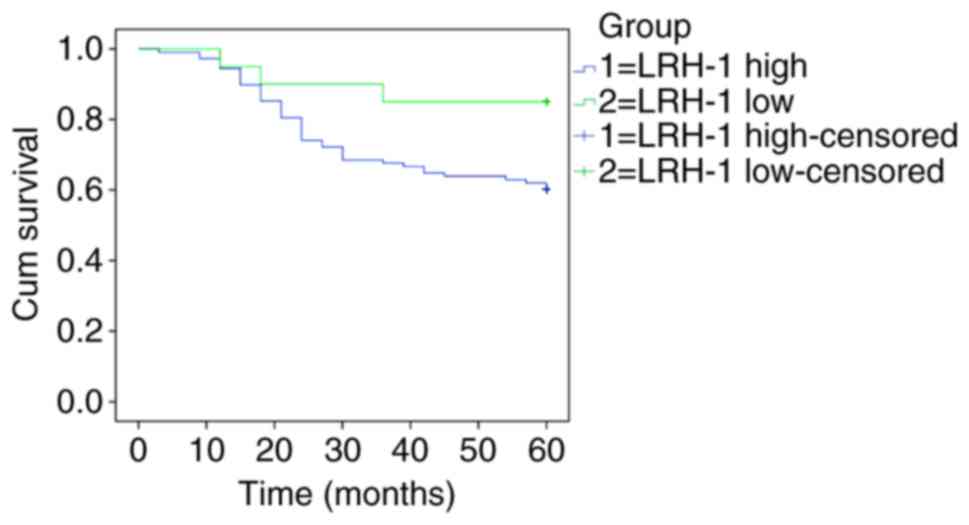
The 5-year OS rate of patients with negative and
positive LRH-1 expression was investigated by Kaplan-Meier survival
curves and the log-rank test, as shown in Fig. 2. A total score of <5 was considered
as negative expression of LRH-1, and a score of ≥5 was considered
as positive expression of LRH-1Among the 128 patients who were
followed up for 5 years, 2 were lost to follow-up. Of the 128
patients, 82 survived this period, and the 5-year OS rate was
64.1%. Moreover, the 5-year OS rates for patients with negative and
positive LRH-1 expression were 85.0 and 60.2%. The median estimated
cumulative survival time was significantly lower in the LRH-1
positive expression group [46 months; 95% confidence interval (CI),
42.729–49.938 months] compared with that in the LRH-1 negative
expression group (54 months; 95% CI, 48.106–60.494 months). These
results suggest that patients with high levels of LRH-1 expression
have a worse prognosis compared with patients with low levels of
LRH-1 expression.
Discussion
The development of tumors and a variety of malignant
behaviors are the outcome of the combined action of multiple genes,
and in general, it is a complex multi-step and multi-factor process
(14). The overexpression and
activation of proto-oncogenes, the inhibition or downregulation of
tumor suppressor genes and the downregulation of mutations serve an
important role in the development of tumorigenesis. Excessive
proliferation and blocked apoptosis are basic characteristics of
tumor cells, which are associated with the regulation of
proliferation, apoptosis and the cell cycle of important signal
pathway disorders, and even loss of function (15,16).
Therefore, it is important to investigate the abnormal expression
of genes associated with malignancy for determining tumor
diagnosis, treatment and prognosis. In view of the abnormal
expression of LRH-1 in a variety of malignant tumors (11), including gastric, breast and
pancreatic cancer, the present study investigated the expression of
LRH-1 in colon cancer and its association with the prognosis of
patients.
LRH-1 is a member of the NR5A subfamily of NRs; it
is expressed in numerous organs, including the liver, pancreas,
colon and ovaries (6). LRH-1 serves
an important role in the body, controlling the development and
differentiation of animal embryos, the production of the steroid
hormone and the metabolism of bile acid (17). LRH-1 exhibits increased expression in
pancreatic cancer cells and can promote its proliferation by
activating cyclin D1, cyclin E1 and c-Myc (18,19).
Furthermore, LRH-1 is highly expressed in breast cancer, promoting
tumor cell growth via an increased synthesis of local estrogen
(20). LRH-1 is involved in the
renewal of intestinal cells in the gastrointestinal tract, and is
expressed in gastric cancer and colon cancer cells. Zhang et
al (21) found that LRH-1 gene
polymorphisms (rs3790843 and rs3790844) increased the risk of local
lymph node and distant metastasis. Kramer et al (22) confirmed the high expression of LRH-1
in colorectal cancer tissues and cells, and indicated that LRH-1
can promote the growth of colorectal cancer cells by inhibiting the
expression of the cyclin-dependent kinase inhibitor 1A gene in the
HCT116 and HT29 cell lines. Bayrer et al (23) showed that the inhibition of LRH-1
expression can weaken the ability of metastases of colon cancer
cells, and can change the expression of associated genes. The
present study found that colon cancer tissue exhibited positive
LRH-1 expression, and the expression rate was 64.7%, higher than
that of the adjacent tissue at 32.2%, which is consistent with the
aforementioned results in pancreatic cancer and breast cancer. This
indicates that the expression level of LRH-1 in colon cancer and
other malignant tumors is abnormally elevated. Further analysis
revealed that LRH-1 expression was associated with
clinicopathological stage, depth of tumor invasion and lymph node
metastasis; for example, the positive expression rate of LRH-1 in
stage III and IV was 95.5%, which was higher than that in stage I
and II, suggesting that if the Tumor-Node-Metastasis (TNM) stage of
colon cancer was higher then the positive expression rate would be
higher. This study also found that the expression rate of LRH-1 in
colon cancer tissues with T3+T4 depth was also higher than that in
patients with lymph node metastasis and distant metastasis, and the
expression of LRH-1 in patients with lymph node metastasis and
distant metastasis was significantly higher than that in patients
without lymph node metastasis. However, there is no cytological
evidence that LRH-1 expression is directly involved in the
malignant transformation of colon cancer and its transformation
mechanism. Our next study intends to verify the effect of LRH-1 on
colon cancer cells. In the present study, the 5-year survival rate
was determined and it was found that LRH-1 positive expression was
associated with poor prognosis. The median OS time of patients with
positive expression was 46 months, which was lower than that of
patients with negative expression at 54 months. The poor prognosis
may be related to positive expression of LRH-1, associated with TNM
stage, depth of invasion and lymph node metastasis.
In summary, LRH-1 is mainly expressed in colon
cancer tissues, and is associated with the depth of tumor invasion,
lymph node metastasis and clinicopathological stage. The prognosis
of patients with positive LRH-1 expression is significantly worse
than that of patients with negative expression. These results
suggest that LRH-1 may serve an important role in the development
and progression of colon cancer. The detection of LRH-1 expression
can be used to assist in the diagnosis and evaluation of colon
cancer.
Acknowledgements
The authors would like to thank Dr Hui Wang (The
First People's Hospital of Changzhou, The Third Affiliated Hospital
of Soochow University, Changzhou, China) for providing help with
immunohistochemistry.
Funding
The present study was supported by the Applied Basic
Research Project of Changzhou Science and Technology Bureau (grant
no. CJ20140047) and Changzhou High-Level Medical Talents Training
Project (grant no. 2016CZBJ046).
Availability of data and materials
The datasets used or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
CW made substantial contribution to the conception
and design of the present study, as well as the writing of the
manuscript. JF made substantial contributions to the acquisition,
analysis and interpretation of data for the present study and
revised this article. ZL made substantial contributions to
conception and design, analysis and interpretation of the data. ZL
was involved in drafting the manuscript and revising it critically
for important intellectual content.
Ethics approval and consent to
participate
The research program used in the study was approved
by the Ethical Committee of The Third Affiliated Hospital of
Soochow University. Written informed consent was obtained from all
study participants.
Consent for publication
Written informed consent was obtained from all study
participants.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Boerboom D, Pilon N, Behdjani R,
Silversides DW and Sirois J: Expression and regulation of
transcripts encoding two members of the NR5A nuclear receptor
subfamily of orphan nuclear receptors, steroidogenic factor-1 and
NR5A2, in equine ovarian cells during the ovulatory process.
Endocrinology. 141:4647–4656. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Ikeda Y, Lala DS, Luo X, Kim E, Moisan MP
and Parker KL: Characterization of the mouse FTZ-F1 gene, which
encodes a key regulator of steroid hydroxylase gene expression. Mol
Endocrinol. 7:852–860. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Stein S and Schoonjans K: Molecular basis
for the regulation of the nuclear receptor LRH-1. Curr Opin Cell
Biol. 33:26–34. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Lazarus KA, Wijayakumara D, Chand AL,
Simpson ER and Clyne CD: Therapeutic potential of liver receptor
homolog-1 modulators. J Steroid Biochem Mol Biol. 130:138–146.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Fayard E, Auwerx J and Schoonjans K:
LRH-1: An orphan nuclear receptor involved in development,
metabolism and steroidogenesis. Trends Cell Biol. 14:250–260. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Lee YK and Moore DD: Liver receptor
homolog-1, an emerging metabolic modulator. Front Biosci.
13:5950–5958. 2008. View
Article : Google Scholar : PubMed/NCBI
|
|
7
|
Out C, Hageman J, Bloks VW, Gerrits H,
Gelpke Sollewijn MD, Bos T, Havinga R, Smit MJ, Kuipers F and Groen
AK: Liver receptor homolog-1 is critical for adequate up-regulation
of Cyp7a1 gene transcription and bile salt synthesis during bile
salt sequestration. Hepatology. 53:2075–2085. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Venteclef N, Haroniti A, Tousaint JJ,
Talianidis I and Delerive P: Regulation of anti-atherogenic
apolipoprotein M gene expression by the orphan nuclear receptor
LRH-1. J Biol Chem. 283:3694–3701. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Oosterveer MH, Mataki C, Yamamoto H,
Harach T, Moullan N, van Dijk TH, Ayuso E, Bosch F, Postic C, Groen
AK, et al: LRH-1-dependent glucose sensing determines intermediary
metabolism in liver. J Clin Invest. 122:2817–2826. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Stergiopoulos A and Politis PK: Nuclear
receptor NR5A2 controls neural stem cell fate decisions during
development. Nat Commun. 7:122302016. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Nadolny C and Dong X: Liver receptor
homolog-1 (LRH-1): A potential therapeutic target for cancer.
Cancer Biol Ther. 16:996–1004. 2015. View Article : Google Scholar
|
|
12
|
Edge SB, Byrd DR, Compton CC, Fritz AG,
Greene FL and Trotti A: AJCC Cancer Staging Manual. 7th edition.
Springer; New York: 2010
|
|
13
|
Kang X, Zhang L, Wu R, Sheng X, Sun J and
Chen X: The expression of prohibitin in gastrointestinal cancer
tissue and its clinical signification. Laborat Med. 21:610–612.
2006.
|
|
14
|
Li YX, Zhang J, Qian Y, Meng CH, Wang HL,
Tao XJ, Zhong S, Cao SX and Li QF: Molecular characterization,
expression, polymorphism of NR5A2 and its relationship with litter
size in Hu sheep. Genet Mol Res. 14:12765–12775. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Tan XH, Cheng R, Hu HP and Bai YP:
Classification of colon cancer based on the expression of randomly
selected genes. Genet Mol Res. 14:12628–12635. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Ding M, Li X and Qiu T: Combination of
multiple gene markers to detect circulating tumor cells in the
peripheral blood of patients with non-small cell lung cancer using
real-time PCR. Genet Mol Res. 14:13033–13040. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Fernandez-Marcos PJ, Auwerx J and
Schoonjans K: Emerging actions of the nuclear receptor LRH-1 in the
gut. Biochim Biophys Acta. 1812:947–955. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Gao W, Tang Z, Zhang YF, Feng M, Qian M,
Dimitrov DS and Ho M: Immunotoxin targeting glypican-3 regresses
liver cancer via dual inhibition of Wnt signalling and protein
synthesis. Nat Commun. 6:65362015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Benod C, Vinogradova MV, Jouravel N, Kim
GE, Fletterick RJ and Sablin EP: Nuclear receptor liver receptor
homologue 1 (LRH-1) regulates pancreatic cancer cell growth and
proliferation. Proc Natl Acad Sci USA. 108:16927–16931. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Falender AE1, Lanz R, Malenfant D,
Belanger L and Richards JS: Differential expression of
steroidogenic factor-1 and FTF/LRH-1 in the rodent ovary.
Endocrinology. 144:3598–3610. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zhang X, Gu D, Du M, Wang M, Cao C, Shen
L, Kuang M, Tan Y, Huo X, Gong W, et al: Associations of NR5A2 gene
polymorphisms with the clinicopathological characteristics and
survival of gastric cancer. Int J Mol Sci. 15:22902–22917. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Kramer HB, Lai CF, Patel H, Periyasamy M,
Lin ML, Feller SM, Fuller-Pace FV, Meek DW, Ali S and Buluwela L:
LRH-1 drives colon cancer cell growth by repressing the expression
of the CDKN1A gene in a p53-dependent manner. Nucleic Acids Res.
44:582–594. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Bayrer JR, Mukkamala S, Sablin EP, Webb P
and Fletterick RJ: Silencing LRH-1 in colon cancer cell lines
impairs proliferation and alters gene expression programs. Proc
Natl Acad Sci USA. 112:2467–2472. 2015. View Article : Google Scholar : PubMed/NCBI
|