Introduction
Thyroid cancer is the most widespread endocrine
malignancy. Papillary thyroid cancer (PTC) is the most common and
accounts for approximately 80–90% of human thyroid cancers
(1–4).
Study of the treatment and pathogenesis of thyroid cancer aids in
increasing the rate of early diagnosis of thyroid cancer and the
development of novel therapies, improving the quality of life and
prognosis, and reducing the mortality of patients with thyroid
cancer.
The dysfunction of microRNA (miRNA/miR) has an
important association with human PTC (5–9).
Transfection of miR-21 into TPC-1 human PTC cells resulted in an
increased proliferation and a decreased apoptosis (8). The plasma exosome miR-21 expression
pattern in patients with PTC and follicular thyroid cancer (FTC) is
distinct from benign tumors (9).
miR-21 acts by targeting the tumor suppressor gene phosphatase and
tensin homolog (PTEN), which suppresses the tumor by
dephosphorylating RAC-α serine/threonine-protein kinase (Akt)
(10–13).
The Chinese traditional medicine matrine exhibits
extensive anti-tumor activities through multiple mechanisms
including pro-apoptotic action, cell cycle arrest, growth
inhibition, alteration of miRNA expression, upregulation of PTEN
and suppression of Akt (14–22). Li et al (23), reported that the natural medicine
matrine may affect miRNA-21 to inhibit MCF-7 breast cancer growth.
This result expands the understanding of the antitumor activities
of natural medicines.
Considering the extensive anti-tumor activity of
matrine and the miR-21 expression pattern in human PTC, the
miR-21-regulated antitumor effect of matrine may also exist in PTC.
Therefore, the present study was designed to determine the effect
of matrine on miR-21 and its target PTEN/Akt pathway in TPC-1 human
PTC cells. Proliferation, cell cycle and apoptosis levels were
tested. The present study was designed to identify alternative
therapeutic methods for treatment of thyroid cancer and provide a
foundation for further research on the treatment of thyroid cancer
using matrine.
Materials and methods
Cell line and drug treatment
Matrine (C15H24N2O;
MW 248.36; CAS:519-02-8; purity >98%) was purchased from Meryer
Chemical Company (Shanghai, China). TPC-1 human thyroid cancer
cells were donated by CIAC of CAS (Changchun, Jilin, China). Cells
were cultured in Dulbecco's modified Eagle's medium (Gibco; Thermo
Fisher Scientific, Inc., Waltham, MA, USA) supplemented with 10%
fetal bovine serum (Sijiqing Inc., Hangzhou, Zhejiang, China) at
37°C in a 5% CO2 incubator.
MTT assay
TPC-1 cells were seeded in 96-well plates at a
density of 105/ml (0.2 ml in each well) for 12 h. Cells
were exposed to matrine at a final concentration of 0 (M0,
control), 1, 2, 5, 10, 20 mg/ml for 24, 48 and 72 h. Methyl
thiazolyl tetrazolium (MTT; Sigma-Aldrich; Merck KGaA, Darmstadt,
Germany) at a final concentration of 0.5 mg/ml was incubated in
each well for another 4 h. The formazan crystals were dissolved in
150 µl dimethyl sulfoxide and the absorbance was measured at a
wavelength of 490 nm by a Multiskan FC microplate reader (Thermo
Fisher Scientific, Inc.). All experiments were repeated three
times. The percentage growth inhibition vs. the M0 control cells
was calculated.
Flow cytometry
For apoptosis analysis, TPC-1 cells in the log phase
were collected following treatment with matrine at 0 (control), 2,
5 and 10 mg/ml for 48 h, fixed with 70% ethanol and washed twice
with cold PBS. The cells were resuspended in 500 µl binding buffer
at a concentration of 106/ml, mixed with 10 µl Annexin V
(Bioworld Technology Inc., Jiangsu, China) for 15 min in the dark
at room temperature and incubated with 5 µl propidium iodide (PI;
Bioworld Technology Inc.) for 10 min in the dark. The cells were
analyzed using a FACSCalibur flow cytometer with the CellQuest 3.0
sampling software (BD Biosciences, Franklin Lakes, NJ, USA). The
apoptosis rate was determined using the FlowJo software (BD
Biosciences).
For cell cycle analysis, TPC-1 cells were cultured
in the serum-free medium for 12 h for starvation. TPC-1 cells were
treated with matrine at 0 (M0, control), 2, 5 and 10 mg/ml for 48
h. Then, 5×105 cells in each group were fixed with 70%
ethanol, washed with cold PBS and incubated with RNase and PI
(Bioworld Technology Inc.) for 30 min in the dark. The cells were
analyzed by a FACSCalibur flow cytometer using CellQuest 3.0
software (BD Biosciences). The proportion of cells in each phase
was determined using the FlowJo software (BD Biosciences). Each
experiment was performed three times.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
For detection of miR-21 levels, the experiments were
performed using the Ambion mirVanaTM qRT-PCR miRNA Detection kit
(Invitrogen; Thermo Fisher Scientific, Inc.) according to the
manufacturer's protocol. miR-21 primer sequences for qPCR were
5′-GGGGTAGCTTATCAGACTGATG-3′ (forward) and
5′-TGTCGTGGAGCGGCAATTG-3′ (reverse) (23). To detect PTEN and Akt mRNA levels,
total RNA was extracted from TPC-1 cells by TRIzol®
Reagent (Invitrogen; Thermo Fisher Scientific, Inc.). The
first-strand cDNA was synthesized using FastQuant RT kit (with
gDNAase; Invitrogen; Thermo Fisher Scientific, Inc.). Subsequently,
double-stranded cDNA was synthesized. Primers for the qPCR were
5′-GCAATATGTTCATAACGATGGCTGTGG-3′ (PTEN forward) and
5′-GAACTGGCAGGTAGAAGGCAACTC-3′ (PTEN reverse);
5′-GCAGGATGTGGACCAACGTGAG-3′ (Akt forward) and
5′-GCAGGCAGCGGATGATGAAGG-3′ (Akt reverse). All primers were
synthesized by Sangon Biotech Co., Ltd (Shanghai, China). RT-qPCR
was performed on a Roche LightCycler® 96 fluorescence
quantification PCR machine (Roche Diagnostics, Basel, Switzerland).
The reaction system included 1.5 µl cDNA, 7.5 µl SYBR Green, 0.3 µl
of primers and PCR-grade water to the total volume of 15 µl. The
PCR thermocycling conditions included a pre-denaturation at 95°C
for 30 sec; 40 cycles of 95°C for 15 sec and 60°C for 30 sec for
amplification; and a default condition for dissociation (23). The cycle threshold (Ct) values were
obtained. Quantitative analyzes of miR-21 or mRNA expression levels
were performed following normalization to U6 RNA or GAPDH mRNA
(Sangon Biotech Co., Ltd., Shanghai, China). Relative
experimental/reference miRNA-21 and mRNA expression was calculated
using the following formula: 2−ΔCt(interest-reference).
The fold change was calculated using 2−∆∆Ct method.
Transfection of miR-21 mimic and
western blotting
TPC-1 cells were transfected with miR-21 mimics
using X-treme reagent following the manufacturer's protocol (Roche
Diagnostics, Basel, Switzerland). At 48 h after transfection and/or
48 h after the treatment with 0 (M0, control), 2 and 5 mg/ml
matrine, the expression of PTEN and p-Akt protein was measured by
western blotting. Briefly, TPC-1 cells were washed with cold PBS
and lysed in lysis radioimmunoprecipitation assay buffer (Pierce;
Thermo Fisher Scientific, Inc.). The concentration of proteins was
determined using a bicinchoninic acid Protein Assay kit (Beijing
Solarbio Science and Technology Co., Ltd., Beijing, China). The
proteins were separated on 10% SDS-PAGE gels. Gels were
transblotted to polyvinylidene difluoride membranes (Beyotime
Institute of Biotechnology, Jiangsu, China). The membranes were
incubated with the mouse anti-PTEN and anti-pAkt monoclonal
antibodies (1:1,000 diluted; Cell Signaling Technology, Inc.,
Danvers, MA, USA) at 4°C overnight, and then with the goat
horseradish peroxidase-labeled second antibody (1:1,000 diluted;
TransGen Biotech, Beijing, China) at 25°C for 2 h. Chromophore DAB
reagent (Beyotime Institute of Biotechnology) was used to develop
color. The grayscale ratio of PTEN or Akt to the internal control
GAPDH was calculated using the software Image Pro 6.0 (Media
Cybernetics, Rockville, MD, USA). The grayscale of test protein
bands was normalized to the GAPDH bands.
Statistical analysis
Data are presented as the mean ± standard deviation.
SPSS software, v.16.0 (SPSS Inc., Chicago, IL, USA) was used to
perform statistical analysis. Student's t-test was used for
comparisons between two groups and one-way analysis of variance
followed by Dunn's test was used for multiple comparisons.
P<0.05 was considered to indicate a statistically significant
difference.
Results
Cell viability
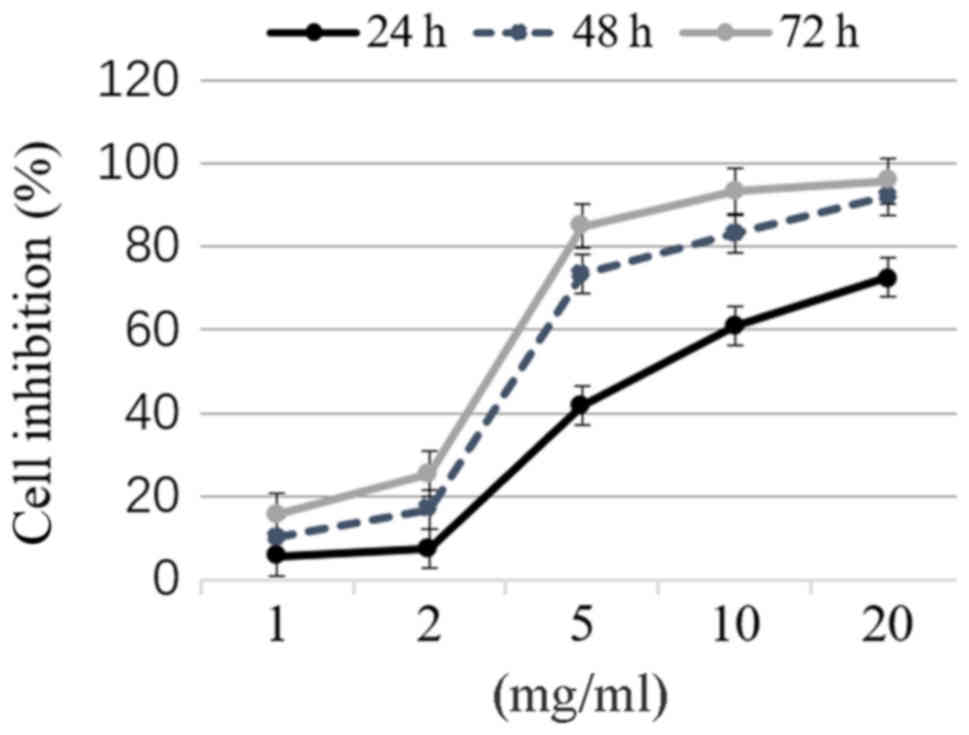
MTT assay was performed to determine the growth of
thyroid cancer TPC-1 cells following treatment with matrine at 0,
1, 2, 5, 10 and 20 mg/ml for 24, 48 and 72 h, respectively. Matrine
inhibited the growth of TPC-1 cells in a concentration- and
time-dependent manner (Fig. 1). The
half maximal inhibitory concentration (IC50) represents
the concentration of a drug that is required for 50% inhibition
in vitro and is a measure of the effectiveness of a drug in
inhibiting cell growth (24). In the
present study, the IC50 of treatment with matrine for 48
h was 3.54 mg/ml.
Apoptosis and cell cycle
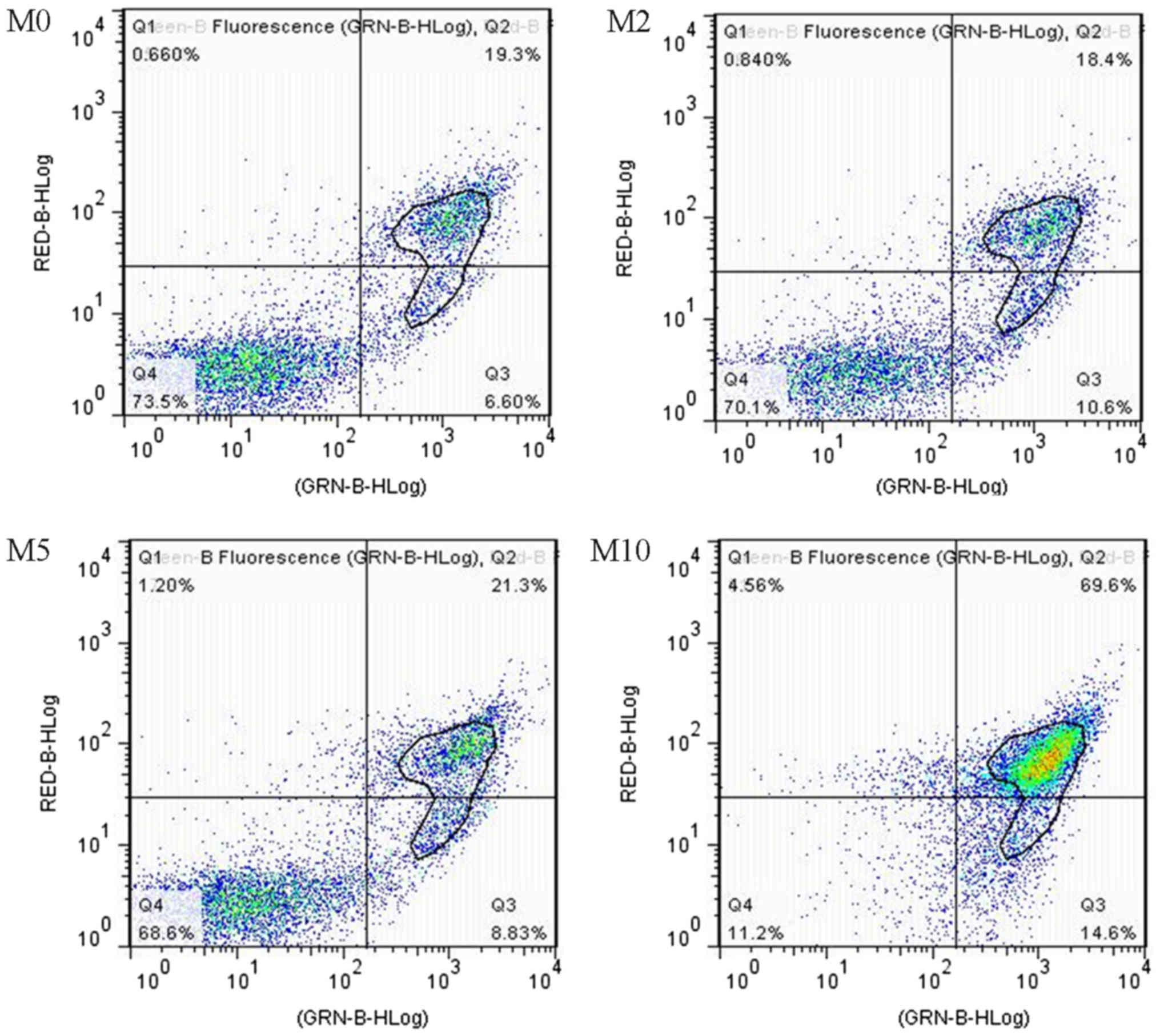
Flow cytometry with FITC-A/PI staining was performed
to detect apoptosis in TPC-1 thyroid cancer cells exposed to 0, 2,
5 and 10 mg/ml matrine. The total percentage of apoptotic cells is
described as the summation of percentages of both early and late
apoptotic subpopulations; the annexin V-FITC positive cells
(25). The effects of matrine on cell
apoptosis are presented in Fig. 2.
Matrine at the concentrations of 2, 5 and 10 mg/ml led to 16.1±1.9,
25.9±2.8 and 61.8±5.4% apoptosis, respectively, all elevated
compared with 15.1±3.1% in the M0 control group (Table I). Matrine significantly induced TPC-1
cell apoptosis.
 | Table I.TPC-1 cell percentage apoptosis and
cell cycle distribution. |
Table I.
TPC-1 cell percentage apoptosis and
cell cycle distribution.
| Matrine (mg/ml) | 0 | 2 | 5 | 10 |
|---|
| Apoptosis % | 15.1±2.1 | 16.1±1.9 | 25.9±2.8a | 61.8±5.4a |
| Cycle phase % |
|
|
|
|
|
G0/G1 | 51.36±2.07 |
60.63±2.57a |
62.96±1.98a |
74.49±3.65a |
| S | 23.37±3.25 |
10.95±2.54a |
11.26±3.47a |
2.42±1.63a |
| G2/M | 18.49±2.46 | 18.23±4.64 | 18.32±2.88 | 18.65±3.19 |
Effects of matrine on cell cycle distribution were
analyzed by flow cytometry in TPC-1 cells. In the M0 control group,
a total of 51.36±2.07% cells were in the G1 phase, 23.37±3.25% in
the S phase and 18.49±2.46% in the G2/M phase (Table I). Following treatment with matrine at
10 mg/ml for 48 h, the percentage of cells in the G1 phase
increased to 74.49±3.65%, and those in the S phase decreased to
2.42±1.63%. No significant alterations in the percentage of cells
in the G2/M phase were observed (Table
I). These results demonstrated that matrine induced cell cycle
arrest at the G0/G1 phase in thyroid cancer cells.
Expression levels of miR-21, PTEN and
Akt
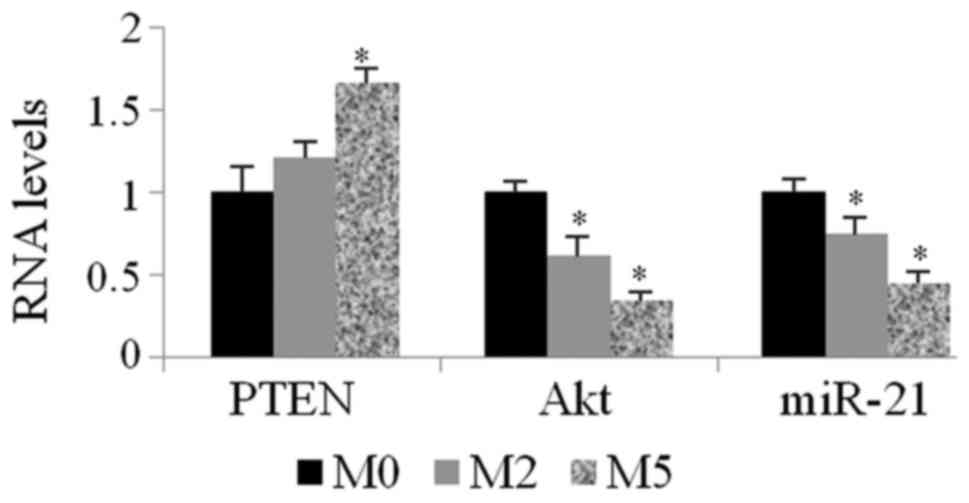
The expression level of miR-21 was determined by
qPCR after TPC-1 cells were treated with matrine at 0, 2 and 5
mg/ml (M0, M2 and M5) for 48 h. As shown in Fig. 3, matrine downregulated the expression
level of miR-21 in TPC-1 cells (0.75±0.09 for M2/M0 and 0.44±0.13
for M5/M0). PTEN mRNA levels increased by 1.21-fold for M2/M0 and
1.66-fold for M5/M0, respectively. Akt mRNA levels decreased by
0.61-fold for M2/M0 and 0.34-fold for M5/M0.
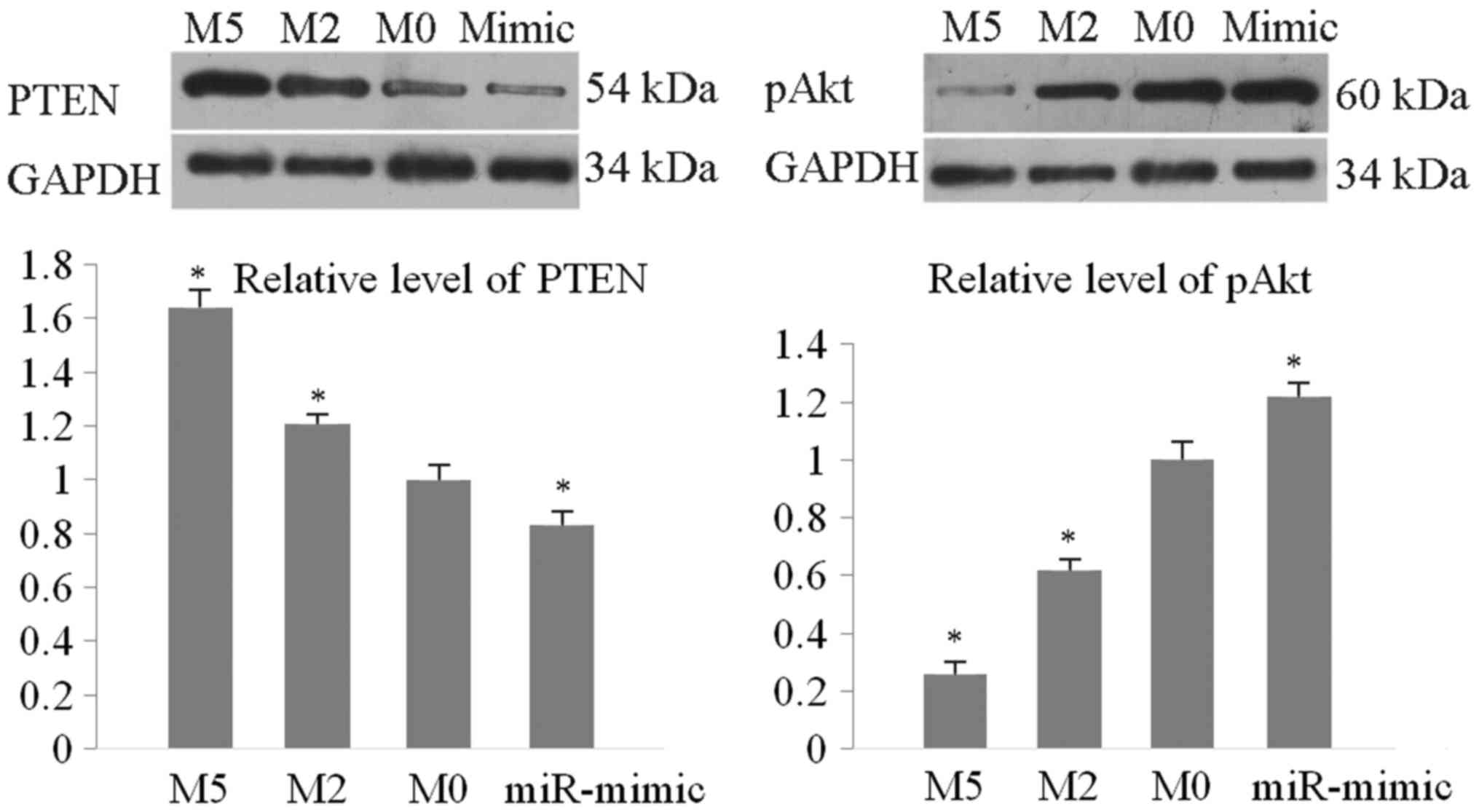
The protein levels of PTEN and phosphorylated (p)Akt
were detected by western blotting. As presented in Fig. 4, PTEN was significantly upregulated by
matrine compared with the M0 control (by 1.64-fold for M5/M0 and
1.21-fold for M2/M0). Levels of pAkt were downregulated (0.61 for
M2/M0 and 0.24 for M5/M0). The miR-21 mimic markedly reduced the
expression level of PTEN protein to 0.83 vs. the M0 blot value and
increased the level of pAkt by 1.24-fold vs. M0 blot value. These
results demonstrated that matrine regulated the miR-21/PTEN/Akt
pathway to induce TPC-1 apoptosis and cell cycle arrest.
Discussion
In the present study, matrine induced apoptosis and
G1 cell cycle arrest through downregulating miR-21 to affect the
PTEN/Akt signaling in TPC-1 human thyroid cancer cells. Matrine can
inhibit cancer cell proliferation by multiple mechanisms in various
tumors (14–23). However, the effect of matrine on
thyroid cancer has not been previously tested. The present study
demonstrated that matrine inhibited growth of TPC-1 cells,
suggesting that this medication may be used to treat thyroid
cancer. Furthermore, matrine induced apoptosis and cell cycle
arrest in TPC-1 cells. These results were consistent with those
previously obtained in studies investigating other cancers
(14,15,17,14).
Dysfunctional miR-21 regulates a tumor suppressor
PTEN to promote cancer cell growth (5,9,11,5). In the present study, miR-21 level in
TPC-1 cells treated with matrine was downregulated, and
subsequently, expression of its target PTEN was increased at both
mRNA and protein levels. Furthermore, miR-21 mimic was transfected
into TPC-1 cells and it was determined that overexpression of
miR-21 downregulated PTEN protein levels. These results indicated
that matrine can regulate miR-21 to prevent PTEN inhibition in
TPC-1 thyroid cancer cells. A similar matrine/miR-21/PTEN
interaction was observed in MCF-7 breast cancer cells as reported
in a previous study (23). PTEN as a
tumor suppressor can dephosphorylate Akt to induce apoptosis and
cell cycle arrest at the G1 and S phase (10,11,20,10). In
the present study, upregulation of PTEN suppressed phosphorylation
of Akt to cause apoptosis and cell cycle arrest in the G1 phase in
TPC-1 cells.
In addition to the miR-21/PTEN/Akt pathway, matrine
may inhibit cancer cells in other ways. Matrine can upregulate
proapoptotic proteins including B cell lymphoma (Bcl)-2 associated
agonist of cell death, Bcl-2 antagonist/killer 1 and Bcl-2
associated X, apoptosis regulator, and inhibit expression of
anti-apoptotic Bcl-2 and Bcl-xl (18,29,30). In
addition to the PTEN/Akt signaling, miR-21 serves important roles
in cancer growth, proliferation, migration and metastasis by
targeting programmed cell death 4 and Sprouty RTK signaling
antagonist 1, or by upregulating Bcl-2 indirectly (10,13,31).
Overexpression of PTEN upregulates the p21/WAF1/CIP1 and p27/KIP1
pathways by dephosphorylating Akt to increase cell apoptosis and/or
induce G1 phase arrest (17,23). These issues should be further
investigated in thyroid cancer cells.
In conclusion, the data presented in the present
study suggested that the miR-21/PTEN/Akt pathway may be one of the
mechanisms for matrine to inhibit TPC-1 thyroid cancer cells.
Matrine may be an alternative potential drug for the treatment of
thyroid cancer.
Acknowledgements
Not applicable.
Funding
The study was funded by the China Natural Science
Foundation (grants no. NSFC81702651) and China Jilin Province
Science Technology Project (grant no. 20180101011JC).
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
ZL cultured cells and performed flow cytometry, PCR,
western blotting and transfection assays. ZX also cultured cells
and performed the MTT assay and statistical analysis. CS designed
the experiment, revised the manuscript and approved the
submission.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Giusti F, Falchetti A, Franceschelli F,
Marini F, Tanini A and Brandi ML: Thyroid cancer: Current molecular
perspectives. J Oncol. 2010:3516792010. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Braun J and Hüttelmaier S: Pathogenic
mechanisms of deregulated microRNA expression in thyroid carcinomas
of follicular origin. Thyroid Res. 4 Suppl 1:S12011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Leonardi GC, Candido S, Carbone M,
Colaianni V, Garozzo SF, Cinà D and Libra M: MicroRNAs and thyroid
cancer: Biological and clinical significance (Review). Int J Mol
Med. 30:991–999. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Benvenga S and Koch CA: Molecular pathways
associated with aggressiveness of papillary thyroid cancer. Curr
Genom. 15:162–170. 2014. View Article : Google Scholar
|
|
5
|
Wang T, Xu H, Qi M, Yan S and Tian X:
miRNA dysregulation and the risk of metastasis and invasion in
papillary thyroid cancer: A systematic review and meta-analysis.
Oncotarget. 9:5473–5479. 2017.PubMed/NCBI
|
|
6
|
Wang Y, Wei T, Xiong J, Chen P, Wang X,
Zhang L, Gao L and Zhu J: Association between genetic polymorphisms
in the promoter regions of Let-7 and risk of papillary thyroid
carcinoma: A case-control study. Medicine (Baltimore).
94:e18792015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Perdas E, Stawski R, Nowak D and Zubrzycka
M: The role of miRNA in papillary thyroid cancer in the context of
miRNA Let-7 family. Int J Mol Sci. 17:E9092016. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Lu X, Jia M, Liu Y, Liu Z, Xu H and Geng
Z: The role of microRNA-21 in papillary thyroid cancer cells. Chin
J Exp Surg. 29:1981–1982. 2012.(In Chinese with English
Abstract).
|
|
9
|
Samsonov R, Burdakov V, Shtam T,
Radzhabova Z, Vasilyev D, Tsyrlina E, Titov S, Ivanov M, Berstein
L, Filatov M, et al: Plasma exosomal miR-21 and miR-181a
differentiates follicular from papillary thyroid cancer. Tumour
Biol. 37:12011–12021. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Meng F, Henson R, Wehbe-Janek H, Ghoshal
K, Jacob ST and Patel T: Microrna-21 regulates expression of the
PTEN tumor suppressor gene in human hepatocellular cancer.
Gastroenterology. 133:647–658. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Qi L, Bart J, Tan LP, Platteel I, Sluis T,
Huitema S, Harms G, Fu L, Hollema H and Berg Av: Expression of
miR-21 and its targets (PTEN, PDCD4, TM1) in flat epithelial atypia
of the breast in relation to ductal carcinoma in situ and invasive
carcinoma. BMC Cancer. 9:1632009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zhu S, Wu H, Wu F, Nie D, Sheng S and Mo
YY: MicroRNA-21 targets tumor suppressor genes in invasion and
metastasis. Cell Res. 18:350–359. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ma X, Kumar M, Choudhury SN, Buscaglia
Becker LE, Barker JR, Kanakamedala K, Liu MF and Li Y: Loss of the
miR-21 allele elevates the expression of its target genes and
reduces tumorigenesis. Proc Natl Acad Sci USA. 108:10144–10149.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Liu Y, Xu Y, Ji W, Li X, Sun B, Gao Q and
Su C: Anti-tumor activities of matrine and oxymatrine: Literature
review. Tumour Biol. 35:5111–5119. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
An Q, Han C, Zhou Y, Li F, Li D, Zhang X,
Yu Z, Duan Z and Kan Q: Matrine induces cell cycle arrest and
apoptosis with recovery of the expression of miR-126 in the A549
non-small cell lung cancer cell line. Mol Med Rep. 14:4042–4048.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wu L, Wang G, Wei J, Huang N, Zhang S,
Yang F, Li M, Zhou G and Wang L: Matrine derivative YF-18 inhibits
lung cancer cell proliferation and migration through
down-regulating Skp2. Oncotarget. 8:11729–11738. 2017.PubMed/NCBI
|
|
17
|
Lu Z, Xiao Y, Liu X, Zhang Z, Xiao F and
Bi Y: Matrine reduces the proliferation of A549 cells via the
p53/p21/PCNA/eIF4E signaling pathway. Mol Med Rep. 15:2415–2422.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Luo C, Zhu Y, Jiang T, Lu X, Zhang W, Jing
Q, Li J, Pang L, Chen K, Qiu F, et al: Matrine induced gastric
cancer MKN45 cells apoptosis via increasing pro-apoptotic molecules
of Bcl-2 family. Toxicology. 229:245–252. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Li H, Xie S, Liu X, Wu H, Lin X, Gu J,
Wang H and Duan Y: Matrine alters microRNA expression profiles in
SGC-7901 human gastric cancer cells. Oncol Rep. 32:2118–2126. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
He M, Jiang L, Li B, Wang G, Wang J and Fu
Y: Oxymatrine suppresses the growth and invasion of MG63 cells by
up-regulating PTEN and promoting its nuclear translocation.
Oncotarget. 8:65100–65110. 2017.PubMed/NCBI
|
|
21
|
Peng X, Zhou D, Wang X, Hu Z, Yan Y and
Huang J: Matrine suppresses proliferation and invasion of SGC7901
cells through inactivation of PI3K/Akt/uPA pathway. Ann Clin Lab
Sci. 46:457–462. 2016.PubMed/NCBI
|
|
22
|
Niu H, Zhang Y, Wu B, Zhang Y, Jiang H and
He P: Matrine induces the apoptosis of lung cancer cells through
downregulation of inhibitor of apoptosis proteins and the Akt
signaling pathway. Oncol Rep. 32:1087–1093. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Li LQ, Li XL, Wang L, Du WJ, Guo R, Liang
HH, Liu X, Liang DS, Lu YJ, Shan HL and Jiang HC: Matrine inhibits
breast cancer growth via miR-21/PTEN/Akt pathway in MCF-7 cells.
Cell Physiol Biochem. 30:631–641. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Oldham ED, Nunes LM, Varela-Ramirez A,
Rankin SE, Knutson BL, Aguilera RJ and Lehmler HJ: Cytotoxic
activity of triazole-containing alkyl β-D-glucopyranosides on a
human T-cell leukemia cell line. Chem Cent J. 9:32015. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Robles-Escajeda E, Lerma D, Nyakeriga AM,
Ross JA, Kirken RA, Aguilera RJ and Varela-Ramirez A: Searching in
mother nature for anti-cancer activity: Anti-proliferative and
pro-apoptotic effect elicited by green barley on leukemia/lymphoma
cells. PLoS One. 8:e735082013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Jin H, Sun Y, Wang S and Cheng X: Matrine
activates PTEN to induce growth inhibition and apoptosis in
V600EBRAF harboring melanoma cells. Int J Mol Sci. 14:16040–16057.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Fragni M, Bonini SA, Bettinsoli P, Bodei
S, Generali D, Bottini A, Spano PF, Memo M and Sigala S: The
miR-21/PTEN/Akt signaling pathway is involved in the anti-tumoral
effects of zoledronic acid in human breast cancer cell lines.
Naunyn Schmiedebergs Arch Pharmacol. 389:529–538. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Lv C, Hao Y and Tu G: MicroRNA-21 promotes
proliferation, invasion and suppresses apoptosis in human
osteosarcoma line MG63 through PTEN/Akt pathway. Tumour Biol.
37:9333–9342. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Yu P, Liu Q, Liu K, Yagasaki K, Wu E and
Zhang G: Matrine suppresses breast cancer cell proliferation and
invasion via VEGF-Akt-NF-kappaB signaling. Cytotechnology.
59:219–229. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Liang CZ, Zhang JK, Shi Z, Liu B, Shen CQ
and Tao HM: Matrine induces caspase-dependent apoptosis in human
osteosarcoma cells in vitro and in vivo through the upregulation of
Bax and Fas/FasL and downregulation of Bcl-2. Cancer Chemother
Pharmacol. 69:317–331. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Yan LX, Wu QN, Zhang Y, Li YY, Liao DZ,
Hou JH, Fu J, Zeng MS, Yun JP, Wu QL, et al: Knockdown of miR-21 in
human breast cancer cell lines inhibits proliferation, in vitro
migration and in vivo tumor growth. Breast Cancer Res. 13:R22011.
View Article : Google Scholar : PubMed/NCBI
|