Introduction
Hepatocellular carcinoma (HCC) is the second cause
of male cancer-related mortality worldwide. In 2012, 782,500 new
patients with HCC and 745,500 cases of death due to HCC were
reported, of which 50% were reported in China (1). HCC with extrahepatic metastasis
seriously impacts survival, and metastatic lung cancer is the most
common extrahepatic metastasis, comprising around 53.8% of all
extrahepatic metastasis (2).
No effective therapy is currently available for lung
metastatic HCC. According to reports, pneumonectomy is the most
effective therapeutic modality. However, prior studies revealed
that only 2.6% of patients with HCC with lung metastasis met the
operative indication, and surgery benefited only those who had less
than four lung metastatic foci with diameters less than 3 cm
(3). In China, HCC is mostly
secondary to hepatitis B virus-related liver cirrhosis, with
multiple lung metastatic foci and poor clinical operability
(4). Chemotherapy exhibits certain
efficacy for lung metastatic HCC. However, the majority of
chemotherapy drugs metabolize in the liver. Hepatotoxicity and bone
marrow suppression are the major limitations of chemotherapy.
Besides, a universally recognized chemotherapy regimen is still
lacking. Molecular targeted therapy of sorafenib is effective for
advanced HCC; however, HCC with lung metastasis responds to it
poorly (5). Researchers have also
explored other therapeutic modalities, such as local radiotherapy,
radiofrequency ablation, and so on, but no assertive efficacy has
been achieved. Overall, no standardized and effective therapeutic
strategy exists for lung metastatic HCC till date.
A recent investigation indicated that interferon
(IFN) prolonged survival in patients with HCC who underwent radical
resection, and the combination of IFN with cytokine-induced killer
adoptive cellular therapy prevented recurrence (6). Complete cure of lung metastatic HCC
using single IFN has not been reported till date.
The present study reported one case of HCC with
multiple lung metastatic lesions. After one time of transcatheter
arterial chemoembolization (TACE) and local chemotherapy via
thoracic aorta, lung metastatic foci evidently increased. Despite
that, 4 months of continuous treatment with peginterferon α 2a
(PEG-IFN α 2a) led to a gradual reduction in alpha-fetoprotein
(AFP) from >1,000 ng/ml to normal prior to treatment and
resulted in the gradual disappearance of metastatic lung cancer and
stability of HCC lesions without recurrence. Till the date of
writing this report, the patient lived a high-quality life for 108
months, reaching a clinical cure according to the World Health
Organization (WHO) prognostic criteria.
Case report
A 53-year-old male patient, living in Tianjing,
China, had a medical history of diabetes, hepatitis B e
antigen-negative chronic hepatitis B, and a family history of
hepatitis B and liver cancer (with the death of his mother and one
brother from HBV-related HCC), and without a history of blood
transfusion. In 2007, the patient started lamivudine, 100 mg qd,
for controlling HBV with HBV DNA 5.3×106 cs/ml and
alanine aminotransferase 102 U/l. In April 2008, radioimmunoassay
estimated a concentration of >1,000 ng/ml of AFP in the patient.
Moreover, color ultrasonography revealed a hypoechoic solid mass of
size 4.0×4.0 cm2 in the right anterior lobe of the liver
with uneven density and irregular edge, which was considered as
HCC. It also showed enlargement of the caudate lobe of the liver,
widening of the hepatic fissure, and widening of the portal vein
with a diameter of 1.1 cm, in addition to splenomegaly, which was
considered as liver cirrhosis. Furthermore, enhanced computed
tomography (CT) of the upper abdomen revealed a soft tissue mass
with a diameter of 2.26×4.16 cm2 in the right anterior
lobe of the liver, presenting a low-density soft tissue in the
plain scanning, mild enhancement of the density in the arterial
phase, and yet nonhomogeneous enhancement of the density in the
portal phase, along with an evident reduction in the mass density
in the equilibrium phase, which was considered as HCC (Fig. 1A). Moreover, chest CT exhibited
multiple small nodules in the right inferior lobe of the lung, with
a maximum diameter of 0.5 cm, which were considered as metastatic
cancer (Fig. 1B). Also, in April
2008, the digital subtraction angiography displayed enlarged and
tortuous anterior and posterior branches of the right hepatic
artery and the image of a tumor with a diameter 4.1×4.0
cm2 in the right anterior lobe of the liver in the
parenchymal phase, along with five small nodules of <0.5 cm in
the right inferior lobe of the lung. In summary, the ultrasound
displayed an intrahepatic nodule more than 2 cm in size. The
typical dynamic change in the nodule was detected using a dynamic
imaging method, presenting vessel density enhancement of the
hepatic mass in the arterial phase and rapid elution in the delayed
phase under enhanced CT. The AFP level was measured to be >200
ng/ml. According to the ‘Clinical Guidelines for Hepatocellular
Carcinoma’ issued in the year 2005 by the American Association for
the Study of Liver Diseases (AASLD), HCC was the definitive
diagnosis without the need of biopsy. Gastroscopy indicated mild
esophageal varices. Therefore, the preliminary diagnosis was
primary HCC, metastatic lung cancer, compensated HBV-related
cirrhosis, and chronic hepatitis B. On the request of the patient
and his kin as well as signed informed consent, TACE (Fig. 1C) was performed. Specifically,
floxuridine (FUDR) 500 mg and cisdiamine dichloroplatinum (CDDP) 20
mg were infused through the proper hepatic artery for chemotherapy,
and superselective embolization of the right hepatic artery was
conducted using 10 mg mitomycin plus 10 ml of ultrafluid lipiodol.
Moreover, 250 mg FUDR and 20 mg CDDP were infused through the
bronchial artery of the thoracic aorta at the bifurcation of
trachea level for chemotherapy of lung metastatic foci. The patient
had HCC with metastatic lung cancer, in phase IV of the TNM
staging, at initial presentation. According to the WHO criteria for
solid tumor response, TACE for local chemoembolization is a partial
remission strategy for treating HCC with lung metastatic foci and
the patient survival is expected to be less than 6 months.
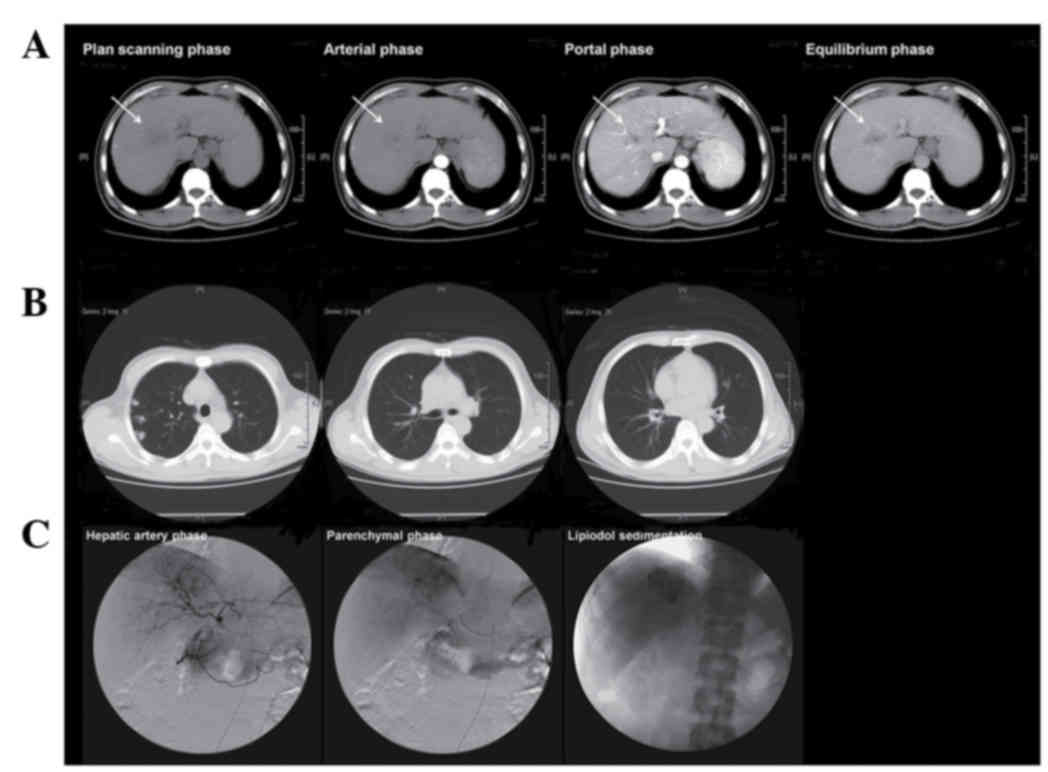 | Figure 1.Enhanced CT scanning of the upper
abdomen and chest CT scanning prior to treatment and TACE imaging.
(A) In April 2008, a soft tissue mass 2.26×4.16 cm2 in
size, with a low density was displayed in the right anterior lobe
of the liver. This was observed in the plain scanning, mild
enhancement of density in the arterial phase and the nonhomogeneous
enhancement of the density in the portal phase, as well as with an
evident reduction in the mass density in the equilibrium phase. (B)
Chest CT exhibited multiple small nodules in the right inferior
lobe of the lung with a maximum diameter of 0.5 cm, which were
considered as metastatic cancer. (C) In April 2008, the digital
subtraction angiography displayed enlarged and tortuous anterior
and posterior branches of the right hepatic artery, and the image
of a tumor with 4.1×4.0 cm2 in size in the right
anterior lobe of the liver in the parenchymal phase, along with
five small nodules of <0.5 cm in the right inferior lobe of the
lung, which was considered as HCC with metastatic lung cancer.
Under the request of the patient and his kin TACE was performed. A
total of 500 mg FUDR and 20 mg CDDP were infused through the proper
hepatic artery for chemotherapy and superselective embolization of
the right hepatic artery was conducted using 20 mg mitomycin 20 mg
plus 10 ml ultrafluid lipiodol. In addition, 250 mg FUDR and 20 mg
CDDP were infused through the bronchial artery of the thoracic
aorta at the bifurcation of trachea level for chemotherapy of lung
metastatic foci. After TACE, good lipiodol sedimentation was
observed in the lesion in the right anterior lobe of the liver. CT,
computed tomography; TACE, transcatheter arterial
chemoembolization; HCC, hepatocellular carcinoma; FUDR,
floxuridine; CDDP, cisdiamine dichloroplatinum. |
In June 2008, following 2 months of TACE, a review
check of the patient showed white blood count of
3.00×109/l, platelet count of 71×109/l, and
AFP of >400 ng/ml. Also, enhanced CT scanning of the upper
abdomen revealed post-TACE change of the soft tissue mass in the
right anterior lobe of the liver, good lipiodol sedimentation, and
relative stability of the HCC lesion. Chest CT scanning displayed
multiple diffuse nodules in the bilateral lung, with the increased
number and enlarged volume. Hence, metastatic cancer was considered
progressive. The patient refused to take targeted therapy of
sorafenib. In systemic consideration of compensated cirrhosis,
Child-Pugh A grade, and normal blood routine, in addition to mild
esophageal varices by gastroscopy, PEG-IFNα 2a (180 µg qw) was
elicited under the informed consent of the patient and his kin.
During treatment, regular monitoring of the patient was done,
including blood routine, AFP, liver function, and IFN-associated
adverse effects, in addition to liver- and lung-imaging changes.
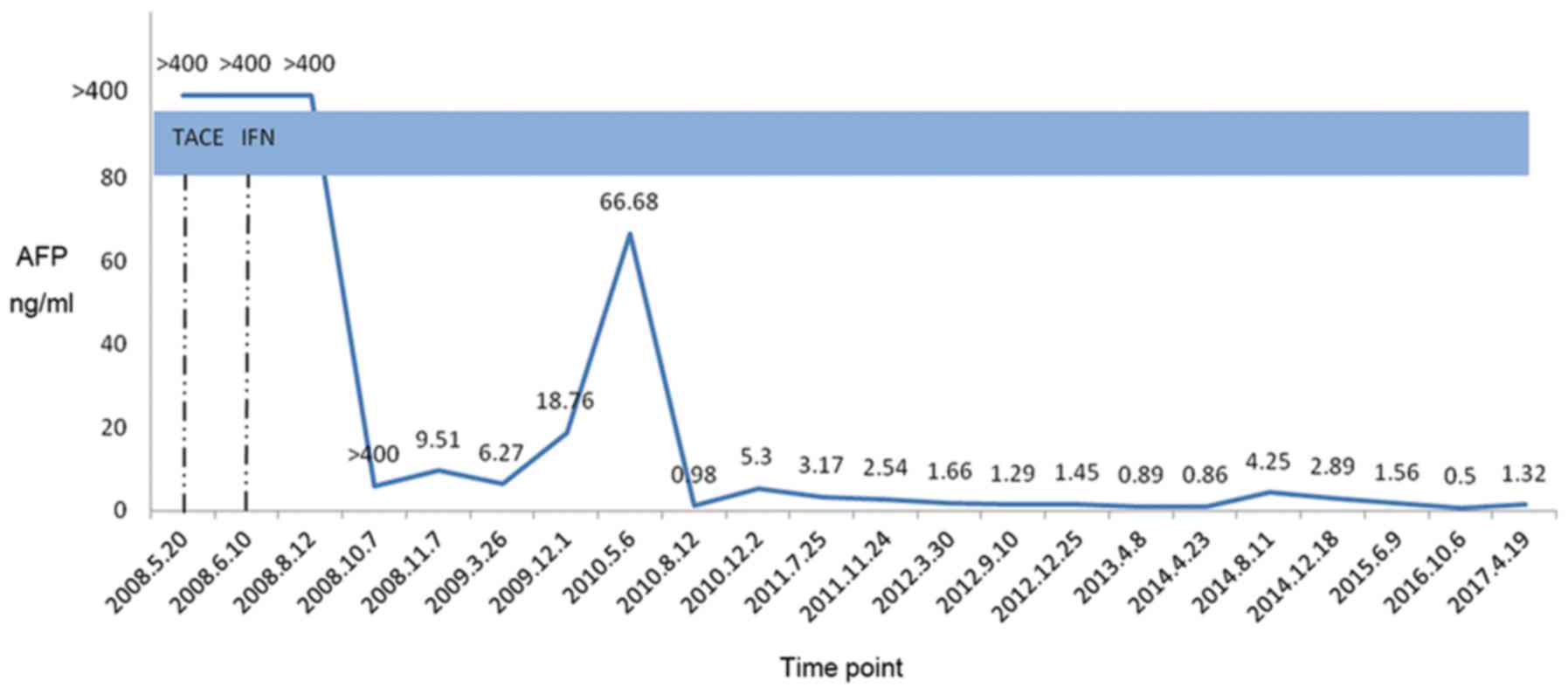
The AFP levels gradually reduced with IFN use. In August 2008, 2
months after the IFN intervention, the AFP level was >400 ng/ml,
which decreased to 5.8 ng/ml after 4 months, in October 2008. Since
then, the AFP level of the patient stayed within the normal range.
The patient once had transient leukopenia and neutropenia, but the
leukocyte and neutrophil levels resumed to normal in 3 days after
temporary subcutaneous administration of the recombinant human
granulocyte colony-stimulating factor (rhGCSF) injection, 75 µg per
day. IFN was continued, and no other IFN-associated adverse effects
were recorded.
In March 2009, 9 months after the IFN intervention,
a full hospital review check of the patient showed an AFP of 6.27
ng/ml. An enhanced CT scanning of the upper abdomen revealed the
post-TACE change of HCC in the right anterior lobe of the liver.
Additionally, chest CT scanning revealed multiple nodules in the
bilateral lung, which was considered as metastatic cancer, but with
marked improvement than before. Both HCC and metastatic lung cancer
met the standard for a complete remission according to the WHO
criteria for solid tumor response. In the following 8 years, IFN
therapy was continued, and the regular review check displayed
stable cancer nodules by enhanced CT scanning of the upper abdomen
and chest CT scanning (Fig. 2). In
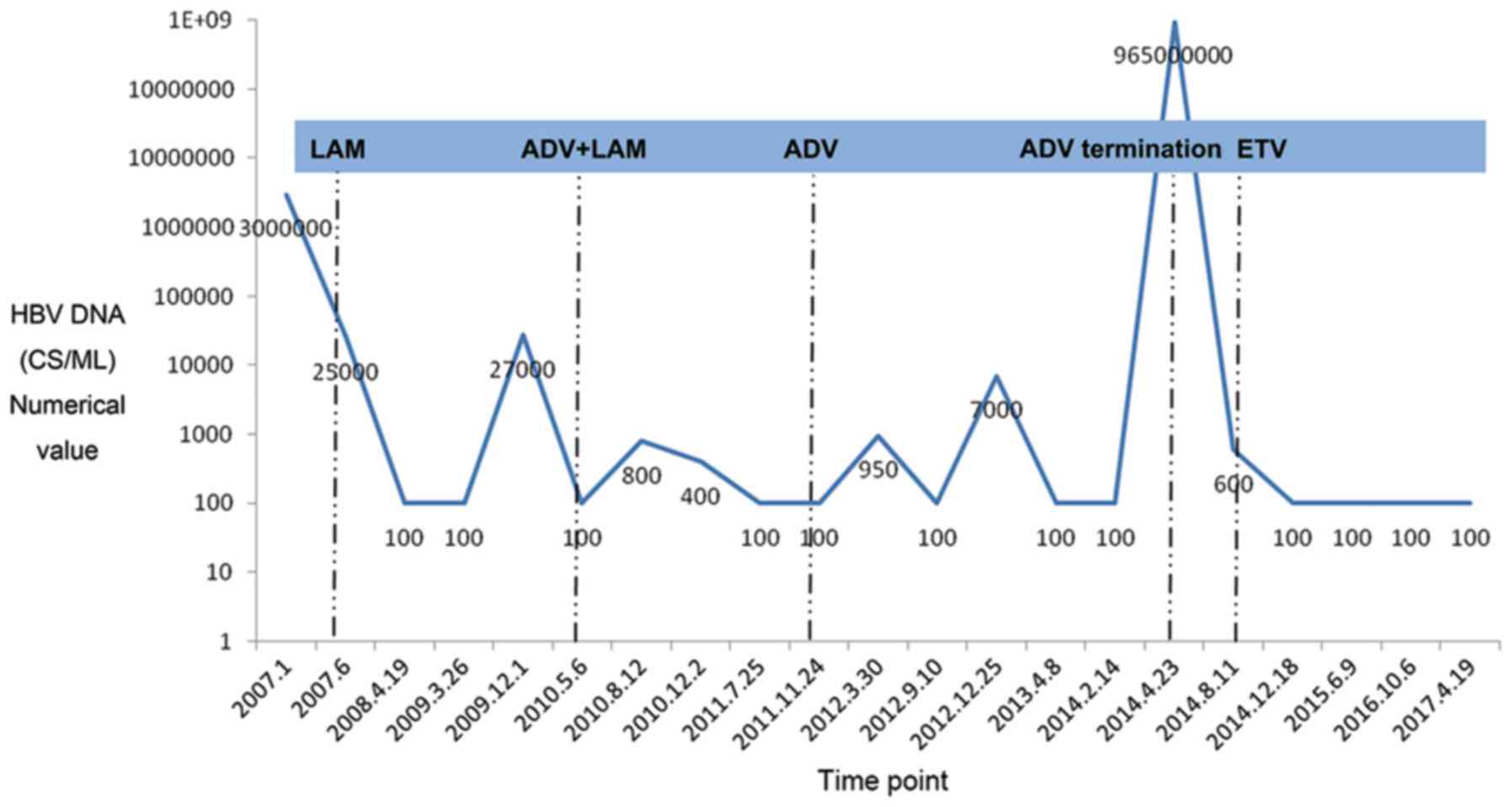
December 2009, HBV mutation was detected in the patient, of which
the 204th residue methionine (M) in the YMDD motif of polymerase C
was replaced by isoleucine (I), with HBV DNA of 2.7×104
cs/ml. The situation improved after adding adefovir dipivoxil
combined with lamivudine to control the virus. In February 2014,
the patient terminated antiviral drugs on his own accord. After 2
months, on April 23, 2014, the review check showed HBV DNA of
9.65×108 cs/ml, and the YMDD motif of the polymerase C
was resumed to wild type. Antiviral therapy with entecavir was
given till date, and HBV DNA was detected to be negative (Fig. 3). Also, the AFP levels were monitored
to be normal. No other HCC-related therapy was enforced ever since
(Fig. 4). In April 2017, the review
check showed AFP of 1.32 ng/ml, hepatitis B surface antigen (HBsAg)
of 45.43 IU/ml, and negative HBV DNA. An enhanced CT scanning of
the upper abdomen displayed a stable HCC nodule, and the chest CT
revealed micronodules in the bilateral lung. According to the WHO
criteria for solid tumor response, the patient reached a complete
remission and survival of up to 9 years, with a good quality of
life (QOL) reflected by the Karnofsky Performance Scale score of
100, performance status grade 0, and QOL score of 60. Currently,
the patient sticks to the average follow-up, once every 3–4 months,
and his general condition, cancer status, and potential adverse
effects are being monitored (Tables I
and II). Continuous attention is
being paid to the disease status of the patient.
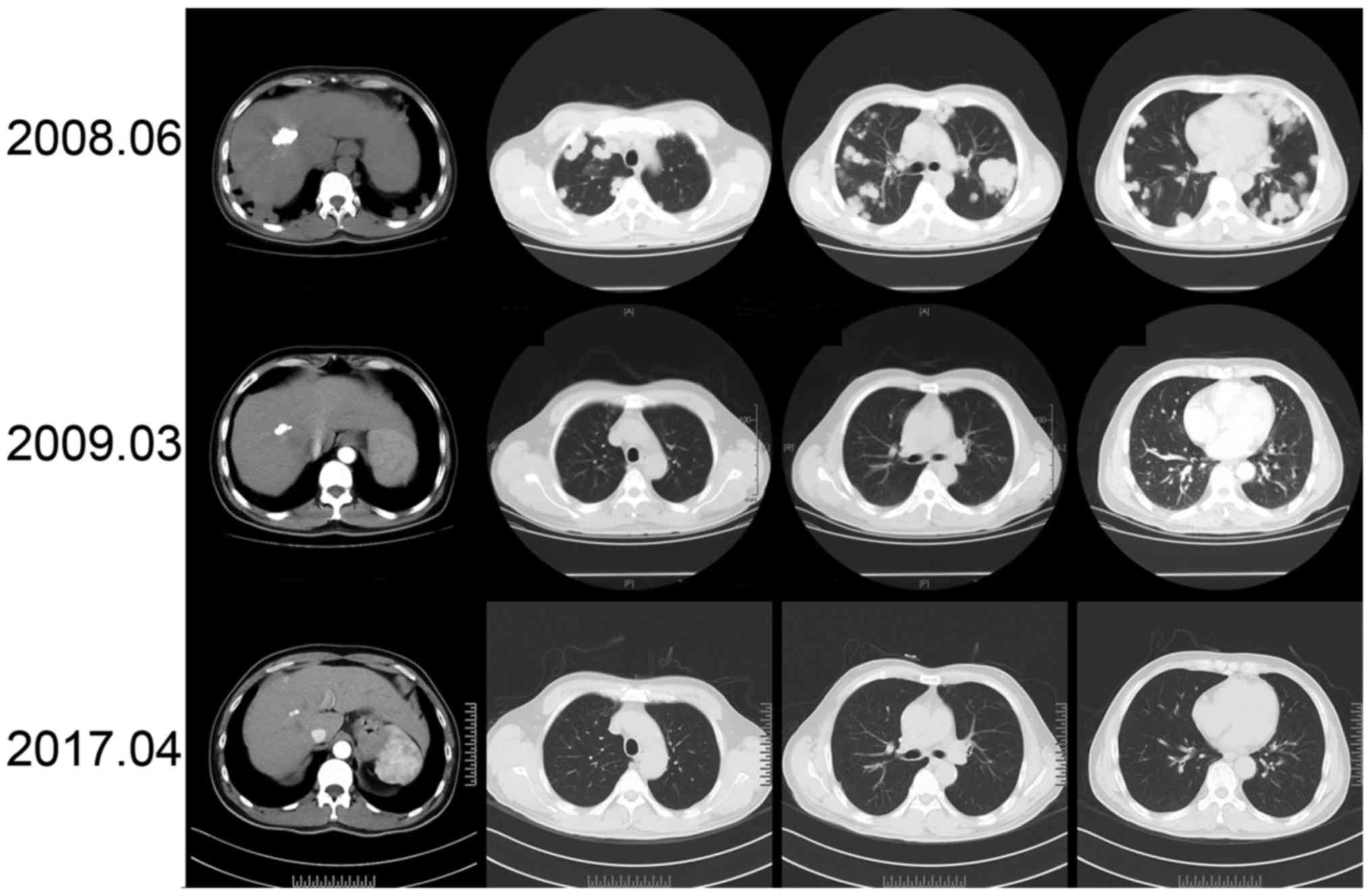 | Figure 2.Change in lesions in the liver and
lung after TACE treatment. Following TACE treatment, a review check
with enhanced CT scanning of the upper abdomen revealed lipiodol
sedimentation in the primary cancer nodule, which decreased each
year and stable nodules without progression, in addition to the
negative incidence of the new intrahepatic lesion. In June 2008, 2
months following TACE, a chest CT scanning revealed multiple
diffuse and scattered nodules in the bilateral lung, which
increased in number and volume compared with those detected in
April 2008. Metastatic lung cancer was considered progressive and
interferon intervention was elicited. In March 2009, 11 months
after TACE and 9 months after interferon therapy, a chest CT
displayed multiple nodules in the bilateral lung with a decrease in
number and evident reduction in volume. Metastatic lung cancer was
considered a retraction. A later review check with a CT still
exhibited micronodules in the bilateral lung, in a stable state
without recurrence. TACE, transcatheter arterial chemoembolization;
CT, computed tomography. |
 | Table I.Monitoring of interferon
therapy-related adverse effects during treatment. |
Table I.
Monitoring of interferon
therapy-related adverse effects during treatment.
| Follow up date
(month/year) | WBC
(3.5–9.5)×109/l | PLT
(100–300)×109/l | IRT | Ca (2.03–2.54)
mmol/l | P (0.90–1.34)
mmol/l | TSH (0.27–4.2)
mIU/l | CD4 (32.8–52.8)% | CD8 (19.7–38.9)% | Gastroscopy |
|---|
| 04/2008 | 3.3 | 67 | All negative | – | – | – | – | – | EV, Li, F1,
RC(−) |
| 06/2008 | 3 | 71 | – | – | – | – | 42.8 | 41.1 | – |
| 04/2009 | 3.01 | 83 | All negative | 2.39 | 0.66 | – | 28.7 | 25.4 | – |
| 05/2010 | 3.81 | 93 | – | – | – | – | 36.3 | 31.3 | – |
| 12/2010 | 3.57 | 86 | – | – | – | – | – | – | EV, Lmi, F2,
RC(−) |
| 07/2011 | 4.13 | 107 | – | – | – | – | 28.4 | 29.1 | – |
| 03/2012 | 3.71 | 140 | – | – | – | – | – | – | – |
| 12/2012 | 4.96 | 112 | – | – | – | – | – | – | EV, Lmi, F2,
RC(−) |
| 04/2013 | 5.25 | 119 | All negative | 2.22 | 0.6 | – | 35.1 | 33.1 | – |
| 08/2014 | 4.77 | 104 | – | 2.19 | 1.03 | 0.21 | – | – | – |
| 12/2014 | 3.87 | 101 | – | 2.08 | 0.64 | – | – | – | Esophagitis,
superficial |
|
|
|
|
|
|
|
|
|
| gastrotis |
| 06/2015 | 4.06 | 88 | All negative | 2.08 | 0.81 | – | – | – | – |
| 10/2016 | 3.54 | 97 | All negative | – | – | 0.75 | – | – | – |
| 04/2017 | 3.63 | 89 | All negative | 2.19 | 0.76 | – | – | – | – |
 | Table II.Clinical follow-up records of
potential adverse effects during IFN therapy. |
Table II.
Clinical follow-up records of
potential adverse effects during IFN therapy.
|
| IFN-associated
adverse effects | Follow-up and
management |
|---|
| Influenza-like
symptoms | Fever
(T>37.3°C) | Transient,
disappeared after 1 week |
|
| Headache | Transient,
disappeared after 1 week |
|
| Muscle and joint
sore | Transient,
disappeared after 1 week |
|
| Whole-body
discomfort | Transient,
disappeared after 1 week |
| Gastrointestinal
reactions | Nausea, vomiting,
inappetence | No occurrence |
| Changes in the skin
and hair | Hair loss | Evident in the
first year and stable in the following years |
|
| Whole-body itching,
rash | No occurrence |
| Symptoms of bone
marrow suppression | Leucopenia | Transient, no
interference with use after symptomatic management |
|
|
Thrombocytopenia | Transient, no
interference with use after symptomatic management |
| Symptoms of the
nervous system | Fatigue, insomnia,
indifference, lack of initiative, depressed to suicide | No occurrence |
| Thyroid dysfunction
symptoms | Change in T3, T4,
and TSH | No occurrence |
|
| Thyroid autoimmune
antibody | No occurrence |
| Autoimmunity
diseases | Diabetes | Stable blood
glucose level |
|
| Thyroiditis,
autoimmune hepatitis, biliary cirrhosis | No occurrence |
| Ocular
abnormalities | Infraorbital
hemorrhage | No occurrence |
|
| Retinal
hemorrhage | No occurrence |
| Hearing
impairment | Tinnitus, hearing
loss | No occurrence |
| Cardiovascular
system | Arrhythmia,
pericarditis | No occurrence |
| Respiratory
system | Interstitial
pneumonia, pulmonary embolism | No occurrence |
Discussion
HCC metastasis and recurrence severely impact
patient survival and QOL. However, no standardized effective
therapy strategy is available for lung metastatic HCC till date.
Personalized therapy deserves further exploration. Radical surgery
is recognized as the cure, but the number of patients who met the
operative indication is limited. Systemic chemotherapy is also one
of the common therapy strategies, but it is not extensively
appreciated because it deteriorates cancer recurrence and showed no
effect on survival improvement in postoperative patients with HCC
and cirrhosis (7). Molecular targeted
therapy of sorafenib is effective for advanced HCC; however, HCC
with lung metastasis responds to it poorly (5). Additionally, the existence of multidrug
resistance gene leads to poor chemotherapeutic efficacy and
accelerated adverse effects, imposing risks on patient's
safety.
In previous studies, the prognosis of HCC with lung
metastasis was poor, but administering IFN was beneficial (Table III) (8–15). A
recent report documented a successful cure of lung metastatic HCC
by chemotherapy, in which Japanese researchers applied S-1 (the
oral fluoropyrimidine), a complex of tegafur, gimeracil, and
oteracil potassium, combined with IFNα to treat 11 patients who
lived for more than 1 year. One of them lived for more than 5 years
with the disappearance of multiple metastatic lesions (16). From the overall perspective, no
standardized effective strategy still exists for systemic
chemotherapy, and adequate evidence-based analysis is lacking. The
patient in the present case study lived up to 108 months without
cancer, taking no subsequent chemotherapy after TACE, except IFN.
This was not reported before and deserved further exploration.
 | Table III.Previous reports of cases with
interferon treatment for hepatocellular carcinoma with lung
metastasis. |
Table III.
Previous reports of cases with
interferon treatment for hepatocellular carcinoma with lung
metastasis.
| Author, ref | Age/sex | History | Location of
HCC | Treatment for
HCC | Recurrence
time | Recurrence
site | Treatment for
recurrence | Type of
interferon | Other therapy | Prognosis |
|---|
| Katsura et
al (8) | 77/male | NA | Left lobe | TACE and left
hepatic lobectomy | 7 months | Multiple bilateral
lungs | S-1 | IFN | No | Alive with the
disease details were not shown, alive with good condition without
recurrence and progression of tumors |
| Oh et al
(9) | 49/male | CHB, cirrhosis, RFA
for a single HCC (4 cm, segment 7) | Intrahepatic
recurrence of HCC with extensive lung metastases | HAIC comprising
epirubicin and cisplatin, and systemic infusion of 5-FU | 13 months | A single small HCC
lesion in the left lobe | HAIC, percutaneous
intratumoral chemoinjection therapy with 5-FU and IFN-γ | IFN-γ | No | Disease-free
intervals for the liver and lung were 41 and 54 months,
respectively |
| Kilickap et
al 10) | 37/male | CHB, HCC | Right lobe, 49×61
mm | Right hepatic
lobectomy | 1 month | Thoracic 6–8
vertebrae | T6-8
laminectomy, | IFN-α | No | Alive without
disease on lamivudine and IFN-α, disease-free for 3 years; followed
up about 10 years after diagnosis |
|
|
|
|
|
| 3 years | The left lower
lobe | local
radiotherapy | IFN-α | Lamuvidine |
|
|
|
|
|
|
| 3 months | of the lung, 1
cm | Resected lung
metastasis |
|
|
|
|
|
|
|
|
| 4 years finding but
stable, progression on 5 years 6 months | The left kidney,
from 13×13 mm increasing to 25 mm | Partial
nephrectomy | Pegylated
IFN-α | Lamuvidine |
|
| Tanaka et al
(11) | 60/male | Hepatitis C | Segment 3, 44 mm in
diameter | Partial
hepatectomy | 9 months after the
surgery | Intra abdominal
solitary lymph node metastasis | Removal of the
lymph node | NA |
| Alive with no
recurrence 19 months, 30 months after the initial operation |
| Nakamura et
al (12) | 54/male | PVT and multiple
intrahepatic metastases | The bilateral lobes
of the liver | Extended left
lobectomy, intra-arterial 5-FU infusion chemotherapy, IFN-α to
treat the lesions in the residual liver | 7 months | Recurrent tumors in
the spleen and residual liver | S-1 | IFN-α |
| Alive with no
recurrence 32 months after initial hepatic resection |
|
|
|
|
|
|
| Recurrent tumors in
the lung | S-1, resected | IFN-α |
|
|
| Nakamura et
al (13) | 56/male | PVT and multiple
intrahepatic metastases | Extended left
lobectomy and a partial resection of the liver | Extended left
lobectomy and a partial resection of the liver, after two weeks,
intra arterial 5-FU infusion, IFN-α | 4 months | Hepatic vein tumor
thrombus, inferior caval vein | TS-1 | IFN-α |
| NA |
|
|
|
|
|
| 8 months | Mutiplle pulmonary
metastases |
|
|
|
|
| Nakamura et
al (14) | 52/male | NA | Right lobe | Right
lobectomy | 5 months | Multiple
recurrences in the live and lung | TAE, UFT and
IFN-α | IFN-α and IFN-β
IFN-α, TS-1/ IFN-β | 5-FU/ CDDP/ | Survived 31 months
with no disturbance in quality of life |
| Kanda et al
(15) | 64/male | HCC with lung
metastasis | NA | γ-interfeorn and
mitoxantrone | NA | NA | NA | IFN-γ | HCFU 400 mg/ body
everyday for 8 months | Still alive |
IFN is a multifunctional cytokine with antiviral and
antitumor activities, which is probably more beneficial for
HBV-related patients with HCC. After surgical resection of HCC, IFN
is the most effective adjuvant therapeutic modality for survival
improvement compared with other common adjuvant therapeutic
strategies for HCC (6). A variety of
meta-analyses indicated that IFN improved the nonrecurrence
survival and overall survival following surgical resection of
virus-related HCC (17–19). Moreover, IFN intervention remarkably
attenuated mortality and recurrence in HBV-related patients with
HCC having a therapeutic history of TACE (20). In the patient in the present case
study, TACE was used comprising CDDP and FUDR for HCC, and
CDDP/FUDR achieved a good tumor response (21). Because of the local and one-time
therapy using CDDP/FUDR, IFN intervention was speculated to be the
key to the successful cure of patients based on several potential
reasons as follows.
First, IFN imparts direct and indirect antitumor
effects. From one aspect, IFN acts directly on tumor cells,
inhibiting cell proliferation and promoting apoptosis possibly via
the mechanisms as follows: i) it is mainly involved in biological
behaviors, such as proliferation, differentiation, and apoptosis,
via a Janus kinase (JAK)/signal transducer and activator of
transcription (STAT) signaling pathway (22); ii) it promotes apoptosis of the HCC
cells via transactivation of P38/mitogen-activated protein kinase
pathway (23); iii) it activates p63
in the S/G2/M phases of the cell cycle and causes cells to remain
quiescent in phase G1, thus inducing apoptosis of the HCC cells
(24) and iv) it induces autophagy
via JAK/STAT and phosphatidylinositol 3-kinase (PI3K)/protein
kinase B (AKT)/mammalian target of rapamycin (mTOR) pathways and is
crucial in the inhibition of cell proliferation (25). From another aspect, IFN exerts an
indirect antitumor effect by activating host antitumor immune
response via mechanisms as follows: i) it transactivates PI3K/Akt
signaling pathway and thus activates natural killer (NK) cells to
execute antitumor activity (26); ii)
it enhances NK cell-mediated antitumor immunity via activation of
host dendritic cells (27); iii) it
promotes tumor-specific CD8+ T cells, thus increasing
the response of cytotoxic T lymphocytes to enhance antitumor
activity (28) and iv) it inhibits
proliferation of regulatory T cells (Treg) and attenuates
Treg-mediated immune suppression to play an indirect antitumor role
(29).
Second, IFN inhibits metastatic lung foci. IFN
intervention could not only continuously inhibit HCC growth but
also block the proliferation of the metastatic foci in the lung.
The epithelium mesenchymal transition (EMT) is closely associated
with tumor metastasis, and matrix metallopeptidase 9
(MMP-9)-mediated EMT is essential in the process, while
downregulation of MMP-9 can hinder HCC metastasis (30). Macrophage in the lung secretes MMP-9
to promote the proliferation and migration of metastatic lung foci,
and IFN intervention decreases MMP-9 levels to control metastatic
lung foci (31). IFN also inhibits
metastasis and recurrence of HCC via downregulation of
VEGF-mediated angiogenesis (32).
Long-term IFN therapy also benefits patients
(33). Drug termination in the
patient who underwent IFN antitumor therapy elicits upregulation of
MMP-9 and macrophages in the lung, explaining the increase in
recurrence and lung metastasis after drug termination according to
clinical observations (34). Of
course, attention should be paid to adverse effects of IFN therapy,
for example hematologic disorder and influence on thyroid functions
and nervous system. Intolerance to these adverse effects interferes
with the extensive and long-term application of IFN in patients
with HCC. With an optimistic attitude toward life, the patient in
the present study took IFN for a long duration. Close monitoring of
the changes in blood routine, blood sugar, thyroid function, and
immunity-related tests exhibited negative abnormality. The patient
responded and tolerated well to IFN intervention, which was also
considered as a critical element for benefits to the patient.
Finally, the antiviral activity of IFN likely
benefits patients. The disappearance of HBsAg is the ultimate goal
of chronic hepatitis B therapy, which contributes to the control of
the incidence rate of HCC. When IFN decreases the HBV DNA levels,
it also regulates the immune response leading to limitation of
HBsAg, and its combinatory therapy with nucleotide analogues
probably imposes a synergistic effect on the maximal reduction in
the prevalence rate of HBV-related HCC (35). In the present study, the patient once
had the HBsAg level as high as 25,000 IU/ml during the last 8 years
or more, which was reduced to 45.43 IU/ml (current) by the
combinatory antiviral therapy of IFN with various nucleotide
analogues, reflecting good immunological control of HBV infection
in the patient system. Although the nucleotide analogue was capable
of improving survival in patients with HCC, it could not treat HCC
on its own. Therefore, IFN was believed to be indispensable in
fighting against HBV and HCC, which was probably the essential
cause leading to the complete clinical cure and high QOL for the
patient. Until now, this is the only case reported with a survival
of 108 months and with regular follow-up. This patient was followed
up regarding disease progression and change.
In summary, this study provided a novel idea for
treating patients with HCC having lung metastasis, particularly
HBV-related HCC. IFN intervention, as the main adjuvant therapy,
resulted in complete disappearance of metastatic lung cancer and
led to stable HCC without progression, in addition to a complete
clinical cure and long-term survival. Prior to treatment, if HBV
whole-genome sequencing from the patient or genetic test of the HCC
tissue had been performed, or the immune state of the patient had
been uncovered, for example, the measurement of IFN receptors, it
would have helped to sort out the beneficial factors of IFN cure of
HBV-related HCC. Given IFN exerts anti-HBV and antitumor effects,
long-term IFN intervention is a promising strategy as a critical
adjuvant therapy for HBV-related HCC with lung metastasis, which is
worth studying further. Also, the IFN dose and treatment course
requires attention, together with its safety and applicable
population.
Acknowledgements
Not applicable.
Funding
This study was funded by the Major Projects of the
Ministry of Science and Technology of China (grant no.
2017ZX10302202) and the Tianjin Municipal Health and Family
Planning Commission (grant nos. 16KG151 and 2014KY03).
Availability of data and materials
The datasets used and analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
FW observed the patient's condition, collected
clinical data and wrote the manuscript. HML, YL, TH, HL, FL and YG
participated in the clinical treatment and follow-up record of the
patient over the past 9 years. KJ conducted the TACE treatment and
image data follow-up of the patient. FMW decided on the patient
diagnosis and treatment as well as the design and revision of the
manuscript. All authors read and approved the final manuscript.
Ethics approval and consent to
participate
The present study was approved by the Ethics
committee of Tianjin Third Central Hospital in accordance with the
ethical standards mentioned in the 1964 Declaration of Helsinki and
its later amendments or comparable ethical standards. Informed
consent was obtained from the patient included in the study.
Patient consent for publication
Informed consent was obtained from the patient for
the publication of their data and associated images.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Torre LA, Bray F, Siegel RL, Ferlay J,
Lortet-Tieulent J and Jemal A: Global cancer statistics, 2012. CA
Cancer J Clin. 65:87–108. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Natsuizaka M, Omura T, Akaike T, Kuwata Y,
Yamazaki K, Sato T, Karino Y, Toyota J, Suga T and Asaka M:
Clinical features of hepatocellular carcinoma with extrahepatic
metastases. J Gastroenterol Hepatol. 20:1781–1787. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Chen F, Sato K, Fujinaga T, Sonobe M,
Shoji T, Sakai H, Miyahara R, Bando T, Okubo K, Hirata T and Date
H: Pulmonary resection for metastases from hepatocellular
carcinoma. World J Surg. 32:2213–2217. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Hu HH, Liu J, Lin YL, Luo WS, Chu YJ,
Chang CL, Jen CL, Lee MH, Lu SN, Wang LY, et al: The rs2296651
(S267F) variant on NTCP (SLC10A1) is inversely associated with
chronic hepatitis B and progression to cirrhosis and hepatocellular
carcinoma in patients with chronic hepatitis B. Gut. 65:1514–1521.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Yau T, Chan P, Ng KK, Chok SH, Cheung TT,
Fan ST and Poon RT: Phase 2 open-label study of single-agent
sorafenib in treating advanced hepatocellular carcinoma in a
hepatitis B-endemic Asian population: Presence of lung metastasis
predicts poor response. Cancer. 115:428–436. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Zhu GQ, Shi KQ, Yu HJ, He SY, Braddock M,
Zhou MT, Chen YP and Zheng MH: Optimal adjuvant therapy for
resected hepatocellular carcinoma: A systematic review with network
meta-analysis. Oncotarget. 6:18151–18161. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ono T, Yamanoi A, El Assal Nazmy O, Kohno
H and Nagasue N: Adjuvant chemotherapy after resection of
hepatocellular carcinoma causes deterioration of long-term
prognosis in cirrhotic patients: Metaanalysis of three randomized
controlled trials. Cancer. 91:2378–2385. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Katsura Y, Wada H, Kobayashi S, Marubashi
S, Eguchi H, Tanemura M, Umeshita K, Wakasa K, Doki Y, Mori M and
Nagano H: [A case of complete response to interferon-alpha and S-1
combination therapy for multiple pulmonary recurrences of
hepatocellular carcinoma after hepatic resection]. Gan To Kagaku
Ryoho. 38:2487–2489. 2011.PubMed/NCBI
|
|
9
|
Oh YJ, Park YM, Kim BH, Kim MJ, Cho JH,
Cha CW, Park SJ and Yeon JW: A case of hepatocellular carcinoma
with pulmonary metastases treated successfully with a combination
of repeated hepatic arterial infusion epirubicin and Cisplatin
chemotherapy and systemic low-dose infusion of 5-Fluorouracil. Gut
Liver. 3:343–348. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kilickap S, Kars A, Hamaloglu E and Simsek
H: A case of recurrent metastatic hepatocellular cancer controlled
with immunotherapy and antiviral therapy following resection. Med
Oncol. 26:501–505. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Tanaka K, Yamada K, Sasaki H, Kishi K,
Noura S, Takachi K, Eguchi H, Miyashiro I, Ohue M, Ohigashi H, Yano
M, Ishikawa O and Imaoka S: [A surgical case of solitary lymph node
metastatic recurrence of hepatocellular carcinoma after
hepatectomy]. Gan To Kagaku Ryoho. 33:1938–1940. 2006.PubMed/NCBI
|
|
12
|
Nakamura M, Nagano H, Wada H, Ota H,
Damdinsuren B, Marubashi S, Miyamoto A, Takeda Y, Umeshita K, Dono
K and Monden M: A case of hepatocellular carcinoma with multiple
lung, spleen, and remnant liver metastasis successfully treated by
combination chemotherapy with the novel oral DPD-inhibiting
chemotherapeutic drug S-1 and interferon-alpha. J Gastroenterol.
41:1120–1125. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Nakamura M, Nagano H, Sakon M, Yamamoto T,
Ota H, Wada H, Yoshioka S, Kato H, Damdinsuren B, Marubashi S, et
al: [A case of HCC with inferior caval vein tumor thrombus and
multiple pulmonary metastases that remarkably responded to
combination therapy of TS-1 and interferon-alpha]. Gan To Kagaku
Ryoho. 32:1824–1828. 2005.PubMed/NCBI
|
|
14
|
Nakamura M, Nagano H, Sakon M, Kondo M,
Yamamoto T, Ota H, Wada H, Damdinsuren B, Yang Y, Marubashi S, et
al: [A case of long-term survivor with multiple pulmonary
metastases of HCC after hepatic resection]. Gan To Kagaku Ryoho.
31:1939–1942. 2004.PubMed/NCBI
|
|
15
|
Kanda Y, Akazawa S, Futatsugi K, Fujiki T,
Saifuku K, Yamamoto K, Suzuki F, Nakajima T, Naoi Y, Muraoka M, et
al: [A case of hepatocellular carcinoma (HCC) with lung metastasis
which responded to chemotherapy with a single use of
1-hexylcarbamoyl-5-fluorouracil (HCFU)]. Gan To Kagaku Ryoho.
16:269–272. 1989.PubMed/NCBI
|
|
16
|
Akita H, Marubashi S, Wada H, Hama N,
Kawamoto K, Kobayashi S, Eguchi H, Doki Y, Mori M and Nagano H:
Combination therapy with S-1 and interferon-α in hepatocellular
carcinoma patients with lung metastasis. Mol Clin Oncol. 3:322–328.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Jiang S, Liu Y, Wang L, Duan C and Liu M:
A meta-analysis and systematic review: Adjuvant interferon therapy
for patients with viral hepatitis-related hepatocellular carcinoma.
World J Surg Oncol. 11:2402013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zhang W, Song TQ, Zhang T, Wu Q, Kong DL,
Li Q and Sun HC: Adjuvant interferon for early or late recurrence
of hepatocellular carcinoma and mortality from hepatocellular
carcinoma following curative treatment: A meta-analysis with
comparison of different types of hepatitis. Mol Clin Oncol.
2:1125–1134. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Huang TS, Shyu YC, Chen HY, Yuan SS, Shih
JN and Chen PJ: A systematic review and meta-analysis of adjuvant
interferon therapy after curative treatment for patients with viral
hepatitis-related hepatocellular carcinoma. J Viral Hepat.
20:729–743. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Yang S, Lin Q, Lin W, Hu W and Wang G:
Effect of adjuvant interferon therapy on hepatitis B virus-related
hepatocellular carcinoma: A systematic review. World J Surg Oncol.
14:1592016. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Eun JR, Lee HJ, Moon HJ, Kim TN, Kim JW
and Chang JC: Hepatic arterial infusion chemotherapy using
high-dose 5-fluorouracil and cisplatin with or without
interferon-alpha for the treatment of advanced hepatocellular
carcinoma with portal vein tumor thrombosis. Scand J Gastroenterol.
44:1477–1486. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
O'Shea JJ, Schwartz DM, Villarino AV,
Gadina M, McInnes IB and Laurence A: The JAK-STAT pathway: Impact
on human disease and therapeutic intervention. Annu Rev Med.
66:311–328. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Quiroga AD, de Lujan Alvarez M, Parody JP,
Ronco MT, Carnovale CE and Carrillo MC: Interferon-alpha2b
(IFN-alpha2b)-induced apoptosis is mediated by p38 MAPK in
hepatocytes from rat preneoplastic liver via activation of NADPH
oxidase. Growth Factors. 27:214–227. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Maeda S, Wada H, Naito Y, Nagano H,
Simmons S, Kagawa Y, Naito A, Kikuta J, Ishii T, Tomimaru Y, et al:
Interferon-α acts on the S/G2/M phases to induce apoptosis in the
G1 phase of an IFNAR2-expressing hepatocellular carcinoma cell
line. J Biol Chem. 289:23786–23795. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Schmeisser H, Bekisz J and Zoon KC: New
function of type I IFN: Induction of autophagy. J Interferon
Cytokine Res. 34:71–78. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Comet NR, Aguiló JI, Rathoré MG, Catalán
E, Garaude J, Uzé G, Naval J, Pardo J, Villalba M and Anel A: IFNα
signaling through PKC-θ is essential for antitumor NK cell
function. Oncoimmunology. 3:e9487052014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Lapenta C, Donati S, Spadaro F, Castaldo
P, Belardelli F, Cox MC and Santini SM: NK cell activation in the
antitumor response induced by IFN-α dendritic cells loaded with
apoptotic cells from follicular lymphoma patients. J Immunol.
197:795–806. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Hervas-Stubbs S, Mancheño U, Riezu-Boj JI,
Larraga A, Ochoa MC, Alignani D, Alfaro C, Morales-Kastresana A,
Gonzalez I, Larrea E, et al: CD8 T cell priming in the presence of
IFN-α renders CTLs with improved responsiveness to homeostatic
cytokines and recall antigens: Important traits for adoptive T cell
therapy. J Immunol. 189:3299–3310. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Pacella I, Timperi E, Accapezzato D,
Martire C, Labbadia G, Cavallari EN, D'Ettorre G, Calvo L, Rizzo F,
Severa M, et al: IFN-α promotes rapid human Treg contraction and
late Th1-like Treg decrease. J Leukoc Biol. 100:613–623. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Wang J, Zhu CP, Hu PF, Qian H, Ning BF,
Zhang Q, Chen F, Liu J, Shi B, Zhang X and Xie WF: FOXA2 suppresses
the metastasis of hepatocellular carcinoma partially through matrix
metalloproteinase-9 inhibition. Carcinogenesis. 35:2576–2583. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Martin MD, Carter KJ, Jean-Philippe SR,
Chang M, Mobashery S, Thiolloy S, Lynch CC, Matrisian LM and
Fingleton B: Effect of ablation or inhibition of stromal matrix
metalloproteinase-9 on lung metastasis in a breast cancer model is
dependent on genetic background. Cancer Res. 68:6251–6259. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Wang L, Wu WZ, Sun HC, Wu XF, Qin LX, Liu
YK, Liu KD and Tang ZY: Mechanism of interferon alpha on inhibition
of metastasis and angiogenesis of hepatocellular carcinoma after
curative resection in nude mice. J Gastrointest Surg. 7:587–594.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Sun HC, Tang ZY, Wang L, Qin LX, Ma ZC, Ye
QH, Zhang BH, Qian YB, Wu ZQ, Fan J, et al: Postoperative
interferon alpha treatment postponed recurrence and improved
overall survival in patients after curative resection of
HBV-related hepatocellular carcinoma: A randomized clinical trial.
J Cancer Res Clin Oncol. 132:458–465. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Gong W, Zhang GM, Liu Y, Lei Z, Li D, Yuan
Y, Huang B and Feng ZH: IFN-gamma withdrawal after immunotherapy
potentiates B16 melanoma invasion and metastasis by intensifying
tumor integrin alphavbeta3 signaling. Int J Cancer. 123:702–708.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Hiramatsu N, Yamada R and Takehara T: The
suppressive effect of nucleos(t)ide analogue treatment on the
incidence of hepatocellular carcinoma in chronic hepatitis B
patients. J Gastroenterol Hepatol. 31:546–552. 2016. View Article : Google Scholar : PubMed/NCBI
|