Introduction
Hepatocellular carcinoma (HCC) develops from chronic
liver diseases and it represents a common cancer type worldwide,
with high incidence in East and South Asia (1,2). Various
HCC treatments exist, such as resection, radiofrequency ablation,
irradiation, and chemotherapy, but transarterial chemoembolization
(TACE) is usually performed (3,4), as this
selective intervention may result in a favorable clinical course
(5). However, the prognosis of
multiple HCCs remains poor due to a high recurrence rate and
resistance to chemotherapy (6). In
order to improve the disease prognosis and evaluate the
effectiveness of TACE, the identification of non-invasive
predictive biomarkers is required.
MicroRNAs (miRNAs) are small non-coding RNAs (17–23
nucleotides) that regulate mRNA post-transcriptionally, and many
miRNAs have been reported to be potential predictive biomarkers
(7). miR-122 is one of the miRNAs
highly expressed in liver (8), and a
decrease in its expression in HCC patients was shown to be
associated with hepatocarcinogenesis and poor prognosis (9). Additionally, cyclin G1, a disintegrin
and metalloprotease 17 (ADAM17), and IGF1R are the targets of
miR-122 (10), shown to be
downregulated in HCC tissues (11).
In contrast, miR-21 was shown to be overexpressed in some
malignancies, and it plays an important role in cell proliferation,
invasion, and migration, by suppressing PTEN expression (12).
Exosomes are extracellular vesicles, 40–100 nm
large, which can contain different molecules, including proteins,
DNA, RNA, and miRNAs, but their content does not necessarily mirror
the RNA expression profile and can change in response to cellular
conditions (13). Although the
exosomal miRNAs found in patient sera were shown to be associated
with the clinical features in different malignances (14,15),
TACE-induced changes in the exosomal miRNA expression levels remain
unknown. Therefore, we hypothesized that the exosomal miR-122 and
miR-21 may play a key role in HCC development and progression and
investigated whether the exosomal miRNA ratio can be used as a
predictive marker in HCC patients treated with TACE.
Materials and methods
Patients and samples
Seventy-five HCC patients who underwent TACE as the
initial treatment at Nagasaki University Hospital (Nagasaki, Japan)
from January 2006 to March 2013 were enrolled in this study. This
study was approved by the Research Ethics Committee of Nagasaki
University Hospital, and we obtained informed consent from all
patients. Liver function before the treatment was preserved in all
patients, and patients with Child-Pugh grade C or with the portal
vein obstruction due to tumor thrombosis were excluded from the
study. We used the Barcelona Clinic Liver Cancer staging
classification (BCLC) for clinical staging. The average age at the
treatment was 73 (range, 38–89) and the number of patients with
liver cirrhosis (LC) was 57 (76%) and with chronic hepatitis, 18
(24%) (Table I). HCC patients were
diagnosed by using contrast-enhanced-computed tomography (CE-CT) or
Gd-EOB-DTPA-enhanced magnetic resonance imaging (MRI), which
revealed the nodules with early enhancement in arterial phase and
washout in portal or venous phase. Serum samples were obtained
before TACE and approximately 7 days after the treatment, and they
were preserved at −80°C until exosome extraction.
 | Table I.Clinical characteristics of 75
patients pre-TACE. |
Table I.
Clinical characteristics of 75
patients pre-TACE.
| Characteristic | Median (range) or
number (%) |
|---|
| Age (years) | 73 (38–89) |
| Sex (%) |
|
| Male | 49 (65) |
|
Female | 26 (35) |
| Liver cirrhosis
(%) | 57 (76) |
| Chronic hepatitis
(%) | 18 (24) |
| Etiology (%) |
|
| HBV | 9 (12) |
|
HBV+HCV | 3 (4) |
| HCV | 39 (52) |
| NBNC | 24 (32) |
| BCLC stage (%) |
|
| 0 | 1 (1) |
| A | 36 (48) |
| B | 34 (46) |
| C | 4 (5) |
| Type 2 diabetes
(%) |
|
|
Positive | 30 (40) |
|
Negative | 45 (60) |
| AST (IU/l) | 47 (13–219) |
| ALT (IU/l) | 33 (9–220) |
| HbA1c (%) | 5.5 (4.1–8.4) |
| T-Bil (mg/dl) | 0.9 (0.3–4.2) |
| ALB (g/dl) | 3.5 (2.3–5.0) |
| PT (%) | 75 (47–118) |
| Plt
(×104/l) | 11.1
(2.6–39.7) |
| Child-Pugh
score | 6 (5–9) |
| AFP (pg/ml) | 15.8
(1–126234) |
| DCP (AU/ml) | 96 (7–225827) |
| Tumor diameter
(cm) | 3.4 (1–19.5) |
| Tumor number | 2 (1–10) |
TACE treatment
The conventional TACE was performed using a mixture
of epirubicin hydrochloride or miriplatin hydrate, mitomycin C,
iodine addition products of the fatty acid ethyl esters obtained
from the poppy-seed oil, and a contrast agent, administered into
the tumor vessels. Subsequently, embolization was performed using
gelatin sponge particles if a massive tumor thrombosis did not
appear following the treatment.
Exosome isolation from human serum
samples
ExoQuick (63 µl; System Biosciences, Palo Alto, CA,
USA) was added to 250 µl of cell-free serum samples, and the
mixture was placed at 4°C overnight. The mixture was centrifuged at
1,500 × g for 30 min and the supernatant was removed to obtain the
exosome pellets. The pellets were resuspended in 300 µl of the
lysis buffer for RNA isolation and 5 µl of Caenorhabditis
elegans microRNA (Cel-miR-39, mirVana miRNA mimic;
Thermo Fisher Scientific, Inc., Waltham, MA, USA) was added to
normalize the levels.
RNA isolation and RT-qPCR assay
MirVana miRNA Isolation Kit (Invitrogen; Thermo
Fisher Scientific, Inc.) was used to isolate RNA. TaqMan MicroRNA
Reverse Transcription Kit (Applied Biosystems; Thermo Fisher
Scientific, Inc.) was used for the reverse transcription of miRNA
to cDNA, using miR-122, miR-21 and cel-miR-39 specific primers
(TaqMan MicroRNA Assays; Applied Biosystems; Thermo Fisher
Scientific, Waltham, Inc.).
After the synthesis of cDNA, RT-qPCR was performed
by using TaqMan Universal Mastermix II (Applied Biosystems; Thermo
Fisher Scientific, Inc.) and RT-qPCR reactions were performed using
LightCycler 480 system II (Roche Diagnostics, Basel, Switzerland),
and Cq values were calculated. The assays were performed in
triplicate, and the relative quantification of miRNA expression
levels was performed using the 2−ΔΔCq method
(ΔCq=CqmiR-Cqcel-miR-39) (16). Because of the non-normal distribution,
the logarithmic transformation of the relative expression levels of
exosomal miRNAs was used for analyses. Exosomal miRNA ratio was
defined as the fold change of ΔCq (miR ratio=ΔCq miR after TACE/ΔCq
miR before TACE).
Statistical analysis
Clinicopathological data are presented as median
(range). JMP Pro 11.2.0 (SAS Institute Inc., Cary, NC, USA) was
used for the statistical analysis. The Student's t-test was used to
analyze paired data, while the correlations were analyzed using the
Pearson's correlation coefficient. The cumulative survival rate was
calculated using Kaplan-Meier method and the difference in the
survival time between two groups was assessed with log-rank test.
Univariate and multivariate Cox proportional hazard analyses were
used to calculate Cox proportional hazard ratio between disease
specific survival and clinical parameter. P-values were bilaterally
tested, and P<0.05 was considered to indicate a statistically
significant difference.
Results
TACE-induced exosomal miRNA
alterations and the correlations between the pre-TACE exosomal
miRNA levels and clinical parameters
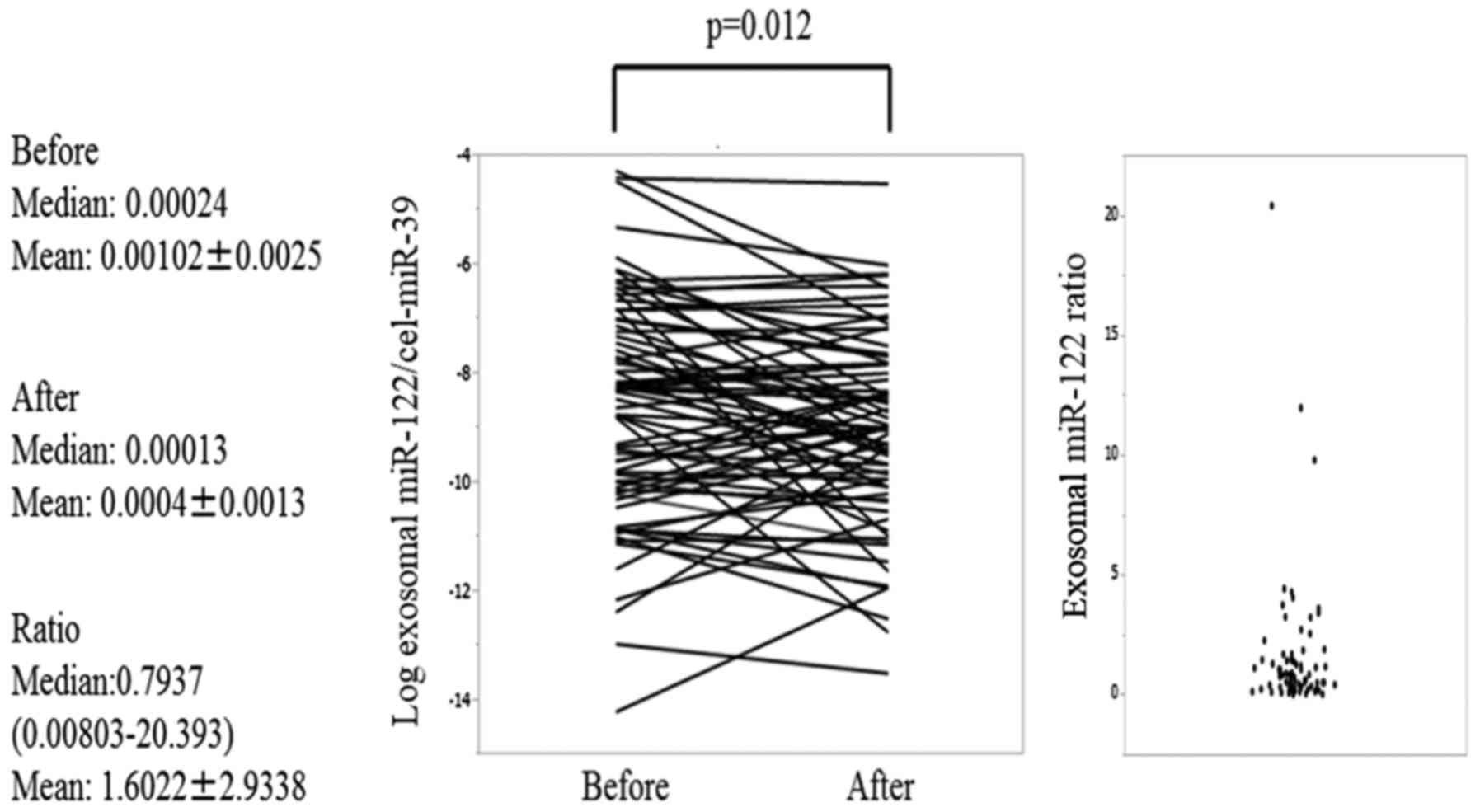
Exosomal miR-122 expression levels were shown to be
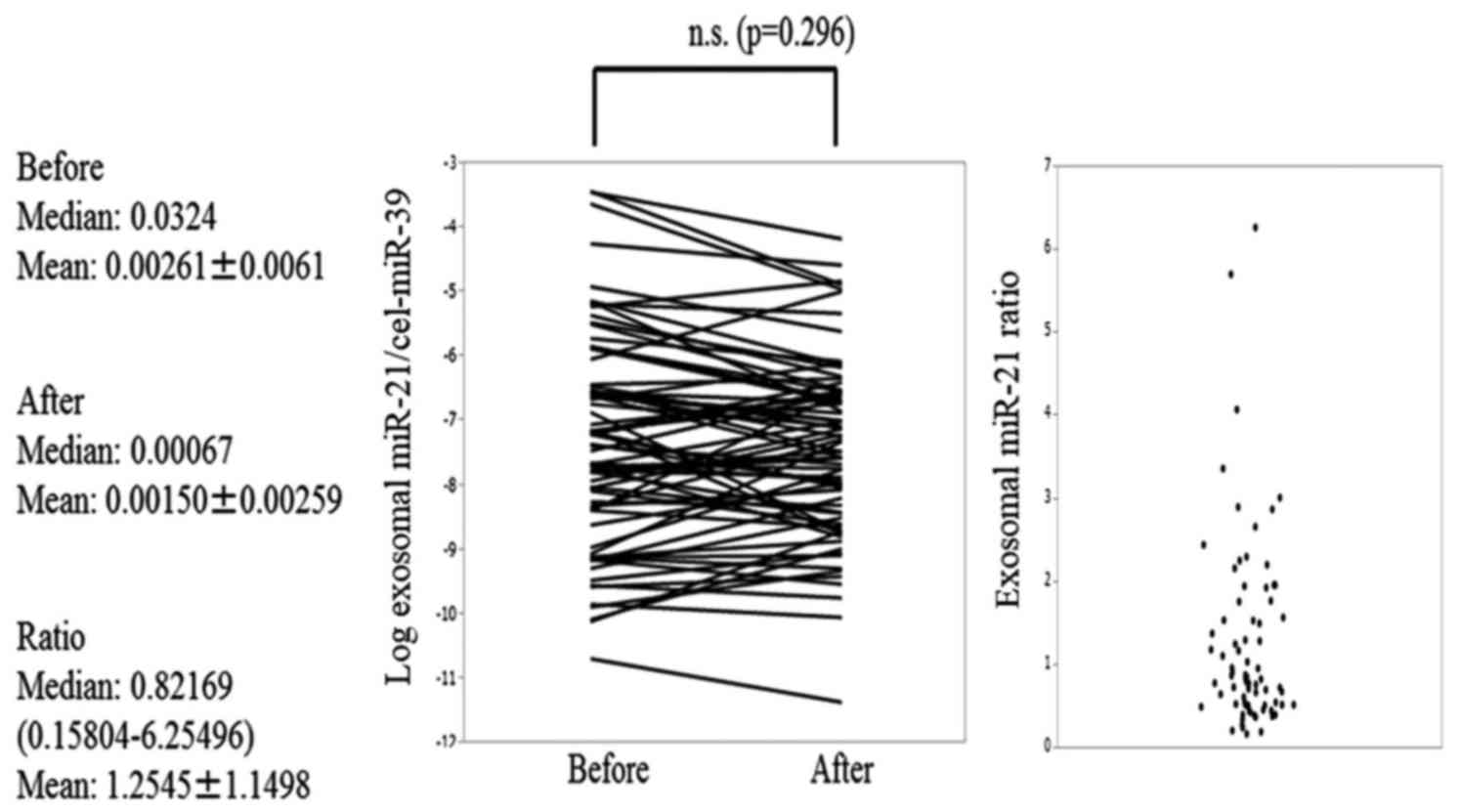
significantly decreased after TACE (P=0.012) (Fig. 1), but the exosomal miR-21 expression
levels did not change (Fig. 2). The
expression levels of exosomal miR-122 before TACE were shown to be
significantly correlated with aspartate aminotransferase (AST)
levels (r=0.31, P=0.004), alanine aminotransferase (ALT) levels
(r=0.33, P=0.003), tumor diameter (r=0.29, P=0.010), and Child-Pugh
score (r=−0.28, P=0.013; Table II).
Exosomal miR-21 pre-TACE levels were shown to correlate with
prothrombin time (r=0.38, P<0.001) and Child-Pugh score
(r=−0.30, P=0.006; Table III).
 | Table II.Correlation between pre-TACE exosomal
miR-122 levels and clinical parameters. |
Table II.
Correlation between pre-TACE exosomal
miR-122 levels and clinical parameters.
| Variable | r | Case number | Lower 95% CI | Upper 95% CI | P-value |
|---|
| Age (years) | −0.1594 | 75 | −0.3729 | 0.0701 | 0.172 |
| HbA1c (%) | 0.1528 | 70 | −0.0852 | 0.3743 | 0.206 |
| AST (IU/l) | 0.3144 | 75 | 0.0942 | 0.5053 | 0.006 |
| ALT (IU/l) | 0.3365 | 75 | 0.1186 | 0.5235 | 0.003 |
| T-Bil (mg/dl) | −0.1892 | 75 | −0.399 | 0.0395 | 0.104 |
| ALB (g/dl) | 0.1092 | 75 | −0.1208 | 0.328 | 0.351 |
| PT (%) | 0.2209 | 73 | −0.0097 | 0.4291 | 0.060 |
| Plt
(×104/l) | 0.1423 | 75 | −0.0874 | 0.3578 | 0.223 |
| Child Pugh
score | −0.2838 | 75 | −0.4798 | −0.0607 | 0.013 |
| AFP (pg/ml) | 0.1893 | 75 | −0.0394 | 0.3991 | 0.103 |
| DCP (AU/ml) | 0.2194 | 73 | −0.0112 | 0.4279 | 0.062 |
| Tumor diameter
(cm) | 0.2933 | 75 | 0.0711 | 0.4878 | 0.010 |
| Tumor number | 0.1461 | 75 | −0.0837 | 0.3611 | 0.211 |
 | Table III.Correlation between pre-TACE exosomal
miR-21 levels and clinical parameters. |
Table III.
Correlation between pre-TACE exosomal
miR-21 levels and clinical parameters.
| Variable | r | Case number | Lower 95% CI | Upper 95% CI | P-value |
|---|
| Age (years) | 0.0487 | 75 | −0.1802 | 0.2727 | 0.678 |
| HbA1c (%) | 0.1242 | 70 | −0.1141 | 0.349 | 0.305 |
| AST (IU/l) | 0.1937 | 75 | −0.0348 | 0.403 | 0.095 |
| ALT (IU/l) | 0.2248 | 75 | −0.0023 | 0.4298 | 0.052 |
| T-Bil (mg/dl) | −0.1781 | 75 | −0.3893 | 0.051 | 0.126 |
| ALB (g/dl) | 0.1932 | 75 | −0.0353 | 0.4025 | 0.096 |
| PT (%) | 0.3846 | 73 | 0.1696 | 0.5647 | <0.001 |
| Plt
(×104/l) | 0.1831 | 75 | −0.0457 | 0.3937 | 0.115 |
| Child Pugh
score | −0.3093 | 75 | −0.5011 | −0.0886 | 0.006 |
| AFP (pg/ml) | 0.1495 | 75 | −0.0802 | 0.3641 | 0.200 |
| DCP (AU/ml) | 0.1168 | 73 | −0.1164 | 0.3378 | 0.325 |
| Tumor diameter
(cm) | 0.1263 | 75 | −0.1036 | 0.3434 | 0.280 |
| Tumor number | −0.0639 | 75 | −0.2867 | 0.1655 | 0.586 |
Exosomal miR-122 and miR-21 pre-TACE expression
levels did not significantly differ between the LC and chronic
hepatitis groups. However, exosomal miR-122 pre-TACE expression
levels were significantly higher in the Child-Pugh grade A patients
than in the Child-Pugh grade B patients (ANOVA, P=0.0090). Similar
results were obtained when miR-21 expression was analyzed (ANOVA,
P=0.0022).
Association between clinical
parameters and exosomal miRNA levels and disease-specific
survival
The MST for all patients was 47 months. Univariate
analysis of factors associated with survival demonstrated that
Child-Pugh grade B (hazard ratio (HR), 2.495; 95% confidence
interval (CI), 1.186–5.218; P=0.016), α-fetoprotein (AFP) levels
>20 pg/ml (HR, 2.186; 95% CI, 1.061–4.609; P=0.034),
des-gammma-carboxyprothrombin (DCP) levels >40 AU/ml (HR, 3.240,
95% CI, 1.248–11.06; P=0.013), and tumor diameter >3 cm (HR,
2.426; 95% CI, 1.132–5.637; P=0.022) represent the risk factors.
Multivariate Cox proportional hazard regression results showed that
Child-Pugh grade B (HR, 5.172; 95% CI, 2.194–12.586; P=0.0002), AFP
>20 pg/ml (HR, 2.667; 95% CI, 1.197–6.147; P=0.0163), DCP >40
AU/ml (HR, 4.695; 95% CI, 1.674–17.051), and tumor diameter >3
cm (HR, 2.844; 95% CI, 1.266–6.931; P=0.0106) represent independent
risk factors. However, exosomal miR-122 and miR-21 expression
levels, exosomal miR-122 ratio, and exosomal miR-21 ratio were not
shown to be associated with the disease-specific survival.
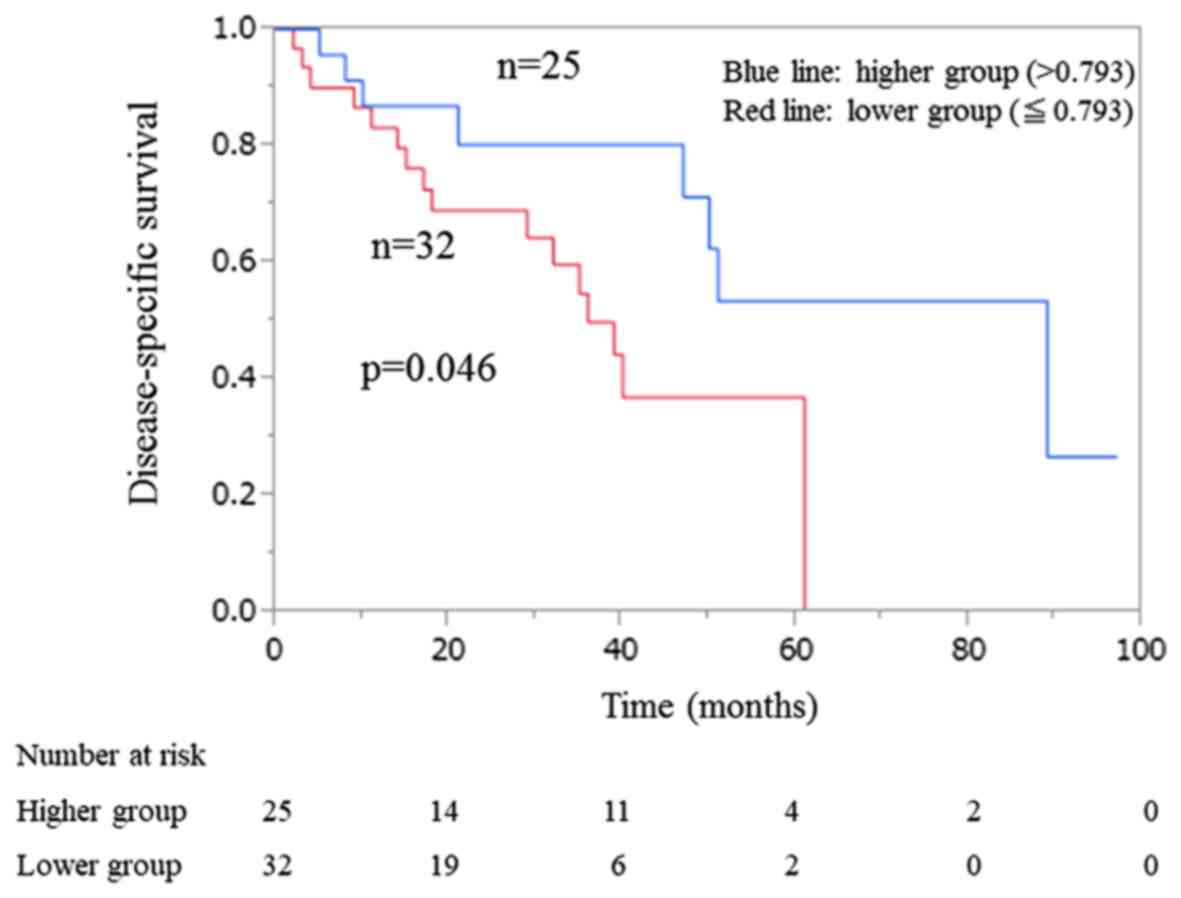
In the limited LC group (n=57), according to median
exosomal miR-122 ratio, the patients with the higher ratio showed a
significantly longer disease-specific survival than that observed
in the group with the lower ratio (P=0.0461) (Fig. 3). No significant difference in
disease-specific survival was observed between the patients with
the higher and lower exosomal miR-21 ratios. Univariate Cox
proportional hazards regression revealed that Child-Pugh grade B
(HR, 2.372; 95% CI, 1.011–5.685; P=0.047), DCP >40 AU/ml (HR,
2.810; 95% CI, 1.046–9.763; P=0.0396), tumor diameter >3 cm (HR,
2.554; 95% CI, 1.101–6.387; P=0.0287), and the decrease in the
exosomal miR-122 ratio (HR, 2.490; 95% CI, 1.028–6.689; P=0.0429)
represent risk factors (Table IV).
Multivariate Cox proportional hazards regression results
demonstrated here that the Child-Pugh grade B (HR, 3.588; 95% CI,
1.446–9.224; P=0.006), tumor diameter >3 cm (HR, 3.606; 95% CI,
1.484–9.505; P=0.004), and lower exosomal miR-122 ratio (HR, 2.720;
95% CI, 1.035–8.022; P=0.042) represent independent factors
associated with poor prognosis.
 | Table IV.Factors associated with the
disease-specific survival in liver cirrhosis patients (n=57). |
Table IV.
Factors associated with the
disease-specific survival in liver cirrhosis patients (n=57).
| Variable | HR (95% CI) | P-value | HR (95% CI) | P-value |
|---|
| Sex (male vs.
female) | 0.545
(0.236–1.320) | 0.172 | – | – |
| Age (years >60
vs. ≤60) | 0.678
(0.300–1.623) | 0.369 | – | – |
| Type 2 diabetes
(positive vs. negative) | 0.433
(0.155–1.044) | 0.063 | – | – |
| HBV (positive vs.
negative) | 2.144
(0.610–5.896) | 0.209 | – | – |
| AST (>50 vs. ≤50
U/l) | 1.158
(0.489–2.664) | 0.731 | – | – |
| ALT (>50 vs. ≤50
U/l) | 0.838
(0.301–2.029) | 0.708 | – | – |
| Child-Pugh grade (B
vs. A) | 2.372
(1.011–5.685) | 0.047 | 3.588
(1.446–9.224) | 0.006 |
| AFP (>20 vs. ≤20
pg/ml) | 2.225
(0.968–5.378) | 0.059 |
|
|
| DCP (>40 vs ≤40
AU/ml) | 2.810
(1.046–9.763) | 0.039 | 2.960
(0.984–11.238) | 0.053 |
| Tumor diameter
(>3 vs. ≤3 cm) | 2.554
(1.101–6.387) | 0.028 | 3.606
(1.484–9.505) | 0.004 |
| Tumor number (≥2
vs. 1) | 1.007
(0.412–2.816) | 0.987 | – | – |
| BCLC (B+C vs
0+A) | 1.108
(0.492–2.585) | 0.804 | – | – |
| Exo miR122
(≤0.00024 vs. >0.00024) | 0.754
(0.329–1.743) | 0.502 | – | – |
| Exo miR122 ratio
(≤0.793 vs. >0.793) | 2.490
(1.028–6.689) | 0.042 | 2.720
(1.035–8.022) | 0.042 |
| Exo miR21 (≤0.00064
vs. >0.00064) | 1.049
(0.466–2.407) | 0.906 | – | – |
| Exo miR21 ratio
(≤0.82169 vs. >0.82169) | 1.293
(0.564–2.996) | 0.539 | – | – |
Discussion
Treatment strategy for HCC patients requires
obtaining the accurate data showing the tumor stage and residual
liver function, due to the potential post-treatment liver failure.
Therefore, biomarkers that may provide liver-specific information
are required. Several studies analyzed the pre-treatment expression
levels of exosomal miRNAs in HCC patients (17,18),
however, there are no reports describing the alterations in miRNA
levels after TACE. To the best of our knowledge, this is the first
report showing that the exosomal miRNA ratio affects the prognosis
of LC patients.
Liver function usually deteriorates after TACE, due
to the damaging of the non-cancerous tissue. We showed that the
post-TACE exosomal miR-122 levels significantly decreased, and no
correlation between exosomal miRNA levels and conventional tumor
marker levels was observed. The expression levels of miR-122 after
TACE significantly inversely correlated with the Child-Pugh score
(r=−0.24, P=0.0344), however, these changes did not correlate with
the changes in the standard liver function tests. These results
suggest that the decline in exosomal miR-122 levels may reflect a
decrease in the liver function, rather than the anti-tumor effects
of this procedure. In contrast, exosomal miR-21 levels were not
significantly altered after TACE. A previous report showed the
increased expression of serum exosomal miR-21 in HCC patients with
HBV (19) but no previous studies
reported the changes in the exosomal miR-21 level following the
TACE treatment.
Recent studies demonstrated that the circulating
miR-122 levels are associated with the liver damage and ALT levels
(20–23). In our study, the expression levels of
serum exosomal miR-122 before TACE were shown to correlate
significantly with AST and ALT levels,. Pre-TACE exosomal miR-122
levels were shown to be negatively correlated with the Child-Pugh
score, indicating that the expression levels of exosomal miR-122
reflect liver function and liver fibrosis rate. In our previous
study, we showed that a decrease in serum miR-122 levels correlates
with the development of severe fibrosis in patients with
non-alcoholic fatty liver disease (NAFLD) (24), while Morita et al (25) showed that the hepatic miR-122 levels
in patients with HCV are negatively correlated with the functional
liver damage. If exosomal miRNA levels mirror those in the parental
cells, the obtained result may indicate that the exosomal miR-122
levels reflect residual liver function and capacity.
Pre-TACE expression levels of exosomal miR-122 did
not significantly differ between the chronic hepatitis and LC
patient groups. miR-122 was shown to be associated with the liver
fibrosis rate (24,26,27) and
viral replication rate (28–30) and therefore, the heterogeneous patient
background may affect the obtained results. Furthermore, no
significant correlation was observed between the expression levels
of exosomal miR-21 and the BCLC stage. Exosomal miR-21 levels were
shown to be associated with prothrombin time and Child-Pugh score,
and therefore, this molecule can represent a less specific
prognostic biomarker than exosomal miR-122 in HCC patients.
The mechanisms of action and functions of miR-122,
especially post-treatment, are not well-known. In hypoxic condition
after embolization, this molecule may induce hypoxia inducible
factor 1α (HIF-1α) expression in non-cancerous liver tissue and
cancer cells. HIF-1α and vimentin represent miR-122 targets in
hepatocytes (31), while the reduced
liver miR-122 levels were shown to be associated with increased
HIF-1α levels in the diet-induced steatohepatitis mouse model.
There is a possibility that the changes in miR-122 levels are
associated with epithelial-mesenchymal transition (EMT), however,
we were not able to show whether the EMT is associated with decline
of miR-122 in this study. A recent study demonstrated that miR-122
inhibits the EMT by targeting Snail and WNT/β-cadherin signaling
pathway (32). Additionally, miR-122
plays a key role in mitochondrial metabolism by indirectly
regulating mitochondrial genes (33),
such as PPARGC1A (PGC-1a). The loss of miR-122 can result in a
damaged liver function (33) and it
was shown to be associated with the HCC patient mortality.
In all patients, exosomal miR-122 and miR-21 levels,
and miRNA ratios were not shown to be independent factors
associated with the disease-specific survival. However, in HCC
patients with LC, lower exosomal miR-122 ratio was shown to be an
independent factor for poor prognosis. A previous report showed
that serum miR-122 levels negatively correlate with the model of
end-stage liver disease (MELD) score (34) and are associated with poor prognosis
in decompensated liver disease patients (35). Moreover, in HCV-induced fibrosis, the
decrease in circulating miR-122 reflects the development of liver
fibrosis and the loss of viable liver cells (36). Therefore, liver fibrosis contributes
to the liver function decline, and our results indicate that
exosomal miR-122 levels, especially in LC patients, may serve as
important post-TACE predictive biomarkers. Since a considerably
higher decline in exosomal miR-122 levels after TACE occurs in
patients with LC than in those with chronic hepatitis, we
hypothesize that the group with a more prominent decrease in
exosomal miR-122 levels after TACE has a lower survival rate.
Several limitations of this study should be noted.
To determine tumor-specific exosomal miRNAs, exosomal miRNA levels
in the sera of patients without HCC, but with chronic liver
diseases, should be determined. Our study was retrospective, with a
somewhat small sample size, and it included advanced chronic
hepatitis cases, because all patients with preserved liver function
underwent the surgical procedure.
In conclusion, exosomal miR-122 levels may reflect
the liver damage and residual liver function levels. This is the
first report showing that the post-TACE expression levels of
exosomal miR-122 decrease, especially in the LC patients.
Additionally, lower miR-122 ratio was shown to be associated with
poor prognosis. Serum exosomal miRNA levels after treatment may
represent novel biomarkers guiding the decision-making during the
treatment of HCC patients.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
HM designed the study, developed the methodology and
reviewed the final version of the manuscript. TS performed data
analyses, curated and visualized data, and wrote the first draft.
TS and YK performed the experiments. KN supervised the study. All
authors read and approved the final version of the manuscript.
Resources: HS, TH, EO, SM, NT and KN. HS, TH, EO, SM, NT and KN
contributed to the acquisition and interpretation of data
Ethics approval and consent to
participate
This study was approved by the Research Ethics
Committee of Nagasaki University Hospital (no. 16042513), and
informed consent was obtained from all patients.
Consent for publication
Written informed consents was obtained from all
patients included in the study.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Hashim D, Boffetta P, La Vecchia C, Rota
M, Bertuccio P, Malvezzi M and Negri E: The global decrease in
cancer mortality: Trends and disparities. Ann Oncol. 27:926–933.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Bertuccio P, Turati F, Carioli G,
Rodriguez T, La Vecchia C, Malvezzi M and Negri E: Global trends
and predictions in hepatocellular carcinoma mortality. J Hepatol.
67:302–309. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Sangiovanni A and Colombo M: Treatment of
hepatocellular carcinoma: Beyond international guidelines. Liver
Int. 36 Suppl 1:S124–S129. 2016. View Article : Google Scholar
|
|
4
|
Han K and Kim JH: Transarterial
chemoembolization in hepatocellular carcinoma treatment: Barcelona
clinic liver cancer staging system. World J Gastroenterol.
21:10327–10335. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Murata S, Mine T, Ueda T, Nakazawa K,
Onozawa S, Yasui D and Kumita S: Transcatheter arterial
chemoembolization based on hepatic hemodynamics for hepatocellular
carcinoma. ScientificWorldJournal. 2013:4798052013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Peck-Radosavljevic M: Drug therapy for
advanced-stage liver cancer. Liver Cancer. 3:125–131. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Karakatsanis A, Papaconstantinou I,
Gazouli M, Lyberopoulou A, Polymeneas G and Voros D: Expression of
microRNAs, miR-21, miR-31, miR-122, miR-145, miR-146a, miR-200c,
miR-221, miR-222, and miR-223 in patients with hepatocellular
carcinoma or intrahepatic cholangiocarcinoma and its prognostic
significance. Mol Carcinog. 52:297–303. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Girard M, Jacquemin E, Munnich A, Lyonnet
S and Henrion-Caude A: miR-122, a paradigm for the role of
microRNAs in the liver. J Hepatol. 48:648–656. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Li C, Deng M, Hu J, Li X, Chen L, Ju Y,
Hao J and Meng S: Chronic inflammation contributes to the
development of hepatocellular carcinoma by decreasing miR-122
levels. Oncotarget. 7:17021–17034. 2016.PubMed/NCBI
|
|
10
|
Fornari F, Gramantieri L, Giovannini C,
Veronese A, Ferracin M, Sabbioni S, Calin GA, Grazi GL, Croce CM,
Tavolari S, et al: miR-122/cyclin G1 interaction modulates p53
activity and affects doxorubicin sensitivity of human
hepatocarcinoma cells. Cancer Res. 69:5761–5767. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Tsai WC, Hsu PW, Lai TC, Chau GY, Lin CW,
Chen CM, Lin CD, Liao YL, Wang JL, Chau YP, et al: MicroRNA-122, a
tumor suppressor microRNA that regulates intrahepatic metastasis of
hepatocellular carcinoma. Hepatology. 49:1571–1582. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Meng F, Henson R, Wehbe-Janek H, Ghoshal
K, Jacob ST and Patel T: MicroRNA-21 regulates expression of the
PTEN tumor suppressor gene in human hepatocellular cancer.
Gastroenterology. 133:647–658. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Huang X, Yuan T, Tschannen M, Sun Z, Jacob
H, Du M, Liang M, Dittmar RL, Liu Y, Liang M, et al:
Characterization of human plasma-derived exosomal RNAs by deep
sequencing. BMC Genomics. 14:3192013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Matsumura T, Sugimachi K, Iinuma H,
Takahashi Y, Kurashige J, Sawada G, Ueda M, Uchi R, Ueo H, Takano
Y, et al: Exosomal microRNA in serum is a novel biomarker of
recurrence in human colorectal cancer. Br J Cancer. 113:275–281.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Manier S, Liu CJ, Avet-Loiseau H, Park J,
Shi J, Campigotto F, Salem KZ, Huynh D, Glavey SV, Rivotto B, et
al: Prognostic role of circulating exosomal miRNAs in multiple
myeloma. Blood. 129:2429–2436. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Fornari F, Ferracin M, Trerè D, Milazzo M,
Marinelli S, Galassi M, Venerandi L, Pollutri D, Patrizi C, Borghi
A, et al: Circulating microRNAs, miR-939, miR-595, miR-519d and
miR-494, Identify Cirrhotic Patients with HCC. PLoS One.
10:e01414482015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Sohn W, Kim J, Kang SH, Yang SR, Cho JY,
Cho HC, Shim SG and Paik YH: Serum exosomal microRNAs as novel
biomarkers for hepatocellular carcinoma. Exp Mol Med. 47:e1842015.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Wang H, Hou L, Li A, Duan Y, Gao H and
Song X: Expression of serum exosomal microRNA-21 in human
hepatocellular carcinoma. Biomed Res Int.
2014:8648942014.PubMed/NCBI
|
|
20
|
Ding X, Ding J, Ning J, Yi F, Chen J, Zhao
D, Zheng J, Liang Z, Hu Z and Du Q: Circulating microRNA-122 as a
potential biomarker for liver injury. Mol Med Rep. 5:1428–1432.
2012.PubMed/NCBI
|
|
21
|
Laterza OF, Lim L, Garrett-Engele PW,
Vlasakova K, Muniappa N, Tanaka WK, Johnson JM, Sina JF, Fare TL,
Sistare FD, et al: Plasma MicroRNAs as sensitive and specific
biomarkers of tissue injury. Clin Chem. 55:1977–1983. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Bala S, Petrasek J, Mundkur S, Catalano D,
Levin I, Ward J, Alao H, Kodys K and Szabo G: Circulating microRNAs
in exosomes indicate hepatocyte injury and inflammation in
alcoholic, drug-induced, and inflammatory liver diseases.
Hepatology. 56:1946–1957. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Bihrer V, Friedrich-Rust M, Kronenberger
B, Forestier N, Haupenthal J, Shi Y, Peveling-Oberhag J, Radeke HH,
Sarrazin C, Herrmann E, et al: Serum miR-122 as a biomarker of
necroinflammation in patients with chronic hepatitis C virus
infection. Am J Gastroenterol. 106:1663–1669. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Miyaaki H, Ichikawa T, Kamo Y, Taura N,
Honda T, Shibata H, Milazzo M, Fornari F, Gramantieri L, Bolondi L
and Nakao K: Significance of serum and hepatic microRNA-122 levels
in patients with non-alcoholic fatty liver disease. Liver Int.
34:e302–e307. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Morita K, Taketomi A, Shirabe K, Umeda K,
Kayashima H, Ninomiya M, Uchiyama H, Soejima Y and Maehara Y:
Clinical significance and potential of hepatic microRNA-122
expression in hepatitis C. Liver Int. 31:474–484. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Nakao K, Miyaaki H and Ichikawa T:
Antitumor function of microRNA-122 against hepatocellular
carcinoma. J Gastroenterol. 49:589–593. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Halász T, Horváth G, Pár G, Werling K,
Kiss A, Schaff Z and Lendvai G: miR-122 negatively correlates with
liver fibrosis as detected by histology and FibroScan. World J
Gastroenterol. 21:7814–7823. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Conrad KD, Giering F, Erfurth C, Neumann
A, Fehr C, Meister G and Niepmann M: MicroRNA-122 dependent binding
of Ago2 protein to hepatitis C virus RNA is associated with
enhanced RNA stability and translation stimulation. PLoS One.
8:e562722013. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Sendi H: Dual role of miR-122 in molecular
pathogenesis of viral hepatitis. Hepat Mon. 12:312–314. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Jangra RK, Yi M and Lemon SM: Regulation
of hepatitis C virus translation and infectious virus production by
the microRNA miR-122. J Virol. 84:6615–6625. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Csak T, Bala S, Lippai D, Satishchandran
A, Catalano D, Kodys K and Szabo G: microRNA-122 regulates
hypoxia-inducible factor-1 and vimentin in hepatocytes and
correlates with fibrosis in diet-induced steatohepatitis. Liver
Int. 35:532–541. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Jin Y, Wang J, Han J, Luo D and Sun Z:
miR-122 inhibits epithelial-mesenchymal transition in
hepatocellular carcinoma by targeting Snail1 and Snail2 and
suppressing WNT/β-cadherin signaling pathway. Exp Cell Res.
360:210–217. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Burchard J, Zhang C, Liu AM, Poon RT, Lee
NP, Wong KF, Sham PC, Lam BY, Ferguson MD, Tokiwa G, et al:
microRNA-122 as a regulator of mitochondrial metabolic gene network
in hepatocellular carcinoma. Mol Syst Biol. 6:4022010. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Köberle V, Kronenberger B, Pleli T, Trojan
J, Imelmann E, Peveling-Oberhag J, Welker MW, Elhendawy M, Zeuzem
S, Piiper A and Waidmann O: Serum microRNA-1 and microRNA-122 are
prognostic markers in patients with hepatocellular carcinoma. Eur J
Cancer. 49:3442–3449. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Waidmann O, Köberle V, Brunner F, Zeuzem
S, Piiper A and Kronenberger B: Serum microRNA-122 predicts
survival in patients with liver cirrhosis. PLoS One. 7:e456522012.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Trebicka J, Anadol E, Elfimova N, Strack
I, Roggendorf M, Viazov S, Wedemeyer I, Drebber U, Rockstroh J,
Sauerbruch T, et al: Hepatic and serum levels of miR-122 after
chronic HCV-induced fibrosis. J Hepatol. 58:234–239. 2013.
View Article : Google Scholar : PubMed/NCBI
|