Introduction
Colorectal cancer (CRC) is one of the most dangerous
malignancies in the world. In recent years, the morbidity and
mortality of CRC showed an upward trend in China (1). The global cancer statistics in 2011
showed that the incidence of CRC was the third of all malignant
tumors in males and the second of all malignant tumors in females,
while the mortality of CRC ranked third and fourth of all malignant
tumors in females and males (2). It
has been reported that 70% of CRC is associated with somatic
mutation and epigenetic variation (3). Therefore, the exploration of relevant
regulators will provide theoretical basis and further research
directions for the early diagnosis and comprehensive treatment of
CRC in the future.
MicroRNAs (miRNAs) are a group of long small
non-coding RNAs with 19–22 nucleotide. They are involved in the
regulation of many important cellular processes, such as
differentiation, cycle regulation, cell stress and apoptosis.
Approximately 30% of human genes are regulated by miRNAs (4). It is reported that about half of the
miRNA-encoding genes are located at the fragile site related to
tumorigenesis, which are easily deleted, amplified or mutated in
cancer cells (5). miR-329 is located
at 14q32.31, and previous studies have shown that the expression of
miR-329 functions a lot in hippocampal neuronal activity-dependent
dendritic growth (6). Decreased
expression of miR-329 is also observed in glioma cells and tumor
tissues (7). However, the
relationship between miR-329 and CRC has not been reported till
now. In this study, we explored the impact of miR-329 on CRC, which
provides the basis for further study on the mechanism of miR-329
and a potential target for the diagnosis and treatment of CRC.
5-Fluorouracil (5-FU) is a first-line chemotherapy
drug for the treatment of CRC. It inhibits the activity of
pyrimidine nucleotide rate-limiting enzyme-thymidylate synthase and
affects the biosynthesis of deoxythymidylate, thereby suppressing
tumor cell proliferation (8,9). However, large doses of drugs make the
tumor cells prone to drug resistance (10). Our study focused on how to improve the
effect of 5-FU.
E2F family included 8 family members (E2F1-8), which
are important regulatory factors in cellular processes. E2Fs encode
transcriptional regulators and exert essential roles in the G1/S
transition of mammalian cell (11).
E2F1 has both cancer-promoting and tumor-suppressive activities. On
the one hand, E2F1 acts as a target gene activator to induce cell
transition from the G0 to the S phase. On the other hand, it
induces apoptosis as a target gene inhibitor. It has been reported
that miRNA-34 targets E2F-related pathways to induce apoptosis in
cancer cells (12). However, the role
of E2F1 and miR-329 in CRC chemotherapy sensitivity have not been
reported. Therefore, the regulation of E2F1 by miR-329 on the
chemotherapeutic effect of 5-FU is particularly significant.
Materials and methods
miR-329 expression analysis
We used RT-qPCR to access the expression of miR-329
in CRC tissues as well as their corresponding adjacent tissues from
56 patients (aged 65.2 ± 69.5 and ranging berween 47–81 years). A
total of 37 male patients and 19 female patients were diagnosed
with colorectal cancer at the First People's Hospital of Wujiang
District (Suzhou, China) between June 2015 and September 2017.
Among these patients, 35 were graded with stage I+II while another
21 were graded with stage III+IV. Additionally, the expression
level of miR-329 in drug-resistant and non-resistant tumor cells
treated with or without 5-FU were also detected. This study was
approved by the Ethics Committee of the First People's Hospital of
Wujiang District (Suzhou, China).
Cell culture
The HCT116 and SW480 cells were cultured in
Dulbecco's modified Eagle's medium (DMEM) containing 10% fetal
bovine serum (FBS) and 1,000 U/ml penicillin. The cells were
maintained at 37°C with 5% CO2. The cells were digested
and seeded into 6-well plates (2×105/well) and cultured
for 24 h. Transfection of miR-329 mimic was performed in accordance
with the instructions.
CCK-8 assays
The cells in logarithmic growth phase were digested
by trypsin and then inoculated into 96-well plates (100 µl) at a
density of 3×104/ml. After 24 h incubation, the original
medium was replaced by a mixture containing 10% CCK-8 working
solution and then incubated at 37°C with 5% CO2 for 4 h.
The absorbance value at 450 nm was read with a microplate reader
(Bio-Rad, Hercules, CA, USA).
Transwell assays
Cells transfected with or without miR-329 mimic were
seeded onto pretreated Matrigel. The wells with a pore size of 8 µm
were placed in a 24-well plate, and 700 µl of RPMI-1640 medium
containing 15% FBS was added to the lower chamber, 200 µl starved
cells were added to the upper chamber at a density of
2×105/ml. Forty-eight hours later, the supernatant fluid
was removed, and the cells were fixed with 4% paraformaldehyde for
20 min followed by stained with 0.1% crystal violet for 20 min.
After removing the cells stranded in chambers, the cells that
passed through the chamber were observed under an optical
microscope (BX-42; Olympus, Tokyo, Japan) (×100 magnification) and
photographed to calculate the number.
Luciferase reporter gene assays
Cells were cultured in 24-well plates and
transfected with 200 ng luciferase reporter plasmids (QIAGEN,
Duesseldorf, Germany), 50 ng Renilla plasmids or 60 ng
target gene plasmids based on different expression levels,
respectively. Six hours after transfection, Wnt3a or LiCl was added
to stimulate for additional 24–48 h, after which the cells were
lysed and harvested by 120 µl of PLB per well. The cells were then
centrifuged at 1,750 × g for 3 min, and 20 µl of cell lysate
supernatant in each well were measured to achieve the Luciferase
and Renilla fluorescence.
Western blot analysis
Protein samples were separated by polyacrylamide
gels with different concentration at 80 V for 2 h and then
transferred to polyvinylidene fluoride (PVDF) membrane. After
blocked in 5% defatted milk at 37°C for 1 h, the membranes were
incubated with specific primary antibodies at 4°C overnight. Rabbit
polyclonal caspase-3 antibody (dilution, 1:500; cat. no. ab13847),
rabbit polyclonal PARP1 antibody (dilution, 1/500; cat. no.
ab32138) and rabbit monoclonal Bax antibody (dilution, 1/500; cat.
no. ab32503) were all purchased from Abcam (Cambridge, MA, USA).
The membranes were washed with 1% Tris-buffered saline-Tween-20
(TBST) and incubated with secondary goat anti-rabbit (HRP) IgG
antibody (dilution, 1/2,000; cat. no. ab6721) at room temperature
for at least 1 h. After that, these protein bands were subjected to
enhanced chemiluminescence (ECL) and then imaged.
Statistical analysis
We used Statistical Product and Service Solutions
(SPSS) 22.0 software (IBM, Armonk, NY, USA) for statistical
analysis. Chi-square test was used to analyze clinical
classification data. Kaplan-Meier survival curves were introduced
for survival analysis. Independent-samples t-tests between two
groups was performed for statistical test. Comparison between
multiple groups was done using One-way ANOVA test followed by post
hoc test (Least Significant Difference). P<0.05 was considered
to indicate a statistically significant difference.
Results
miR-329 expression decreased in
CRC
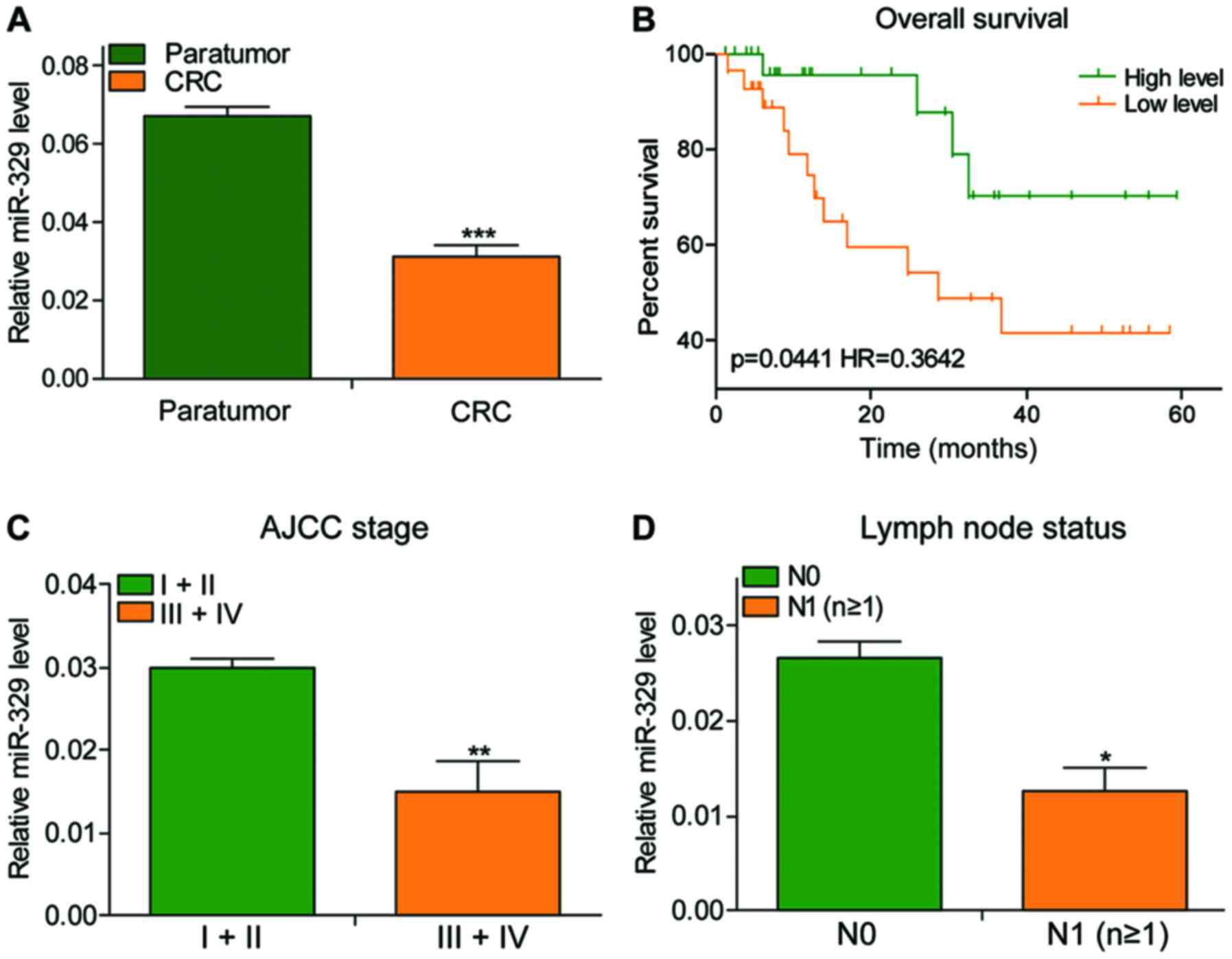
The expression of miR-329 showed a significant
decrease in CRC tissues, especially the tumor tissues at stage
III+IV with lymph node metastasis (Fig.
1A, C and D). The patients' total survival time was positively
associated with the expression of miR-329 (Fig. 1B; P=0.0441, HR=0.3642).
miR-329 inhibited the viability and
invasion of CRC cells
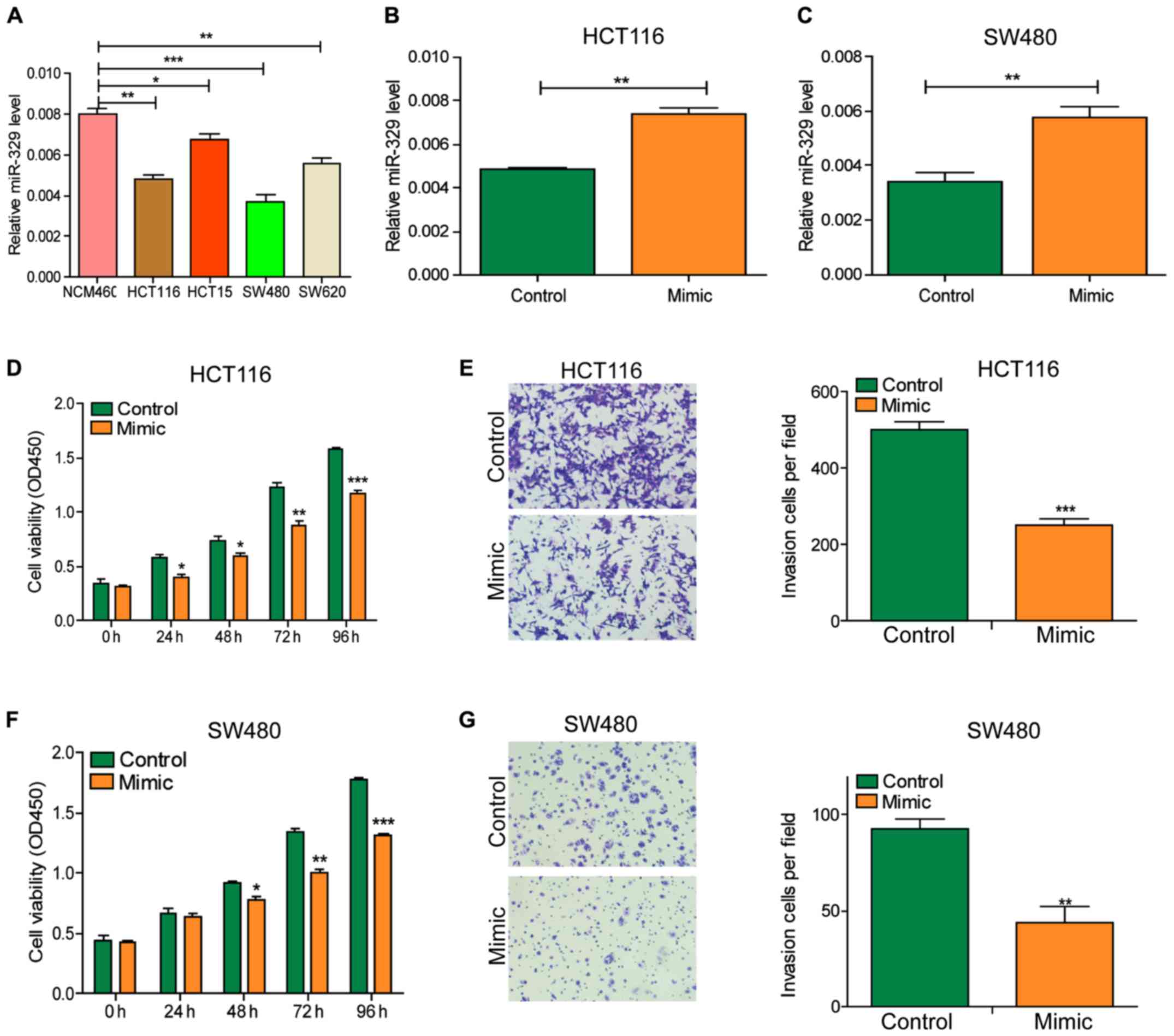
After initially identifying the expression of
miR-329 in CRC tissues, we detected the expression level of miR-329
in cells with RT-qPCR (Fig. 2A). The
results showed that the miR-329 level in tumor cell lines was
significantly lower than that in normal cell lines. Subsequently,
we exogenously increased the level of miR-329 in cells by
transfecting miR-329 mimic into HCT116 and SW480 cells (Fig. 2B and C). CCK-8 and Transwell assays
revealed that the viability and invasion ability of cells were
significantly decreased after miR-329 mimic transfection (Fig. 2D-G).
miR-329 expression level was
positively associated with the sensitivity of cancer cells to
5-FU
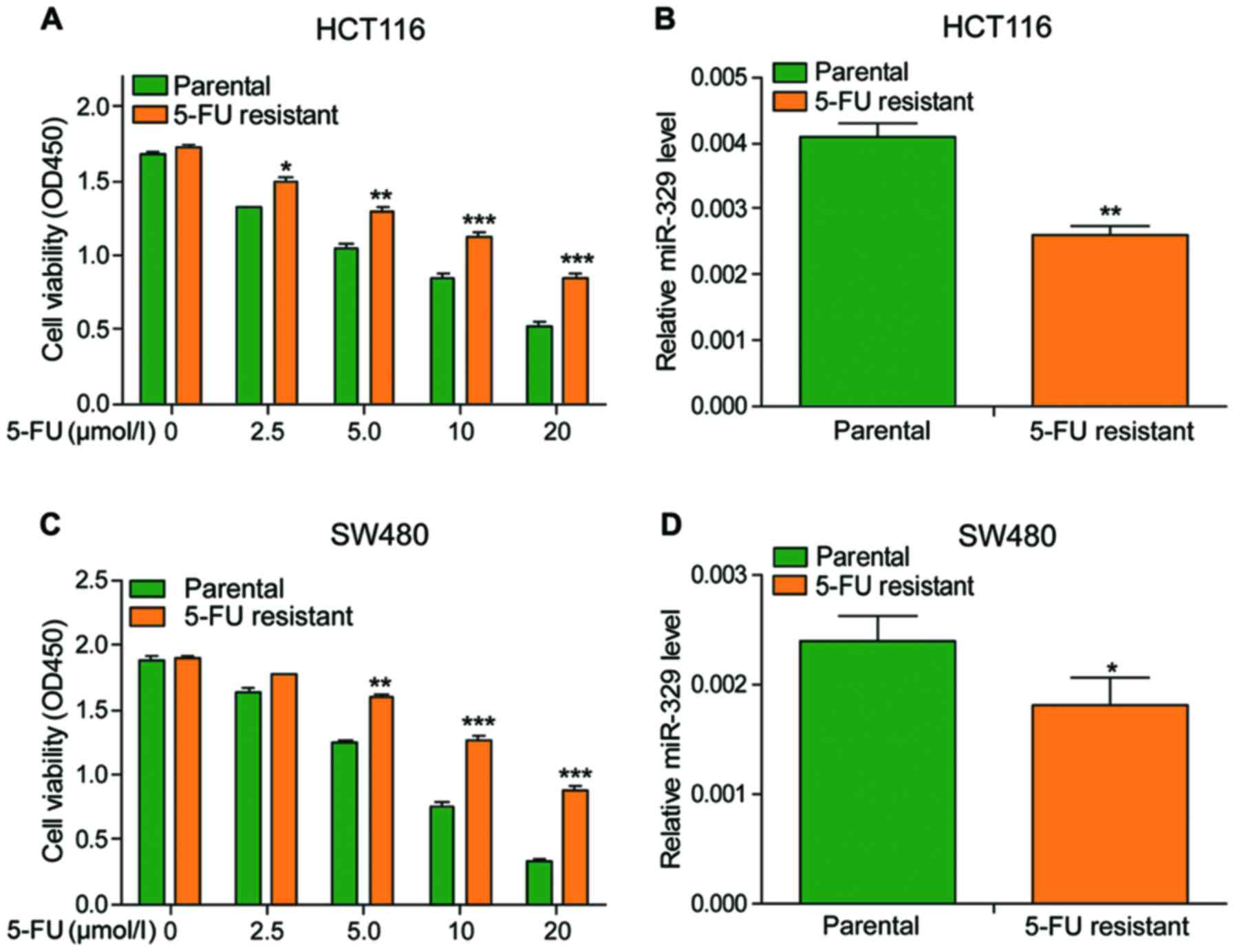
To determine the effects of 5-FU, different
concentrations of 5-FU were used to treat the cells. The viability
of cells declined in a dose-dependent manner and the viability of
drug-resistant cells was significantly higher than that of
non-resistant cells under the same concentration of 5-FU treatment
(Fig. 3A and C). Besides, the
expression of miR-329 was also observed much lower in
drug-resistant cells when compared to non-resistant cells (Fig. 3B and D). The above data indicated that
5-FU could inhibit the vitality of cancer cells, and the
sensitivity of cancer cells to 5-FU is positively associated with
the expression of miR-329.
miR-329 increased the sensitivity of
CRC cells to 5-FU by decreasing the expression of E2F1
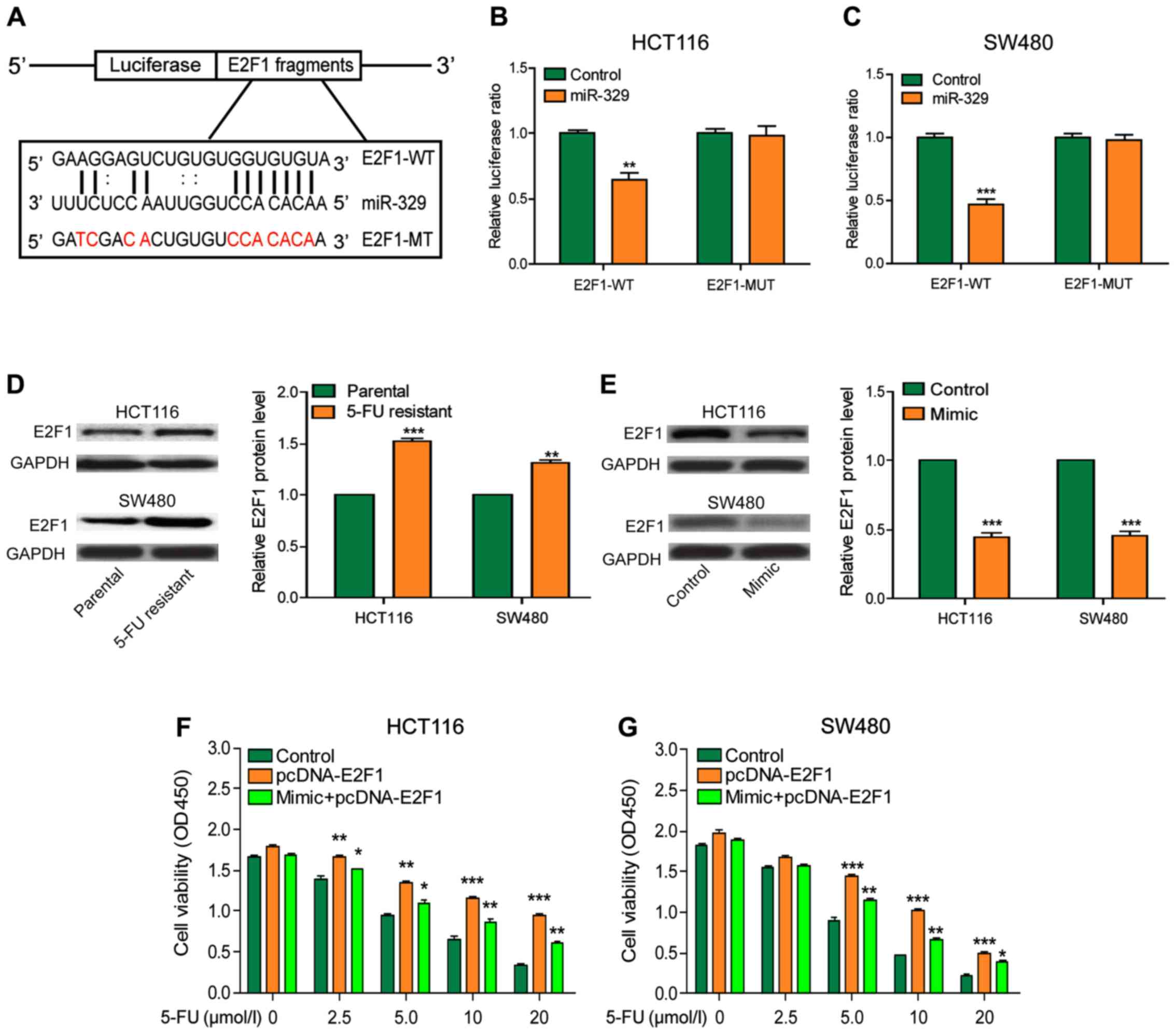
To further explore the mechanism of miR-329 in CRC,
we predicted that E2F1 may be a potential target gene of miR-329 by
searching the bioinformatics website (DIANA, miRanda, PicTar)
(Fig. 4A). The results of luciferase
reporter assay illustrated that miR-329 reduced the relative
luciferase activity of wild-type E2F1 gene but had no effect on the
luciferase activity of mutant reporter plasmids, indicating that
E2F1 was the direct target of miR-329 (Fig. 4B and C). E2F1 expression was
significantly higher in drug-resistant cells than that in the
non-resistant cells (Fig. 4D). After
overexpression of miR-329, E2F1 expression was significantly
decreased in cells (Fig. 4E). The
results of CCK-8 assay indicated that cell viability was markedly
upregulated by the overexpression of E2F1, while the enhanced
expression of miR-329 could partially reverse this effect. The
result of this section indicated that E2F1 could reduce the
chemotherapy effect on CRC, but the effect would be improved after
the upregulation of miR-329 (Fig. 4F and
G).
Discussion
CRC is one of the most common digestive system
malignancies (13). With the progress
of molecular biology, cell biology, immunology and other
disciplines, people's understanding of CRC has gradually extended
to deeper fields. Although chemotherapeutic drugs have been widely
used in the treatment of malignant tumors, the survival status of
patients with tumor metastasis/recurrence remains unsatisfactory
due to the drug resistance of tumor cells and their significant
differences in drug sensitivity (14). Therefore, the deep exploration of the
biological characteristics of CRC will help to further optimize the
treatment strategy and improve the efficacy of the chemotherapeutic
drugs. Recently, miRNAs have been reported to participate in the
development of CRC.
Accumulating evidence indicated that miRNAs may play
an extremely important regulatory role in cells. Various
protein-coding genes can be regulated by only one single miRNA
(15). miRNAs are found to be
dysregulated in many diseases, including cancer. The first study
confirming that miRNAs are tumor-related is in the research on
chronic lymphocytic leukemia (CLL), and the expression level of
miR-15a and miR-16a are downregulated or absent in ~68% of CLL
patients as reported (16).
Subsequent studies also found that miRNA expression is altered in
many other types of tumors, such as gastric cancer, prostate
cancer, esophageal cancer and CRC (17). Our study found that the expression
level of miR-329 was decreased in CRC. The abnormal expression of
miR-329 could significantly reduce the viability and invasion
ability of CRC cells.
In addition to its role in CRC pathology, miRNAs may
also be involved in affecting the sensitivity of CRC to
chemotherapeutic drugs. Lower expression of miR-329 in
drug-resistant cells was observed than that in the non-resistant
cells. Similar to our conclusion, some studies have found that the
upregulation of miR-140 was also related to drug resistance in
tumor treatment, which meant that miRNAs also play an indispensable
role in CRC chemoresistance (18).
The abnormal expression of E2F1 has different
functions in different tumors as reported. Previous studies have
shown that the overexpression of E2F1 results in abnormal
proliferation of cells and promotes tumorigenesis (19). Besides, the E2F1 expression is
elevated in a variety of tumors, for example, lung and breast
cancer (20). In this study, E2F1 was
also observed highly expressed in drug-resistant cells and the
overexpression of E2F1 significantly increased the viability of
cells. However, these phenomena were partially reversed after the
overexpression of miR-329. Moreover, miR-329 reduced the expression
of E2F1 via directly targeting its 3′UTR, thereby enhancing the
killing effect of 5-FU to CRC cells.
In conclusion, our data indicated that the
expression level of miR-329 was reduced in CRC, and the
overexpression of miR-329 promoted the sensitivity of 5-FU in the
chemotherapy of CRC by degrading the target gene E2F1. These
results may provide a theoretical basis for the gene therapy of CRC
and provide an experimental basis for further study of CRC.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
All data generated or analyzed during this study are
included in this published study.
Authors' contributions
JY and HL were responsible for the design and
preparation of the study. XS was responsible for data collection.
ML was responsible for data analysis. FN was responsible for data
interpretation. LX performed RT-qPCR. All authors have read and
approved the final study.
Ethics approval and consent to
participate
This study was approved by the Ethics Committee of
The First People's Hospital of Wujiang District (Suzhou, China).
Signed written informed consents were obtained from the patients
and/or guardians.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Chen W, Zheng R, Baade PD, Zhang S, Zeng
H, Bray F, Jemal A, Yu XQ and He J: Cancer statistics in China,
2015. CA Cancer J Clin. 66:115–132. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Jemal A, Bray F, Center MM, Ferlay J, Ward
E and Forman D: Global cancer statistics. CA Cancer J Clin.
61:69–90. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Hu T, Li Z, Gao CY and Cho CH: Mechanisms
of drug resistance in colon cancer and its therapeutic strategies.
World J Gastroenterol. 22:6876–6889. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Rajewsky N: MicroRNA target predictions in
animals. Nat Genet. 38 Suppl:S8–S13. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Wijnhoven BP, Michael MZ and Watson DI:
MicroRNAs and cancer. Br J Surg. 94:23–30. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Brower JV, Clark PA, Lyon W and Kuo JS:
MicroRNAs in cancer: Glioblastoma and glioblastoma cancer stem
cells. Neurochem Int. 77:68–77. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Møller HG, Rasmussen AP, Andersen HH,
Johnsen KB, Henriksen M and Duroux M: A systematic review of
microRNA in glioblastoma multiforme: Micro-modulators in the
mesenchymal mode of migration and invasion. Mol Neurobiol.
47:131–144. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Yadunandam AK, Yoon JS, Seong YA, Oh CW
and Kim GD: Prospective impact of 5-FU in the induction of
endoplasmic reticulum stress, modulation of GRP78 expression and
autophagy in Sk-Hep1 cells. Int J Oncol. 41:1036–1042. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Deng YH, Pu XX, Huang MJ, Xiao J, Zhou JM,
Lin TY and Lin EH: 5-Fluorouracil upregulates the activity of Wnt
signaling pathway in CD133-positive colon cancer stem-like cells.
Chin J Cancer. 29:810–815. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Hotta T, Takifuji K, Arii K, Yokoyama S,
Matsuda K, Higashiguchi T, Tominaga T, Oku Y and Yamaue H: Clinical
impact of adjuvant chemotherapy on patients with stage III
colorectal cancer: l-LV/5FU chemotherapy as a modified RPMI regimen
is an independent prognostic factor for survival. Anticancer Res.
26(2B): 1425–1432. 2006.PubMed/NCBI
|
|
11
|
Ogawa H, Ishiguro K, Gaubatz S, Livingston
DM and Nakatani Y: A complex with chromatin modifiers that occupies
E2F- and Myc-responsive genes in G0 cells. Science. 296:1132–1136.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Dong F and Lou D: MicroRNA-34b/c
suppresses uveal melanoma cell proliferation and migration through
multiple targets. MOL VIS. 18:537–546. 2012.PubMed/NCBI
|
|
13
|
Westphalen CB, Asfaha S, Hayakawa Y,
Takemoto Y, Lukin DJ, Nuber AH, Brandtner A, Setlik W, Remotti H,
Muley A, et al: Long-lived intestinal tuft cells serve as colon
cancer-initiating cells. J Clin Invest. 124:1283–1295. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Tournigand C, André T, Achille E, Lledo G,
Flesh M, Mery-Mignard D, Quinaux E, Couteau C, Buyse M, Ganem G, et
al: FOLFIRI followed by FOLFOX6 or the reverse sequence in advanced
colorectal cancer: A randomized GERCOR study. J Clin Oncol.
22:229–237. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Wurdinger T and Costa FF: Molecular
therapy in the microRNA era. Pharmacogenomics J. 7:297–304. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Calin GA, Dumitru CD, Shimizu M, Bichi R,
Zupo S, Noch E, Aldler H, Rattan S, Keating M, Rai K, et al:
Frequent deletions and down-regulation of micro-RNA genes miR15 and
miR16 at 13q14 in chronic lymphocytic leukemia. Proc Natl Acad Sci
USA. 99:15524–15529. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Visone R and Croce CM: miRNAs and cancer.
Am J Pathol. 174:1131–1138. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Song B, Wang Y, Xi Y, Kudo K, Bruheim S,
Botchkina GI, Gavin E, Wan Y, Formentini A, Kornmann M, et al:
Mechanism of chemoresistance mediated by miR-140 in human
osteosarcoma and colon cancer cells. Oncogene. 28:4065–4074. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Yamasaki L, Bronson R, Williams BO, Dyson
NJ, Harlow E and Jacks T: Loss of E2F-1 reduces tumorigenesis and
extends the lifespan of Rb1(+/-) mice. Nat Genet. 18:360–364. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zacharatos P, Kotsinas A, Evangelou K,
Karakaidos P, Vassiliou LV, Rezaei N, Kyroudi A, Kittas C,
Patsouris E, Papavassiliou AG, et al: Distinct expression patterns
of the transcription factor E2F-1 in relation to tumour growth
parameters in common human carcinomas. J Pathol. 203:744–753. 2004.
View Article : Google Scholar : PubMed/NCBI
|