Introduction
Papillary thyroid carcinoma (PTC) is one of the most
common types of thyroid cancer, accounting for ~80% of cases
(1). At present, surgery remains the
primary treatment for PTC; however, for patients with tumors <1
cm, or multifocal, highly aggressive or metastasizing tumors, the
outcome of surgery may be unsatisfactory. Therefore, other
therapeutic methods are necessary, particularly immunotherapy
(2).
Studies have indicated that the tumor
microenvironment, which is necessary for maintaining tumor growth,
is comprised of a variety of non-malignant stromal cells.
Macrophages within the tumor microenvironment, termed
tumor-associated macrophages (TAMs), are some of its most important
components (3). The presence of TAMs
is associated with the invasion, angiogenesis, hypoxia and early
metastasis of tumors, and the suppression of adaptive immunity in
various types of tumor, including PTC (4–6). Evidence
from clinical and epidemiological studies also indicates a strong
association between the increased density of TAMs and a poor
prognosis in various kinds of cancer, including breast cancer,
ovarian cancer, glioma, lymphoma and PTC (6–8). Commonly,
TAMs are subdivided into two types, including the classically
activated type 1 macrophages (M1) and the alternatively activated
type 2 macrophages (M2), which are distributed throughout PTC
tissues (9,10). The M1 phenotype macrophages are
activated by interferon (IFN)-γ, lipopolysaccharides (LPS) and
tumor necrosis factor-α (TNF-α) and are characterized by the
production of pro-inflammatory cytokines, including TNF-α,
interleukin (IL)-1β, IL-6, IL-12 and inducible nitric oxide
synthase (iNOS). Furthermore, this phenotype is associated with an
extended survival time in patients with non-small-cell lung cancer
(11,12). However, M2 macrophages, described as
inhibitors of inflammation, are associated with tumor initiation
and progression (11,12). Anti-inflammatory cytokines produced by
M2 macrophages, including IL-10, can reduce the expression of iNOS,
and inhibit antigen presentation and T cell proliferation (13,14).
Furthermore, M2 macrophages are responsible for the growth and
survival of various tumor cells (15,16). TAMs
within a number of types of malignant cancer predominantly exhibit
an M2-like phenotype, which promotes tumor growth and progression
by stimulating tumor cell proliferation; therefore, inhibiting the
functions of TAMs will inhibit tumorigenesis (6,17–19). Thus, it is hypothesized that the
reversal of the polarization of TAMs may be a novel direction for
the treatment of inoperable PTCs.
Bleomycin (BLM), a mixture of cytotoxic glycopeptide
antibiotics isolated from Streptomyces verticillus, is
widely used for anti-tumor treatment, including testicular cancer,
malignant lymphoma, head and neck squamous cell carcinoma, cervical
cancer and skin cancer (20–23). As BLM does not induce pronounced
hepatic, renal or bone marrow toxicity, it is usually used in
combination with other anticancer drugs (24,25).
However, high dose, long-term treatment with BLM can lead to
pulmonary fibrosis (26,27) through the induction of DNA damage
(28,29). Previous studies have demonstrated that
BLM can stimulate the production of proinflammatory cytokines
(IL-18, IL-6 and IL-1β) and chemokines in THP-1 acute monocytic
leukemia cells by activating Toll-like receptor (TLR)2 (30–32),
suggesting that BLM may potentially enhance the anti-tumor role of
macrophages and regulate their polarization. To verify the effect
of low dose BLM treatment on macrophage polarization and the
resulting anti-cancer effects, a model of human macrophage
polarization was established using THP-1 cells. It was determined
that BLM may inhibit the proliferation and invasion of TPC-1 cells,
and facilitate apoptosis via promoting the transition of M2
phenotype macrophages to the M1-like phenotype.
Materials and methods
Reagents
Recombinant human (rh)IL-4, rhIFN-γ and rhIL-13 were
purchased from R&D Systems, Inc., (Minneapolis, MN, USA).
Phorbol myristate acetate (PMA), BLM and LPS were supplied by
Sigma-Aldrich; Merck KGaA (Darmstadt, Germany). BLM solutions were
prepared with endotoxin-free saline to a final concentration of 10
U/ml and stored at −20°C. The anti-human-CD14-peridinin chlorophyll
protein complex (PerCP; cat. no: 340585), CD68-fluorescein
isothiocyanate (FITC; cat. no: 562117), CD80-allophycocyanin (APC;
cat. no: 561134), CCR7-phycoerythrin (PE)-cyanine (cy)7(cat. no:
560922), CD206-PE(cat. no: 555954) and atched isotype antibodies
were all supplied by BD Biosciences (Franklin Lakes, NJ, USA).
FITC-dextran was purchased from Sigma-Aldrich; Merck KGaA.
Cell culture and macrophage
polarization
THP-1 human monocyte cells and TPC-1 human thyroid
carcinoma cells were cultured in RPMI 1640 medium (Invitrogen;
Thermo Fisher Scientific, Inc., Waltham, MA, USA) supplemented with
10% heat-inactivated fetal bovine serum (Invitrogen; Thermo Fisher
Scientific, Inc.) and incubated at 37°C in an atmosphere with 5%
CO2. THP-1 cells were induced to differentiate into an
attached macrophage-like phenotype by stimulation with 100 nM PMA
for 24 h. Subsequently, the cells were cultured in serum-free
RPMI-1640 for an additional 24 h to eliminate the effects of PMA.
The attached THP-1 cells, which corresponded to M0 macrophages,
were polarized to M1 by adding 1 µg/ml LPS and 20 ng/ml rhIFN-γ, or
to M2 by adding 20 ng/ml rhIL-4 and 20 ng/ml rhIL-13. Serum-free
medium was added for a further 24 h to eliminate the effects of
cytokines. The resulting types of macrophages were used in the
subsequent experiments.
BLM cytotoxicity assay against M0
macrophages
The cytotoxic effect of BLM on M0 macrophages was
analyzed by a lactate dehydrogenase (LDH) cytotoxicity assay kit
(Beyotime Institute of Biotechnology, Haimen, China) according to
the manufacturer's protocol. Briefly, 2×104 M0
macrophages were incubated with various concentrations (5, 10, 15,
20 and 25 mU/ml) of BLM. Supernatant was collected after 24 h and
LDH release was quantified. Absorbance was measured at 490 nm by a
microplate reader. All cell culture experiments were performed in
duplicate and repeated five times.
Co-culture of TPC-1 cells with THP-1
macrophages
BLM (5 or 10 mU/ml) was added to successfully
induced M0, M1 or M2 macrophages for 3 days. Subsequently, 5,000
cells TPC-1 cells and macrophages were co-cultured in a Transwell
apparatus (pore size, 0.4 µm; Costar; Corning Incorporated,
Corning, NY, USA) for 3 days.
Flow cytometry
The expression of the cell surface markers CD68,
CD14, CD206, CD80 and CCR7 was used to determine the macrophage
subtypes using a flow cytometer (FACS Canto II; BD Biosciences).
For the apoptosis assays, TPC-1 cells were analyzed by flow
cytometry using an Annexin V-FITC kit (Beyotime Institute of
Biotechnology)-based assay according to the manufacturer's
protocol. For the phagocytosis assay, macrophages were incubated
with FITC-dextran at 4°C and 37°C, and analyzed by flow cytometry.
The phagocytosis rate was determined as follows: Phagocytosis of
cells (%)=Phagocytosis of cells at 37°C (%)-Phagocytosis of cells
at 4°C (%).
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Total RNA was extracted using the RNeasy mini kit
with DNase (Qiagen GmbH, Hilden, Germany) according to the
manufacturer's protocol. Total RNA (2 µg) was reverse-transcribed
using a Transcriptor first strand cDNA synthesis kit (Roche
Diagnostics, Basel, Switzerland). SYBR Green qPCR was performed
using an ABI Prism 7900HT sequence detection system (Applied
Biosystems; Thermo Fisher Scientific, Inc.). The PCR primers were
5′-GAGGCTACGGCGCTGTCA-3′ (forward) and 5′-TCCACGGCCTTGCTCTTG-3′
(reverse) for IL-10; 5′-CAGTGGCAATGAGGATGACTTG-3′ (forward) and
5′-AGTGGTGGTCGGAGATTCGT-3′ (reverse) for IL-1β;
5′-CTTCTGCCTGCTGCACTTTG-3′ (forward) and
5′-GGCCAGAGGGCTGATTAGAGA-3′ (reverse) for TNF-α; and
5′-GGTGAAGGTCGGTGTGAACG-3′ (forward) and 5′-CTCGCTCCTGGAAGATGGTG-3′
(reverse) for GADPH. The fold-change for each gene was calculated
using the 2−ΔΔCq method (33), and the expression levels were
normalized to GADPH.
Wound-healing and proliferation
assays
For in vitro wound-healing assays, wounds
were made in confluent monolayers of TPC-1 cells using a pipette
tip, and images were captured following co-culture with macrophages
for 12 h. For cell proliferation assays, TPC-1 cells were
co-cultured with macrophages at 37°C for 1, 3 and 5 days. MTT
assays were performed at each time point using an MTT kit (Beyotime
Institute of Biotechnology, Shanghai, China) according to the
manufacturer's protocol.
Cell invasion assays
Assays were performed in 24-well plates using a
Transwell apparatus (pore size, 8 µm; Costar; Corning
Incorporated). A total of 5,000 TPC-1 cells were seeded in
serum-free medium in the upper chamber and 50,000 macrophages were
seeded in the lower chamber with medium containing 10% serum. After
incubation for 24 h at 37°C, cells in the upper chamber were
carefully removed with a cotton swab, and lower chamber cells were
fixed in methanol and stained with crystal violet. Images were
captured under a microscope and cells were counted in 5 randomly
selected fields of view.
Statistical analysis
Flow cytometry data analysis was performed using
FlowJo software v.7.6 (Tree Star, Inc., Ashland, OR, USA). All data
were analyzed by one-way analysis of variance (bonfferoni: compare
all pairs of columns) or Mann-Whitney test (non-parametric) using
GraphPad Prism v.5.0 software (GraphPad Software, Inc, La Jolla,
CA, USA). P<0.05 was considered to indicate a statistically
significant difference.
Results
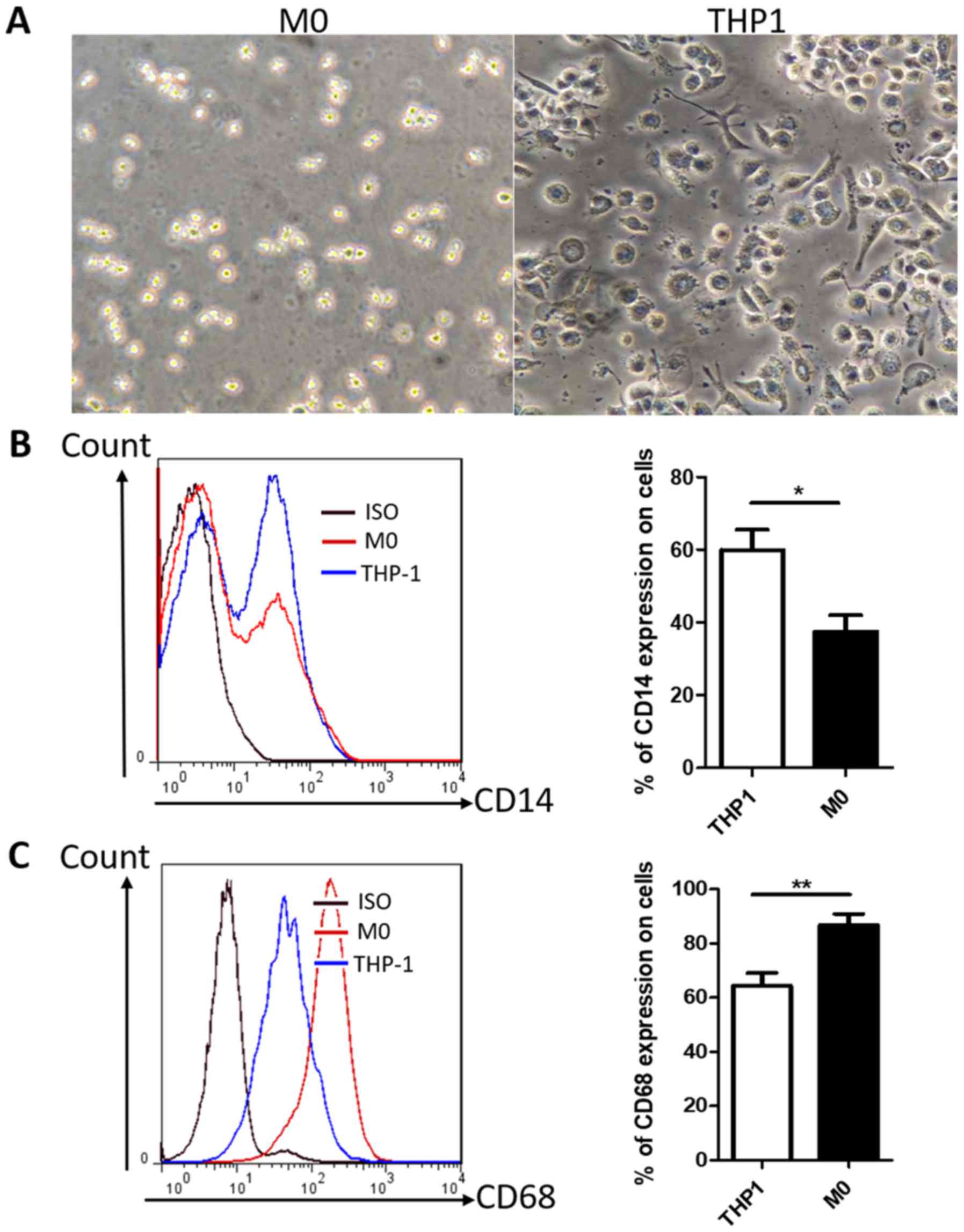
PMA promotes the differentiation of
human THP-1 monocytes into M0 phenotype macrophages
Differentiation of THP-1 monocytes has been widely
used as an in vitro model of human macrophages. Light
microscopy demonstrated that THP-1 cells exhibited a round shape
and a non-adherent growth pattern (Fig.
1A), whereas following treatment with PMA for 24 h, cells
became adherent with the typical flat, amoeboid-shaped, elongated
and branching macrophage morphology (Fig.
1A). The macrophage-like THP-1 cells, hereafter designated as
M0 macrophages, exhibited the downregulation of CD14 and the
upregulation of CD68 compared with untreated THP-1 cells (Fig. 1B and C); this result is consistent
with previous reports (33,34). In addition, the majority of M0
macrophages expressed the CD68 marker, indicating that CD68 may be
a suitable marker for M0 macrophages. Thus, THP-1 monocytes were
successfully differentiated into M0 macrophages by treatment with
PMA.
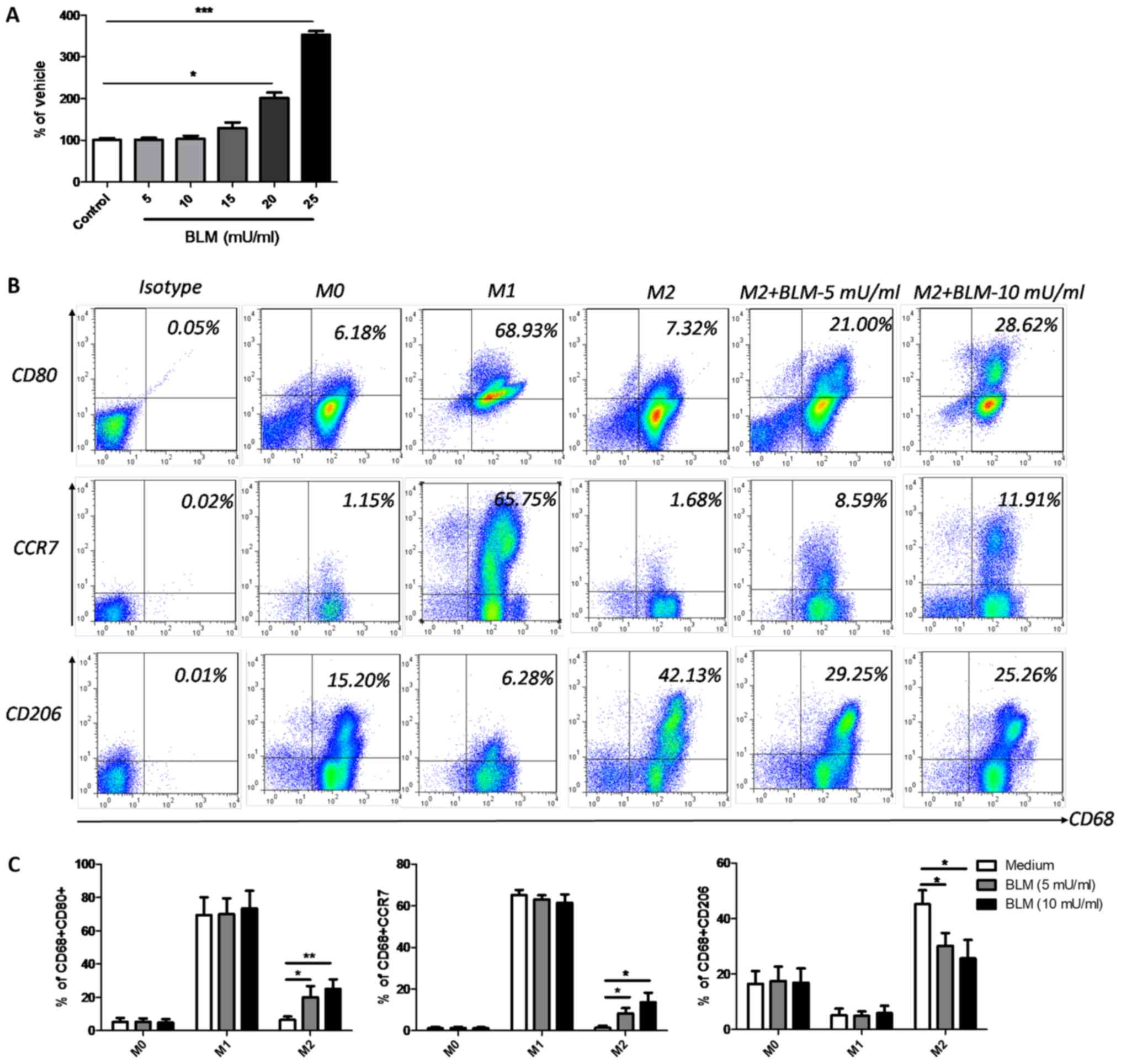
BLM reverses the phenotype of M2
macrophages to M1
THP-1-dervied M0 macrophages were used to further
determine the effects of BLM on macrophage polarization. Firstly,
an LDH cytotoxicity assay was performed using M0 macrophages to
determine the optimal concentration of BLM. The results
demonstrated that BLM at doses lower than 15 mU/ml induced no
significant cytotoxic effects on M0 macrophages. However,
significant cytotoxic effects were observed when BLM was
administered at a concentration of 20 or 25 mU/ml (Fig. 2A). Therefore, low doses of BLM (5 or
10 mU/ml) were selected for further experiments. Following a
previously described protocol (35,36), M1
and M2 macrophages were successfully induced from M0 macrophages by
incubation with LPS and rhIFN-γ, or rhIL-4 and rhIL-13, for 24 h.
The phenotype markers CD80 and CCR7 indicated the successful
induction of the M1 phenotype, while the CD206 marker indicated the
presence of M2 macrophages (Fig. 2B and
C). When 5 or 10 mU/ml BLM was incubated with M0 or M1
macrophages for a further 24 h, no notable alterations of CD80,
CCR7 and CD206 were detected (Fig.
2C). However, when M2 macrophages received the same treatment,
the expression levels of CD80 and CCR7 markedly increased, while
CD206 was downregulated (Fig. 2B and
C), indicating that low dose BLM treatment could reverse M2
macrophages to M1.
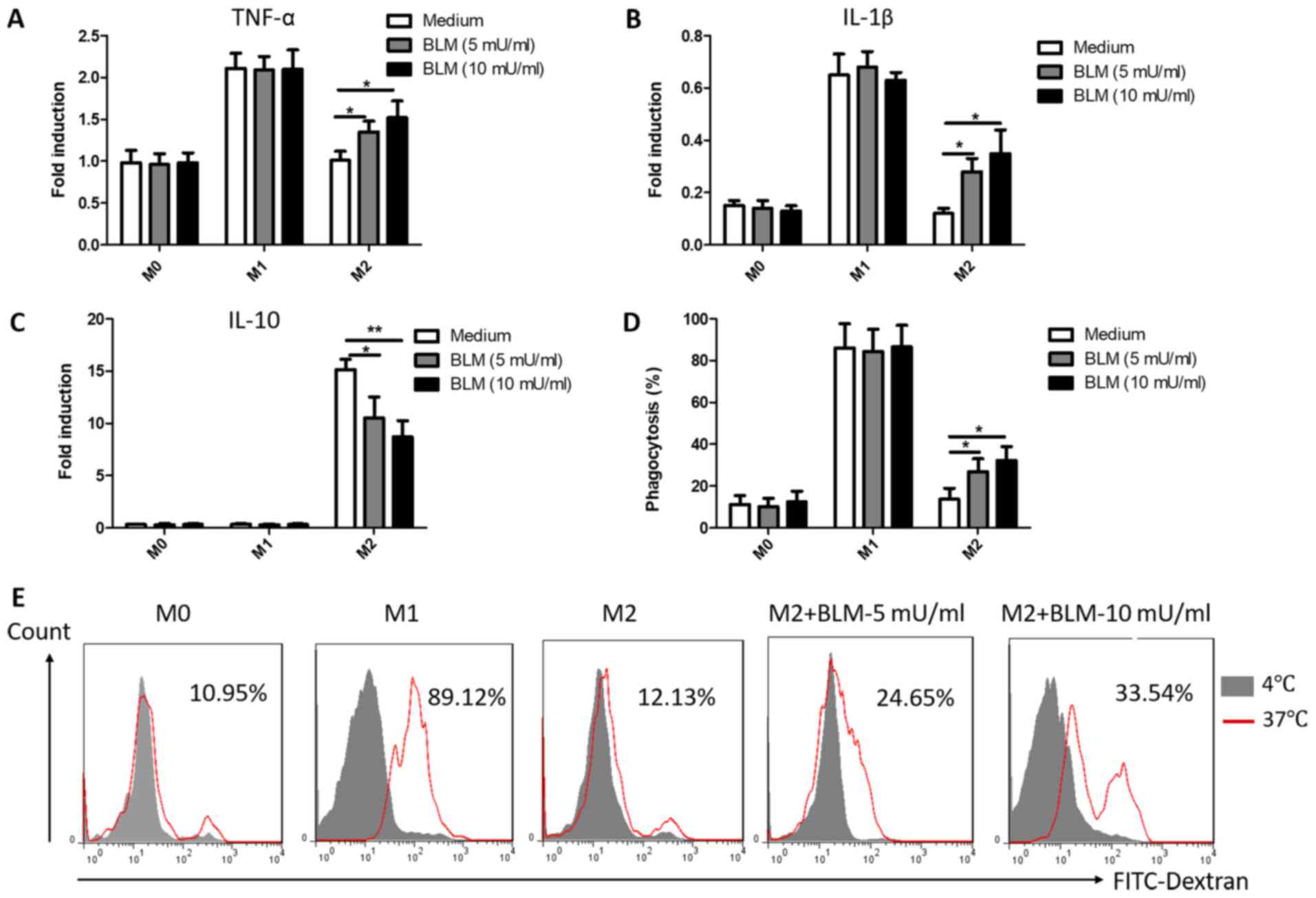
BLM reverses the function of M2
macrophages into M1
TAMs regulate immune and inflammatory responses in
the tumor microenvironment through the secretion of cytokines
(37). M1 macrophages primarily
secrete pro-inflammatory cytokines (TNF-α, IL-1β and IL-6), while
M2 macrophages secrete anti-inflammatory cytokines (IL-10 and
TGF-β) (37). To further observe the
effects of BLM on cytokine secretion in macrophages, the cytokine
mRNA levels were measured in all types of macrophages following
treatment with 5 or 10 mU/ml BLM for 24 h. As presented in Fig. 3A-C, the expression of TNF-α, IL-1β and
IL-10 in M0 and M1 macrophages was not affected by treatment with
BLM. However, the expression levels of TNF-α and IL-1β markedly
increased and the expression of IL-10 decreased in M2 macrophages
in a concentration-dependent manner following treatment with BLM
(Fig. 3A-C). In addition,
phagocytosis, an important function of macrophages, mediates innate
immunity and antigen processing. To further determine the effects
of BLM on macrophages, the phagocytic capacity of macrophages was
detected by flow cytometry. Consistent with the previous data, the
results indicated that M2 macrophages exhibited an elevated
FITC-dextran uptake capacity following incubation with BLM
(Fig. 3D and E). However, the
phagocytic capacity of M1 and M0 macrophages treated with BLM
remained unaltered (Fig. 3D).
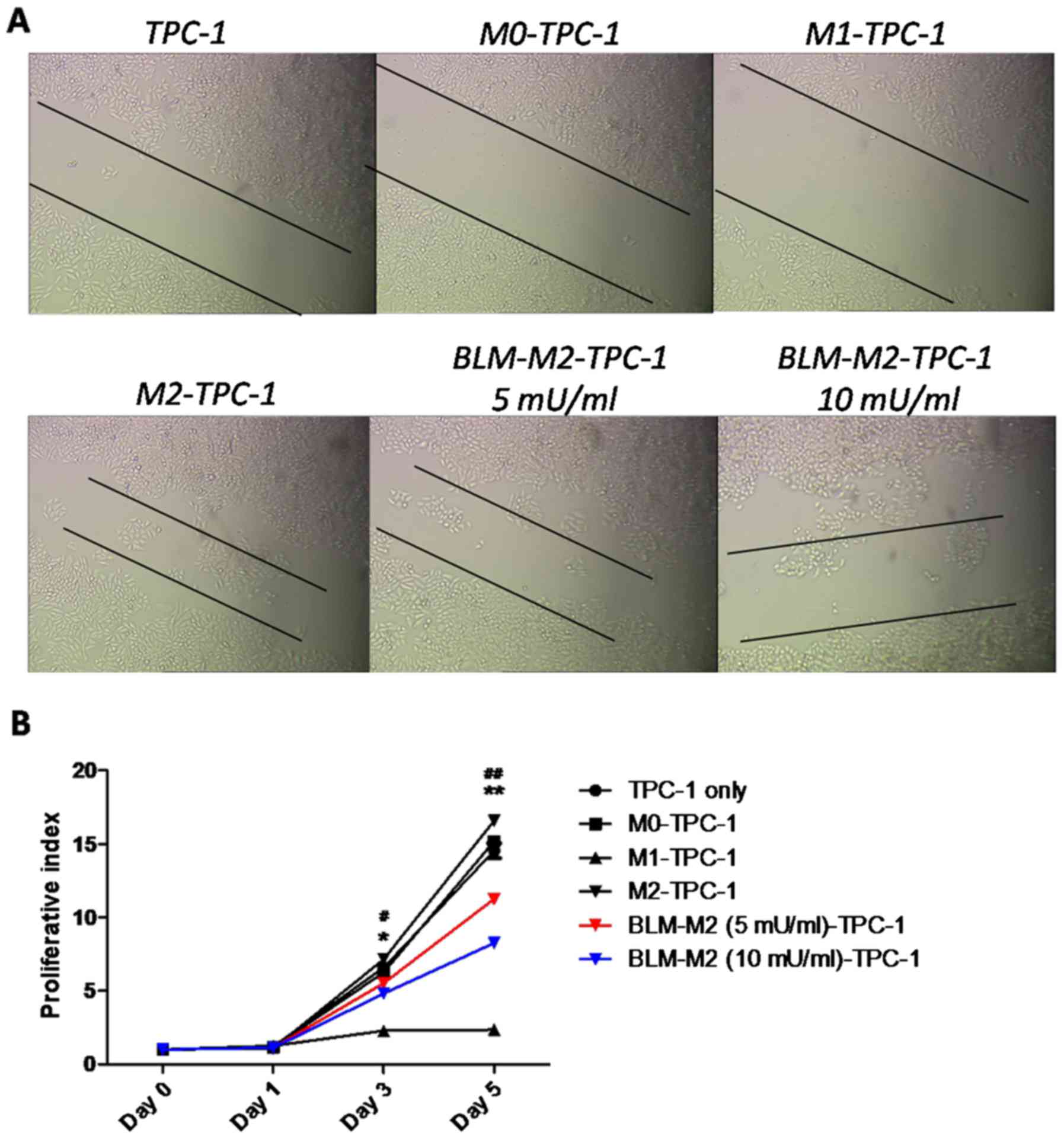
BLM inhibits the migration and
proliferation of TPC-1 cells by reversing M2 macrophage
polarization
Since M2 macrophages were previously reported to
increase the growth and survival rate of various types of cancer
cell (15,16), a co-culture experiment with TPC-1
cells and M2 macrophages was used to study the effects of BLM on
the migration and proliferation of TPC-1 cells via reversing M2
macrophage polarization. Wound-healing assays indicated that the
migration of TPC-1 cells was inhibited following co-culture with
BLM-treated M2 macrophages (Fig. 4A).
Furthermore, MTT assays demonstrated that co-culture with
BLM-treated M2 macrophages inhibited the proliferation of TPC-1
cells compared with M2 macrophages without induction by BLM
(Fig. 4B).
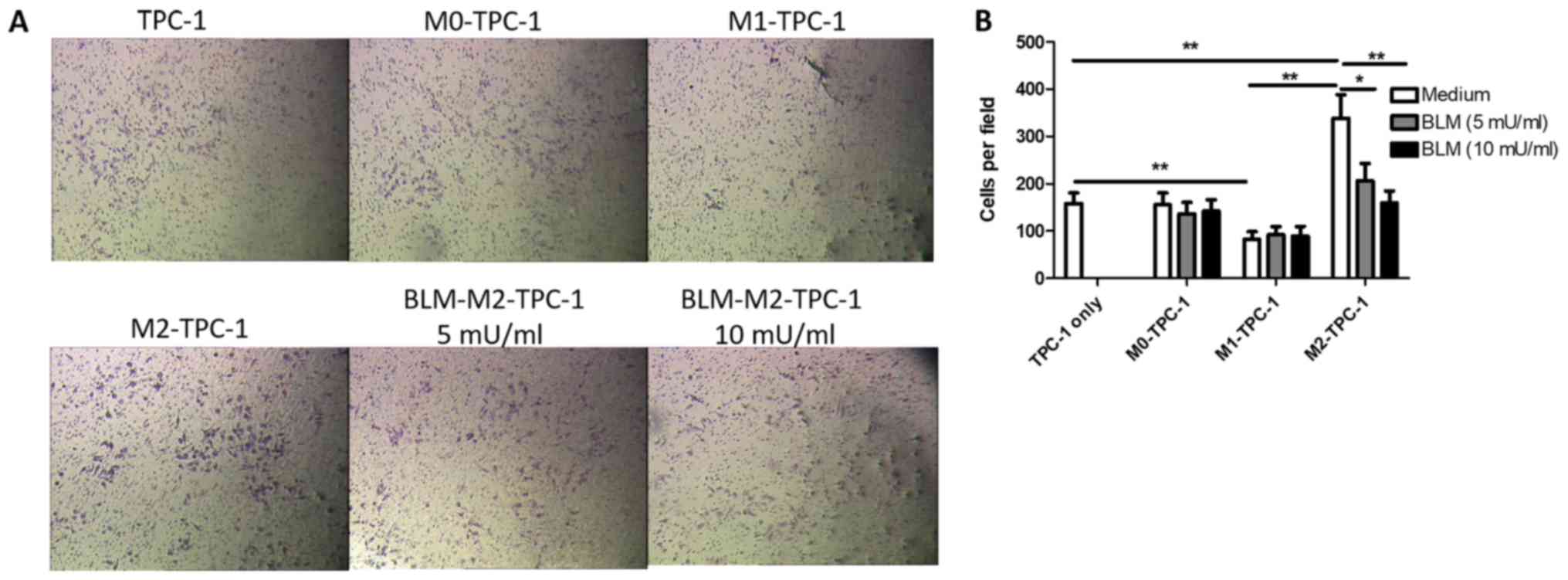
BLM inhibits the invasion of TPC-1
cells by reversing M2 macrophage polarization
To determine whether BLM-treated M2 macrophages
could regulate the invasion of TPC-1 cells, the number of invading
TPC-1 cells was counted following co-culture with BLM-treated M2
macrophages. As presented in Fig. 5,
the invasion ability of TPC-1 cells co-cultured with BLM-treated M2
macrophages significantly decreased; however, this effect was not
observed following co-culture with BLM-treated M1 and M0
macrophages.
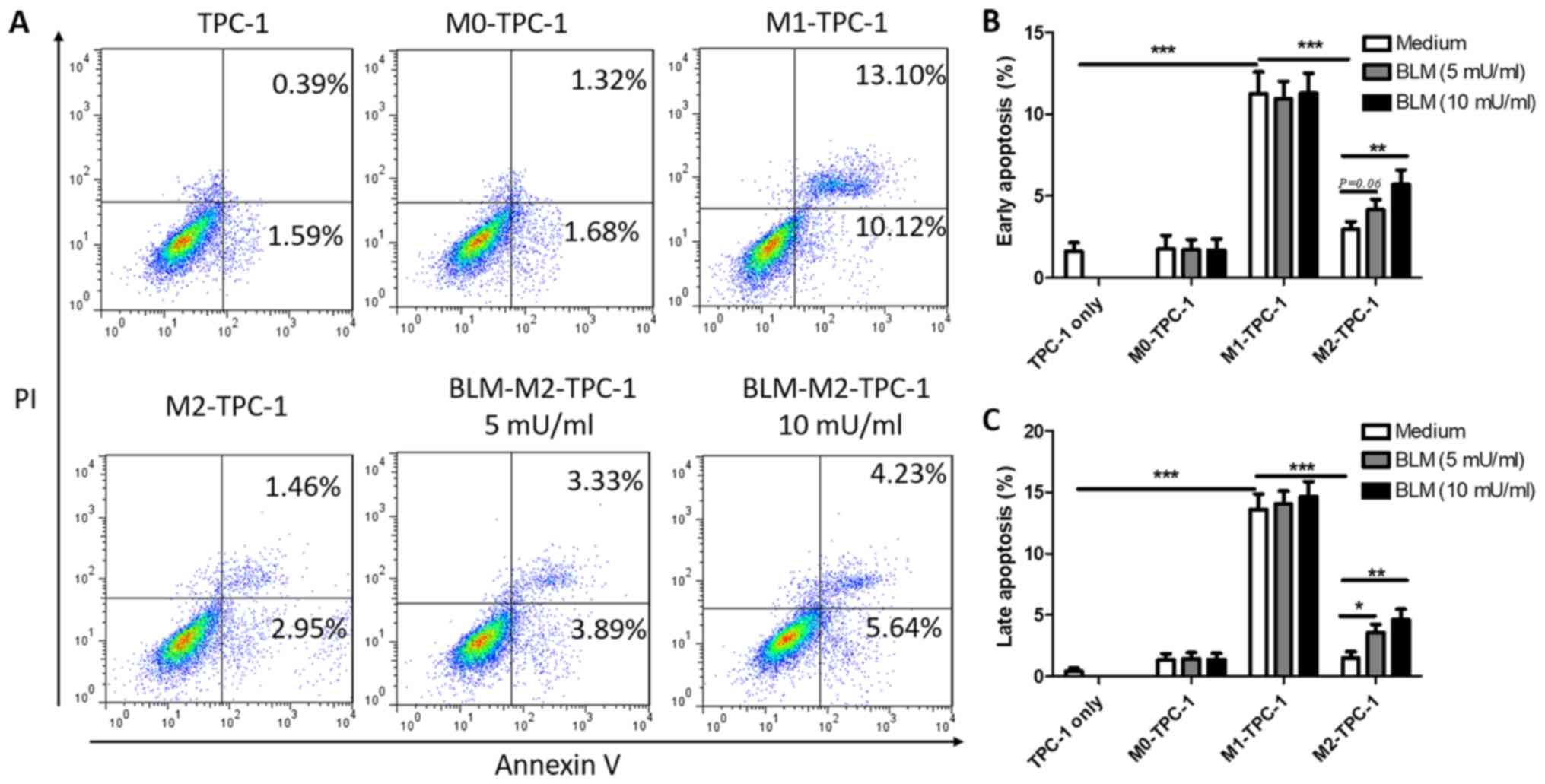
BLM promotes the apoptosis of TPC-1
cells by reversing M2 macrophage polarization
To further verify the anti-tumor effects of
BLM-induced M2 macrophage depolarization, the rate of apoptosis
induced by co-culture of macrophages with TPC-1 cells was
determined. The results indicated that M2 macrophages exhibited a
significantly reduced ability to facilitate the early and late
apoptosis of TPC-1 cells (2.95 and 1.46%) compared with M1
macrophages (10.12 and 13.10%; Fig.
6A). Although the pro-apoptotic ability of M2 cells treated
with either 5 or 10 mU/ml BLM was weaker compared with M1
macrophages, the early apoptosis rates were significantly
upregulated compared with untreated M2 macrophages (Fig. 6A-C). Furthermore, BLM-treated M1 or M0
macrophages did not affect the early or late apoptosis of TPC-1
cells (Fig. 6B and C).
Discussion
TAMs have been proposed to promote tumor progression
by facilitating angiogenesis, lymphogenesis, stroma remodeling and
immune suppression in various types of cancer (36,38–40). TAMs
also serve roles in tumor invasion and metastasis by secreting
enzymes, including plasmin, urokinase-type plasminogen activator,
matrix metalloproteinases and cathepsin B (41–43). An
increased density of TAMs in tumor tissues is associated with a
poor prognosis (44). TAMs are
commonly divided into M1 and M2 subtypes, which exhibit anti-cancer
effects, and potentiate tumorigenesis and tumor progression,
respectively. Previous studies have indicated that TAMs in numerous
types of malignant cancer predominantly exhibit an M2-like
phenotype. Since M2 macrophages in tumor tissue contribute to an
immunosuppressive microenvironment and fuel tumor progression,
reversing M2-TAM polarization could be a novel therapeutic strategy
against cancer (45). In the present
study, low doses of BLM could reverse M2 macrophages to an M1-like
phenotype, as demonstrated by the upregulation of the M1 surface
markers CD80 and CCR7 and the increased expression of predominantly
M1-secreted cytokines (TNF-α and IL-1β). Furthermore, BLM could
also enhance the phagocytic function of M2 macrophages in a
dose-dependent manner. However, the phagocytic capacity was not
significantly altered in M1 and M0 macrophages treated with BLM,
suggesting that BLM could re-polarize M2 macrophage phenotype to M1
phenotype, regulate the balance of M1/M2 TAMs in the tumor
microenvironment and activate the Th1 cell-based anti-cancer immune
response (46,47). BLM may induces the tumor suppression
ability via selectively reversing M2 macrophages to the tumoricidal
M1 phenotype without affecting M1 or M0 macrophages.
The presence of TAMs in tumor cores has been found
to be associated with progressive PTC features and TAM-conditioned
medium-enhanced PTC cell invasion (9). Furthermore, Kim et al (48) identified that primary PTC tumors with
lymph node metastasis and higher TAM density were associated with
larger tumor sizes, suggesting a tumorigenic role for TAMs in human
PTC (4). Although a number of studies
have demonstrated that M2-TAMs induce tumor proliferation,
migration and invasion in hepatocellular carcinoma, human basal
carcinoma, pancreatic cancer and prostate cancer (49,50), to
the best of our knowledge, the reversed polarization of TAMs in the
context of PTC was not previously reported. Since BLM induced TAM
depolarization, a co-culture system of TPC-1 cells and BLM-treated
macrophages was developed in the present study. As expected, the
proliferation, migration and invasion of TPC-1 cells were inhibited
following co-culture with BLM-treated M2 macrophages, indicating
that BLM could inhibit PTC progression by regulating TAM
polarization.
It was previously demonstrated that the tumor
suppression capacity of M1 macrophages is associated with the
activation of the NF-κB signaling pathway (51), which serves a key role in modulating
the apoptosis of tumor cells. The present study also indicated that
BLM-induced M1-like macrophages facilitated both early and late
apoptosis of TPC-1 cells. However, it was previously reported that
BLM can directly induce cancer cell apoptosis and inhibit
proliferation (51–54), and the proposed mechanism was through
affecting the G2 and M phase cell cycle progression of
cancer cells (55,56). Furthermore, an increased expression of
p21, independent of p53, was identified in fibrotic lungs following
direct induction with BLM (57).
Recent data also revealed the crucial roles of NF-κB and p21 in the
p53-independent apoptosis of cancer cells, in response to
DNA-damaging drugs (51,52). The interaction between the functions
of p21 and mediators involved in macrophage differentiation,
including NF-κB, could be an important consideration in attempt to
elucidate the apoptotic cell response to BLM. Nevertheless, the
results of the present study indicated that BLM could indirectly
inhibit TPC-1 cell proliferation and induce apoptosis by
depolarizing M2 macrophages to M1 macrophages. This result is
supported by previous reports that M1 macrophages inhibit cell
proliferation and induce apoptosis (58–60). BLM
is widely used for tumor treatment, but high dose and long-term
exposure to BLM may lead to pulmonary fibrosis due to DNA damage
(27,28). In the present study, tumor suppression
was indirectly induced by a low dose of BLM though the effect on
macrophages.
In conclusion, the results indicate that low dose,
indirect treatment with BLM can suppress PTC by selectively
reversing M2 macrophage polarization to M1. However, further
studies are necessary to identify the signaling pathways induced by
BLM in TAM polarization, which may contribute to improved treatment
methods for a variety of cancer types.
Acknowledgements
Not applicable.
Funding
The present study was finically supported by the
Beijing CSCO Clinical Cancer Research Foundation (grant no.
201510784).
Availability of data and materials
The analyzed data sets generated during the study
are available from the corresponding author, on reasonable
request.
Authors' contributions
LH, LZ and MX designed the study. LH, DH and MX
performed the experiments. LH, JL and LZ analyzed the data.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Cancer Genome Atlas Network: Integrated
genomic characterization of papillary thyroid carcinoma. Cell.
159:676–690. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Walker S: Immunotherapy and the treatment
of non-small cell lung cancer. J Adv Pract Oncol. 7:304–306.
2016.PubMed/NCBI
|
|
3
|
Pusztaszeri MP, Faquin WC and Sadow PM:
Tumor-associated inflammatory cells in thyroid carcinomas. Surg
Pathol Clin. 7:501–514. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Qing W, Fang WY, Ye L, Shen LY, Zhang XF,
Fei XC, Chen X, Wang WQ, Li XY, Xiao JC and Ning G: Density of
tumor-associated macrophages correlate with lymph node metastasis
in papillary thyroid carcinoma. Thyroid. 22:905–910. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Quatromoni JG and Eruslanov E:
Tumor-associated macrophages: Function, phenotype and link to
prognosis in human Lung Cancer. Am J Transl Res. 4:376–389.
2012.PubMed/NCBI
|
|
6
|
Mantovani A, Sozzani S, Locati M, Allavena
P and Sica A: Macrophage polarization: Tumor-associated macrophages
as a paradigm for polarized M2 mononuclear phagocytes. Trends
Immunol. 23:549–555. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Allavena P, Sica A, Solinas G, Porta C and
Mantovani A: The inflammatory micro-environment in tumor
progression: The role of tumor-associated macrophages. Crit Rev
Oncol Hematol. 66:1–9. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Can NY, Ayturk S, Celik M, Sezer YA,
Ozyilmaz F, Tastekin E, Sut N, Ustun F, Bulbul BY, Puyan FO and
Guldiken S: Histological perspective on the effects of
tumor-associated macrophages in the tumor microenvironment
surrounding papillary thyroid carcinoma. Pol J Pathol. 67:332–344.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Fang W, Ye L, Shen L, Cai J, Huang F, Wei
Q, Fei X, Chen X, Guan H, Wang W, et al: Tumor-associated
macrophages promote the metastatic potential of thyroid papillary
cancer by releasing CXCL8. Carcinogenesis. 35:1780–1787. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Chang WC, Chen JY, Lee CH and Yang AH:
Expression of decoy receptor 3 in diffuse sclerosing variant of
papillary thyroid carcinoma: Correlation with M2 macrophage
differentiation and lymphatic invasion. Thyroid. 23:720–726. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Lopez-Gonzalez JS, Avila-Moreno F,
Prado-Garcia H, Aguilar-Cazares D, Mandoki JJ and Meneses-Flores M:
Lung carcinomas decrease the number of monocytes/macrophages (CD14+
cells) that produce TNF-alpha. Clin Immunol. 122:323–329. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Ohri CM, Shikotra A, Green RH, Waller DA
and Bradding P: Macrophages within NSCLC tumour islets are
predominantly of a cytotoxic M1 phenotype associated with extended
survival. Eur Respir J. 33:118–126. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Redente EF, Dwyer-Nield LD, Merrick DT,
Raina K, Agarwal R, Pao W, Rice PL, Shroyer KR and Malkinson AM:
Tumor progression stage and anatomical site regulate
tumor-associated macrophage and bone marrow-derived monocyte
polarization. Am J Pathol. 176:2972–2985. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zea AH, Rodriguez PC, Atkins MB, Hernandez
C, Signoretti S, Zabaleta J, McDermott D, Quiceno D, Youmans A,
O'Neill A, et al: Arginase-producing myeloid suppressor cells in
renal cell carcinoma patients: A mechanism of tumor evasion. Cancer
Res. 65:3044–3048. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Chang CI, Liao JC and Kuo L: Macrophage
arginase promotes tumor cell growth and suppresses nitric
oxide-mediated tumor cytotoxicity. Cancer Res. 61:1100–1106.
2001.PubMed/NCBI
|
|
16
|
Biswas SK, Sica A and Lewis CE: Plasticity
of macrophage function during tumor progression: Regulation by
distinct molecular mechanisms. J Immunol. 180:2011–2057. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Qian BZ and Pollard JW: Macrophage
diversity enhances tumor progression and metastasis. Cell.
141:39–51. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Mehrpour M, Esclatine A, Beau I and
Codogno P: Overview of macroautophagy regulation in mammalian
cells. Cell Res. 20:748–762. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Ruffell B, Affara NI and Coussens LM:
Differential macrophage programming in the tumor microenvironment.
Trends Immunol. 33:119–126. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Batschinski K, Dervisis N, Kitchell B,
Newman R and Erfourth T: Combination of bleomycin and cytosine
arabinoside chemotherapy for relapsed canine lymphoma. J Am Anim
Hosp Assoc. 54:150–155. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Ng K, Duncan S, Shamash J and Alifrangis
C: Dose intense chemotherapy in the management of poor prognosis
and relapsed testicular cancer: Experiences and controversies.
Expert Rev Anticancer Ther. 18:431–436. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Porwal PK, Dubey KP, Morey A, Singh H,
Pooja S and Bose A: Bleomycin sclerotherapy in lymphangiomas of
head and neck: prospective study of 8 cases. Indian J Otolaryngol
Head Neck Surg. 70:145–148. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Racnik J, Svara T, Zadravec M, Gombac M,
Cemazar M, Sersa G and Tozon N: Electrochemotherapy with bleomycin
of different types of cutaneous tumours in a ferret (Mustela
Putorius Furo). Radiol Oncol. 52:98–104. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Cai Y, Sun R, Wang R, Ren JG, Zhang W,
Zhao YF and Zhao JH: The activation of Akt/mTOR pathway by
bleomycin in Epithelial-to-mesenchymal transition of human
submandibular gland cells: A treatment mechanism of bleomycin for
mucoceles of the salivary glands. Biomed Pharmacother. 90:109–115.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Mouratis MA and Aidinis V: Modeling
pulmonary fibrosis with bleomycin. Curr Opin Pulm Med. 17:355–361.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Weng D, Chen JX, Li HH, Liu F, Zhou LD,
Liu HP, Zheng RJ, Jiang Y, Liu ZH and Ge B: 2-aminopurine
suppresses the TGF-β1-induced epithelial-mesenchymal transition and
attenuates bleomycin-induced pulmonary fibrosis. Cell Death Discov.
4:172018. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Jung WJ, Lee SY, Choi SI, Kim BK, Lee EJ,
In KH and Lee MG: Toll-like receptor expression in pulmonary
sensory neurons in the bleomycin-induced fibrosis model. PLoS One.
13:e01931172018. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Bolzan AD and Bianchi MS: DNA and
chromosome damage induced by bleomycin in mammalian cells: An
update. Mutat Res. 775:51–62. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Massonneau J, Ouellet C, Lucien F, Dubois
CM, Tyler J and Boissonneault G: Suboptimal extracellular pH values
alter DNA damage response to induced double-strand breaks. FEBS
Open Bio. 8:416–425. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Huang W, Wang G, Phelps DS, Al-Mondhiry H
and Floros J: Combined SP-A-bleomycin effect on cytokines by THP-1
cells: Impact of surfactant lipids on this effect. Am J Physiol
Lung Cell Mol Physiol. 283:L94–L102. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Razonable RR, Henault M and Paya CV:
Stimulation of toll-like receptor 2 with bleomycin results in
cellular activation and secretion of pro-inflammatory cytokines and
chemokines. Toxicol Appl Pharmacol. 210:181–189. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Hoshino T, Okamoto M, Sakazaki Y, Kato S,
Young HA and Aizawa H: Role of proinflammatory cytokines IL-18 and
IL-1beta in bleomycin-induced lung injury in humans and mice. Am J
Respir Cell Mol Biol. 41:661–670. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Genin M, Clement F, Fattaccioli A, Raes M
and Michiels C: M1 and M2 macrophages derived from THP-1 cells
differentially modulate the response of cancer cells to etoposide.
BMC Cancer. 15:5772015. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Steinbach F and Thiele B: Phenotypic
investigation of mononuclear phagocytes by flow cytometry. J
Immunol Methods. 174:109–122. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Murdoch C, Muthana M, Coffelt SB and Lewis
CE: The role of myeloid cells in the promotion of tumour
angiogenesis. Nat Rev Cancer. 8:618–631. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Yuan A, Hsiao YJ, Chen HY, Chen HW, Ho CC,
Chen YY, Liu YC, Hong TH, Yu SL, Chen JJ and Yang PC: Opposite
effects of M1 and M2 macrophage subtypes on lung cancer
progression. Sci Rep. 5:142732015. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Reeves AR, Spiller KL, Freytes DO,
Vunjak-Novakovic G and Kaplan DL: Controlled release of cytokines
using silk-biomaterials for macrophage polarization. Biomaterials.
73:272–283. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Pang L, Han S, Jiao Y, Jiang S, He X and
Li P: Bu Fei Decoction attenuates the tumor associated macrophage
stimulated proliferation, migration, invasion and immunosuppression
of non-small cell Lung Cancer, partially via IL-10 and PD-L1
regulation. Int J Oncol. 51:25–38. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Kang H, Zhang J, Wang B, Liu M, Zhao J,
Yang M and Li Y: Puerarin inhibits M2 polarization and metastasis
of tumor-associated macrophages from NSCLC xenograft model via
inactivating MEK/ERK 1/2 pathway. Int J Oncol. 50:545–554. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Cho SW, Kim YA, Sun HJ, Kim YA, Oh BC, Yi
KH, Park DJ and Park YJ: CXCL16 signaling mediated macrophage
effects on tumor invasion of papillary thyroid carcinoma. Endocr
Relat Cancer. 23:113–124. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Komohara Y, Fujiwara Y, Ohnishi K and
Takeya M: Tumor-associated macrophages: Potential therapeutic
targets for anti-cancer therapy. Adv Drug Deliv Rev. 99:180–185.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Gocheva V, Wang HW, Gadea BB, Shree T,
Hunter KE, Garfall AL, Berman T and Joyce JA: IL-4 induces
cathepsin protease activity in tumor-associated macrophages to
promote cancer growth and invasion. Genes Dev. 24:241–255. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Wang R, Zhang J, Chen S, Lu M, Luo X, Yao
S, Liu S, Qin Y and Chen H: Tumor-associated macrophages provide a
suitable microenvironment for non-small Lung Cancer invasion and
progression. Lung Cancer. 74:188–196. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Komohara Y, Jinushi M and Takeya M:
Clinical significance of macrophage heterogeneity in human
malignant tumors. Cancer Sci. 105:1–8. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Rolny C, Mazzone M, Tugues S, Laoui D,
Johansson I, Coulon C, Squadrito ML, Segura I, Li X, Knevels E, et
al: HRG inhibits tumor growth and metastasis by inducing macrophage
polarization and vessel normalization through downregulation of
PlGF. Cancer Cell. 19:31–44. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Almatroodi SA, McDonald CF, Darby IA and
Pouniotis DS: Characterization of M1/M2 tumour-associated
macrophages (TAMs) and Th1/Th2 cytokine profiles in patients with
NSCLC. Cancer Microenviron. 9:1–11. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Muraille E, Leo O and Moser M: TH1/TH2
paradigm extended: Macrophage polarization as an unappreciated
pathogen-driven escape mechanism. Front Immunol. 5:6032014.
View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Kim S, Cho SW, Min HS, Kim KM, Yeom GJ,
Kim EY, Lee KE, Yun YG, Park DJ and Park YJ: The expression of
tumor-associated macrophages in papillary thyroid carcinoma.
Endocrinol Metab (Seoul). 28:192–198. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Mellor AL and Munn DH: Creating immune
privilege: Active local suppression that benefits friends, but
protects foes. Nat Rev Immunol. 8:74–80. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Sica A, Schioppa T, Mantovani A and
Allavena P: Tumour-associated macrophages are a distinct M2
polarised population promoting tumour progression: Potential
targets of anti-cancer therapy. Eur J Cancer. 42:717–727. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Liu CP, Zhang X, Tan QL, Xu WX, Zhou CY,
Luo M, Li X, Huang RY and Zeng X: NF-κB pathways are involved in M1
polarization of RAW 264.7 macrophage by polyporus polysaccharide in
the tumor microenvironment. PLoS One. 12:e01883172017. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Gong H, Cao Y, Han G, Zhang Y, You Q, Wang
Y and Pan Y: p53/microRNA-374b/AKT1 regulates colorectal cancer
cell apoptosis in response to DNA damage. Int J Oncol.
50:1785–1791. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Yang L, Zhao W, Wei P, Zuo W and Zhu S:
Tumor suppressor p53 induces miR-15a processing to inhibit neuronal
apoptosis inhibitory protein (NAIP) in the apoptotic response DNA
damage in breast cancer cell. Am J Transl Res. 9:683–691.
2017.PubMed/NCBI
|
|
54
|
Liu JF, Nie XC, Shao YC, Su WH and Ma HY:
Bleomycin suppresses the proliferation and the mobility of human
gastric cancer cells through the smad signaling pathway. Cell
Physiol Biochem. 40:1401–1409. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Gao N, Shang B, Zhang X, Shen C, Xu R,
Chen R and He Q: Potent antitumor actions of the new antibiotic
boningmycin through induction of apoptosis and cellular senescence.
Anticancer Drugs. 22:166–175. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Uchida R, Yokota S and Tomoda H: Structure
elucidation of meroterpenoid habiterpenol, a novel abrogator of
bleomycin-induced G2 arrest in Jurkat cells, produced by
Phytohabitans suffuscus 3787_5. J Antibiot (Tokyo). 67:783–786.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Wang Q, Cui K, Espin-Garcia O, Cheng D,
Qiu X, Chen Z, Moore M, Bristow RG, Xu W, Der S and Liu G:
Resistance to bleomycin in cancer cell lines is characterized by
prolonged doubling time, reduced DNA damage and evasion of G2/M
arrest and apoptosis. PLoS One. 8:e823632013. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Lofdahl A, Rydell-Tormanen K,
Larsson-Callerfelt AK, AUID- and Wenglen Oho C: Pulmonary fibrosis
in vivo displays increased p21 expression reduced by 5-HT2B
receptor antagonists in vitro-a potential pathway affecting
proliferation. Sci Rep. 8:19272018. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Park HR, Lee EJ, Moon SC, Chung TW and Kim
KJ: Inhibition of lung cancer growth by HangAmDan-B is mediated by
macrophage activation to M1 subtype. Oncol Lett. 13:2330–2336.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Li Y, Poppoe F, Chen J, Yu L and Deng F:
Macrophages polarized by expression of toxoGRA15II inhibit growth
of hepatic carcinoma. Front Immunol. 8:1372017.PubMed/NCBI
|