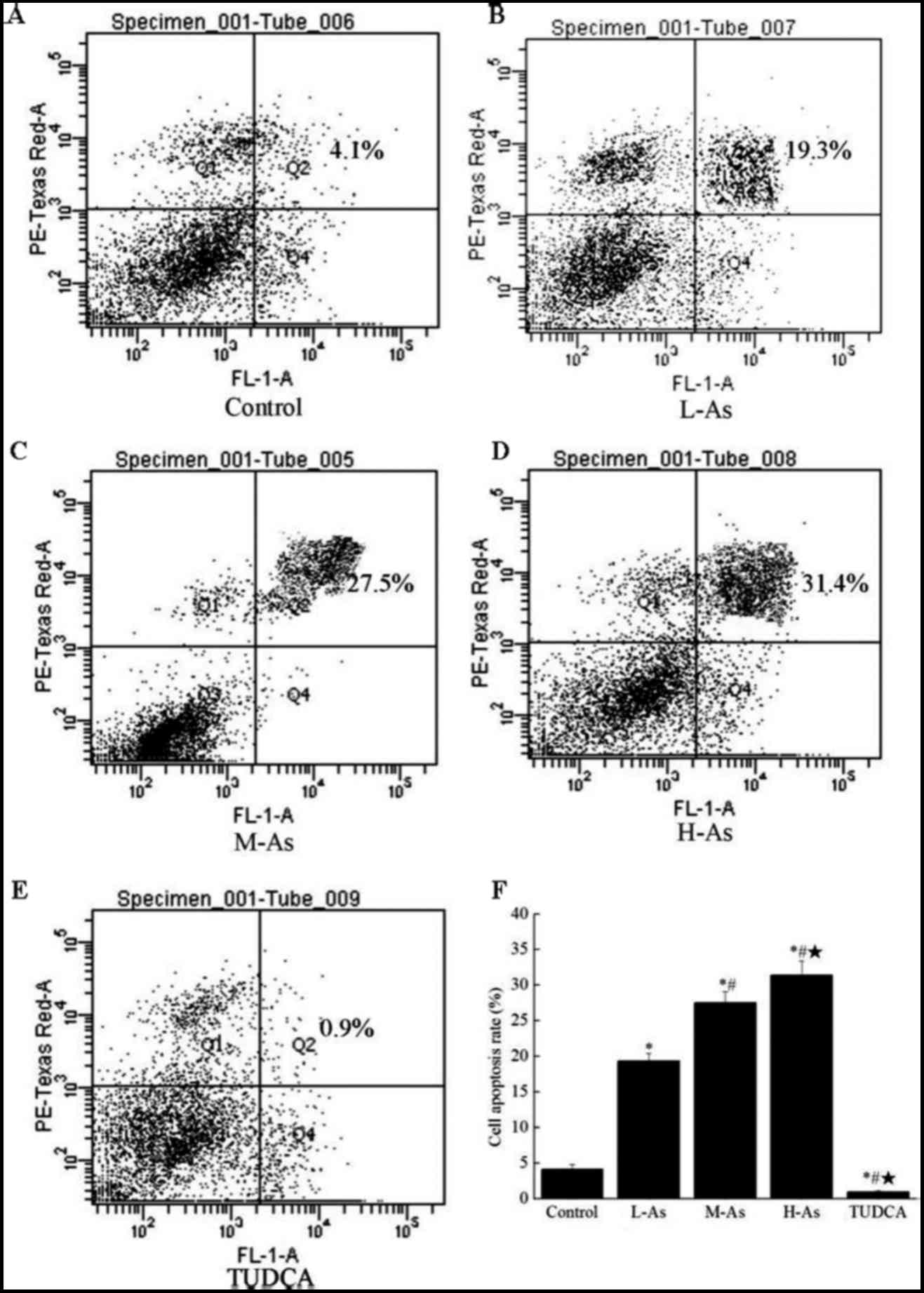
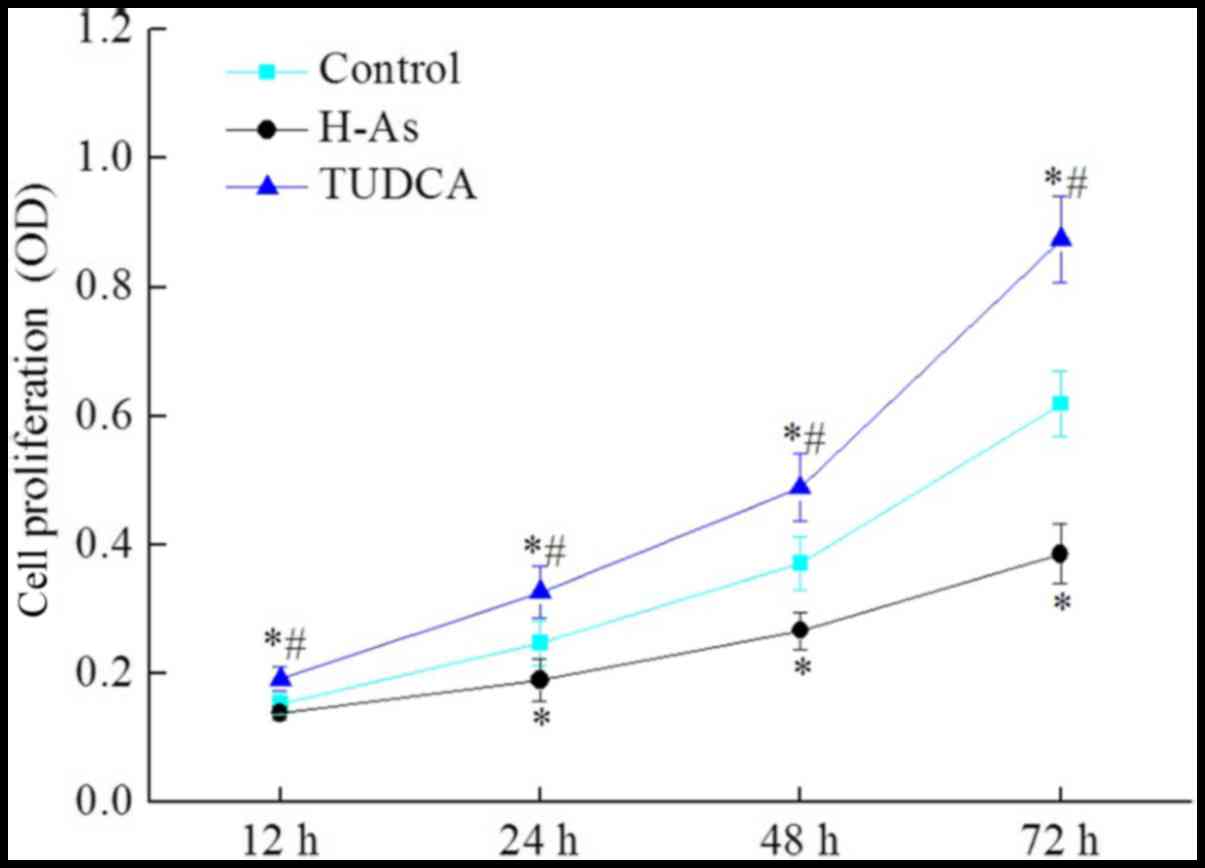
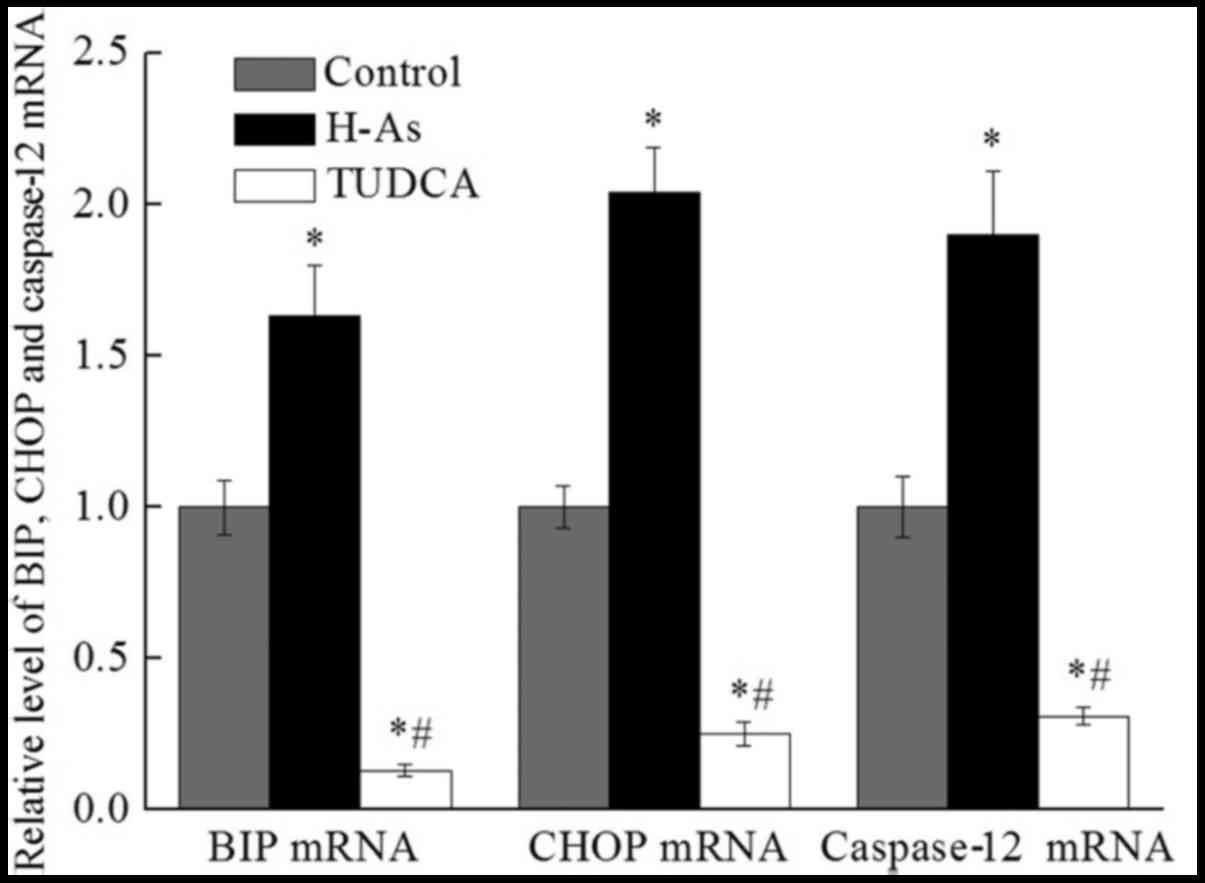
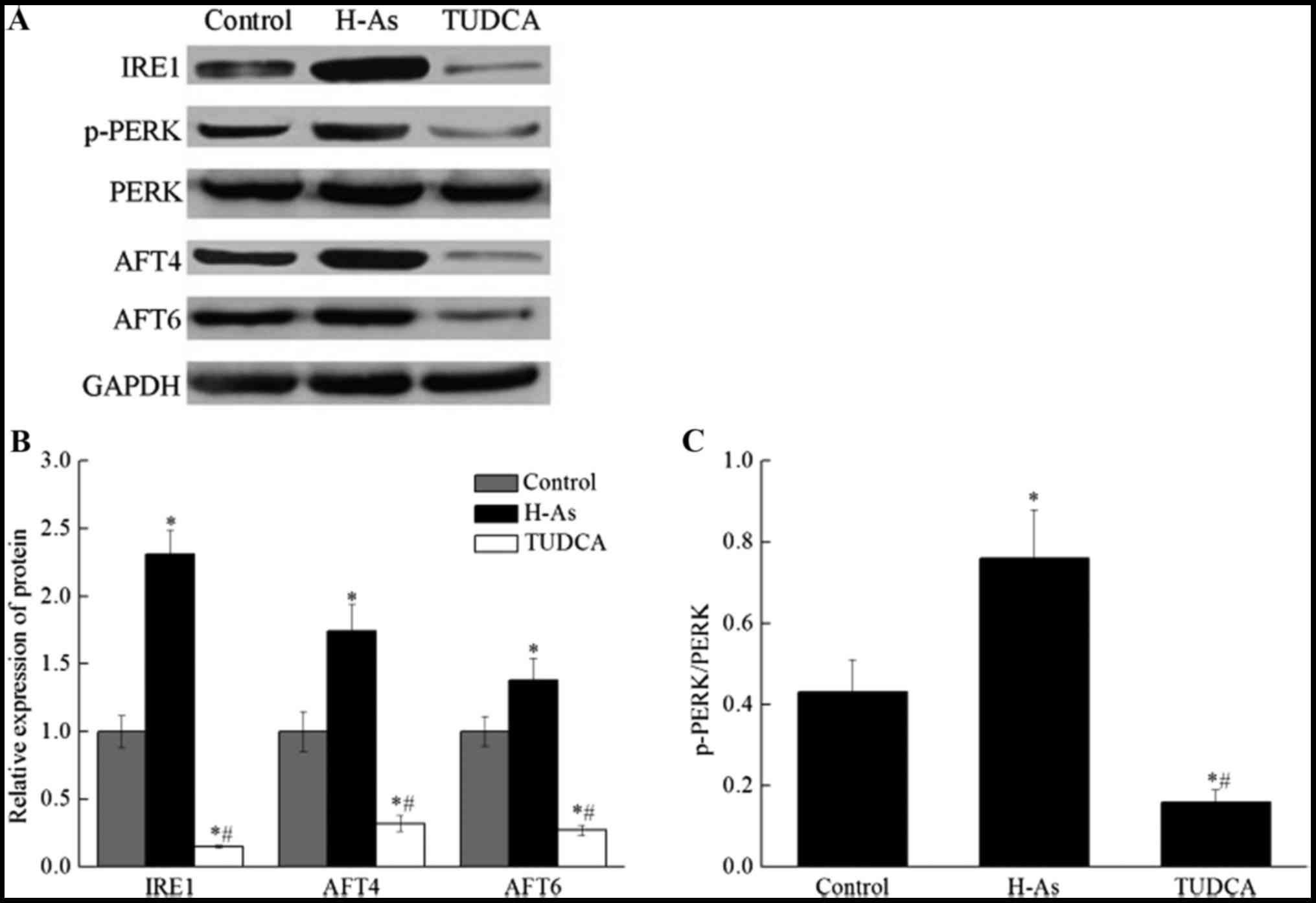
|
1
|
DeSantis CE, Lin CC, Mariotto AB, Siegel
RL, Stein KD, Kramer JL, Alteri R, Robbins AS and Jemal A: Cancer
treatment and survivorship statistics, 2014. CA Cancer J Clin.
64:252–271. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Nyquist MD and Dehm SM: Interplay between
genomic alterations and androgen receptor signaling during prostate
cancer development and progression. Horm Cancer. 4:61–69. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Hamid AR, Hoogland AM, Smit F, Jannink S,
van Rijt-van de Westerlo C, Jansen CF, van Leenders GJ and Verhaegh
GW: The role of HOXC6 in prostate cancer development. Prostate.
75:1868–1876. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Crawford ED, Stone NN, Evan YY, Koo PJ,
Freedland SJ, Slovin SF, Gomella LG, Berger ER and Keane TE:
Challenges and recommendations for early identification of
metastatic disease in prostate cancer. Urology. 83:664–669. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Rustin GJ, Bast RC Jr, Kelloff GJ, Barrett
JC, Carter SK, Nisen PD, Sigman CC, Parkinson DR and Ruddon RW: Use
of CA-125 in clinical trial evaluation of new therapeutic drugs for
ovarian cancer. Clin Cancer Res. 10:3919–3926. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Victorson DE, Brucker PS, Bode RK, Eton
DT, Talcott JA, Clark JA, Knight SJ, Litwin MS, Moinpour CM, Reeve
BB, et al: Ensuring comprehensive assessment of urinary problems in
prostate cancer through patient-physician concordance. Urol Oncol.
32(26): e25–e31. 2014.
|
|
7
|
Schröder FH, Hugosson J, Roobol MJ,
Tammela TL, Zappa M, Nelen V, Kwiatkowski M, Lujan M, Määttänen L,
Lilja H, et al: Screening and prostate cancer mortality: results of
the European Randomised Study of Screening for Prostate Cancer
(ERSPC) at 13 years of follow-up. Lancet. 384:2027–2035. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Chen W, Zheng R, Baade PD, Zhang S, Zeng
H, Bray F, Jemal A, Yu XQ and He J: Cancer statistics in China,
2015. CA Cancer J Clin. 66:115–132. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Freytag SO, Stricker H, Lu M, Elshaikh M,
Aref I, Pradhan D, Levin K, Kim JH, Peabody J, Siddiqui F, et al:
Prospective randomized phase 2 trial of intensity modulated
radiation therapy with or without oncolytic adenovirus-mediated
cytotoxic gene therapy in intermediate-risk prostate cancer. Int J
Radiat Oncol Biol Phys. 89:268–276. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Wang S, Tang L and Chen F: Astragaloside
III from Astragalus membranaceus antagonizes breast cancer
growth. AJTCAM. 12:183–186. 2015. View Article : Google Scholar
|
|
11
|
Li B, Wang F, Liu N, Shen W and Huang T:
Astragaloside IV inhibits progression of glioma via blocking
MAPK/ERK signaling pathway. Biochem Biophys Res Commun. 491:98–103.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Dai PC, Liu DL, Zhang L, Ye J, Wang Q,
Zhang HW, Lin XH and Lai GX: Astragaloside IV sensitizes non-small
cell lung cancer cells to gefitinib potentially via regulation of
SIRT6. Tumour Biol. 39:10104283176975552017. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Yongping M, Zhang X, Xuewei L, Fan W, Chen
J, Zhang H, Chen G, Liu C and Liu P: Astragaloside prevents
BDL-induced liver fibrosis through inhibition of notch signaling
activation. J Ethnopharmacol. 169:200–209. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Huang X, Tang L, Wang F and Song G:
Astragaloside IV attenuates allergic inflammation by regulation
Th1/Th2 cytokine and enhancement CD4+ CD25+ Foxp3 T cells in
ovalbumin-induced asthma. Immunobiology. 219:565–571. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Chen Y, Gui D, Chen J, He D, Luo Y and
Wang N: Down-regulation of PERK-ATF4-CHOP pathway by Astragaloside
IV is associated with the inhibition of endoplasmic reticulum
stress-induced podocyte apoptosis in diabetic rats. Cell Physiol
Biochem. 33:1975–1987. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zhao Y, Li Q, Zhao W, Li J, Sun Y, Liu K,
Liu B and Zhang N: Astragaloside IV and cycloastragenol are equally
effective in inhibition of endoplasmic reticulum stress-associated
TXNIP/NLRP3 inflammasome activation in the endothelium. J
Ethnopharmacology. 169:210–218. 2015. View Article : Google Scholar
|
|
17
|
Wang ZS, Xiong F, Xie XH, Chen D, Pan JH
and Cheng L: Astragaloside IV attenuates proteinuria in
streptozotocin-induced diabetic nephropathy via the inhibition of
endoplasmic reticulum stress. BMC Nephrol. 16:442015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Cao SS and Kaufman RJ: Endoplasmic
reticulum stress and oxidative stress in cell fate decision and
human disease. Antioxid Redox Sign. 21:396–413. 2014. View Article : Google Scholar
|
|
19
|
Gardner BM, Pincus D, Gotthardt K,
Gallagher CM and Walter P: Endoplasmic reticulum stress sensing in
the unfolded protein response. Cold Spring Harb Perspect Biol.
5:a0131692013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Deegan S, Saveljeva S, Gorman AM and
Samali A: Stress-induced self-cannibalism: On the regulation of
autophagy by endoplasmic reticulum stress. Cell Mol Life Sci.
70:2425–2441. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Liu KC, Yen CY, Wu RS, Yang JS, Lu HF, Lu
KW, Lo C, Chen HY, Tang NY, Wu CC and Chung JG: The roles of
endoplasmic reticulum stress and mitochondrial apoptotic signaling
pathway in quercetin-mediated cell death of human prostate cancer
PC-3 cell. Environ Toxicol. 29:428–439. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Hetz C: The unfolded protein response:
Controlling cell fate decisions under ER stress and beyond. Nat Rev
Mol Cell Biol. 13:89–102. 2012. View
Article : Google Scholar : PubMed/NCBI
|
|
24
|
Tabas I and Ron D: Integrating the
mechanisms of apoptosis induced by endoplasmic reticulum stress.
Nat Cell Biol. 13:184–190. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Nawrocki ST, Carew JS, Dunner K Jr, Boise
LH, Chiao PJ, Huang P, Abbruzzese JL and McConkey DJ: Bortezomib
inhibits PKR-like endoplasmic reticulum (ER) kinase and induces
apoptosis via ER stress in human pancreatic cancer cells. Cancer
Res. 65:11510–11519. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Zou P, Zhang J, Xia Y, Kanchana K, Guo G,
Chen W, Huang Y, Wang Z, Yang S and Liang G: ROS generation
mediates the anti-cancer effects of WZ35 via activating JNK and ER
stress apoptotic pathways in gastric cancer. Oncotarget.
6:5860–5876. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Cheng X, Gu J, Zhang M, Yuan J, Zhao B,
Jiang J and Jia X: Astragaloside IV inhibits migration and invasion
in human lung cancer A549 cells via regulating PKC-α-ERK1/2-NF-κB
pathway. Int Immunopharmacol. 23:304–313. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Bouman L, Schlierf A, Lutz AK, Shan J,
Deinlein A, Kast J, Galehdar Z, Palmisano V, Patenge N, Berg D, et
al: Parkin is transcriptionally regulated by ATF4: Evidence for an
interconnection between mitochondrial stress and ER stress. Cell
Death Differ. 18:769–782. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Zhao Y, Li Q, Zhao W, Li J, Sun Y, Liu K,
Liu B and Zhang N: Astragaloside IV and cycloastragenol are equally
effective in inhibition of endoplasmic reticulum stress-associated
TXNIP/NLRP3 inflammasome activation in the endothelium. J
Ethnopharmacol. 169:210–218. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Liu ZW, Zhu HT, Chen KL, Dong X, Wei J,
Qiu C and Xue JH: Protein kinase RNA-like endoplasmic reticulum
kinase (PERK) signaling pathway plays a major role in reactive
oxygen species (ROS)-mediated endoplasmic reticulum stress-induced
apoptosis in diabetic cardiomyopathy. Cardiovasc Diabetol.
12:1582013. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Jheng JR, Ho JY and Horng JT: ER stress,
autophagy and RNA viruses. Front Microbiol. 5:3882014. View Article : Google Scholar : PubMed/NCBI
|