Introduction
The incidence of Hodgkin lymphoma (HL) in human
immunodeficiency virus (HIV)-infected individuals has been
increasing across countries since the advent of combination
antiretroviral therapy (cART), and HL is presently one of the most
frequent non-AIDS defining malignancies (1–3).
Therefore, HIVpositive HL is currently an important
complication of HIV infection in the cART era.
Our previous nationwide survey in Japan demonstrated
that most HIVpositive HL patients are EBV-positive
(EBV+) (4). Epstein-Barr
virus (EBV) is considered to play a role in the pathogenesis of an
HL subset (5,6); however, the frequency of EBV association
in HL is markedly different between HIVpositive HL
(80–100%) and HIVnegative HL (20–50%) (5–8). In HIV
negative cases, EBV positivity is demonstrated to have a male
predominance, a high incidence of mixed cellularity classical
Hodgkin lymphoma (MCCHL), and advanced clinical stages (9). The prognostic impact of EBV positivity
in HL remains controversial (9–12). It has
been recently reported that HIV infection has no prognostic impact
on advanced-stage HL (13). However,
to the best of our knowledge, there has been no comparative
clinical study that focused on the differences in the clinical
features of EBV+ HL between HIV positive and negative
cases. Thus, we planned an expanded survey to compare the clinical
characteristics between HIVpositive HL and
HIVnegative HL with pathologically detectable EBV in a
Japanese population.
Patients and methods
In our previous retrospective nationwide study in
Japan between 1991 and 2010 from 511 institutions among all
regional centers and all educational hospitals certified by the
Japanese Society of Hematology (4,14), there
were only 19 HIVpositive HL patients. Among them, we
found 16 evaluable EBV+ HIVpositive HL
patients for analysis in this study. The criteria for
EBV-positivity were defined by EBER in situ hybridization
and/or LMP-1 immunostaining (4). In
addition, data of newly obtained 123 HIVnegative HL
patients who visited three regional hospitals (i.e., Cancer
Institute Hospital of JFCR, National Hospital Organization Nagoya
Medical Center, and Tokyo Medical University) between 2001 and 2010
were used for this study. Chart reviews were performed for all
identified patients (Table I). We
further re-assessed the pathological diagnosis and performed
additional immunostaining for EBV assessment, as defined by EBER
in situ hybridization and/or LMP-1 immunostaining, and
finally identified 34 HIVnegative EBV+ HL
patients as a control. This study was approved by the Ethics
Committee of Tokyo Medical University Hospital (no. 2610; February
4, 2014), Cancer Institute Hospital of JFCR, and National Hospital
Organization Nagoya Medical Center.
 | Table I.Baseline characteristics of EBV+ HL
patients with and without HIV. |
Table I.
Baseline characteristics of EBV+ HL
patients with and without HIV.
| Variable | EBV+
HIVpositive HL (n=16) | EBV+
HIVnegative HL (n=34) | P-value |
|---|
| Male/female | 14/2 | 25/9 | 0.232A |
| Median age, years
(range) | 45 (31–66) | 60.5 (20–85) |
0.0158B |
| Absolute
CD4+ cell count, cells × 109/l (range) | 231 (1–567) | ND |
|
| Viral load <500
copies/ml | 10/14a | ND |
|
| On ART | 13/16 (81.3%) | 0/34 |
|
| Histological
subtype n (%) |
|
|
|
|
NSCHL | 3 (18.8%) | 8 (23.5%) |
|
|
MCCHL | 11
(68.8%)b | 21 (61.8%) | 0.631C |
|
LRCHL | 0 (0%) | 5 (14.7%) |
|
|
LDCHL | 1
(6.3%)c | 0 (0%) |
|
|
Non-specific | 1 (6.3%) | 0 (0%) |
|
| Ann Arbor stage n
(%) |
|
|
|
|
Localized stage | 3 (18.8%) | 11 (32.4%) | 0.258A |
|
Advanced stage | 13
(81.3%)d | 23 (67.6%) |
|
| Symptoms in
category B of Ann Arbor stagingD | 8
(50.0%)e | 14 (41.2%) | 0.388A |
| Extranodal
lesion | 9
(56.3%)f | 7 (20.6%) | 0.015A |
| Bone
marrow involvement | 7/16
(47.8%)g | 1 (2.9%) |
0.000748A |
| IPS |
|
|
|
|
0–2 | 7 (43.8%) | 16 (47.1%) | 0.498A |
| ≥3 | 9
(56.3%)h | 17 (50.0%) |
|
|
Unknown |
| 1 (2.9%) |
|
| Treatment |
|
|
|
|
Localized stage |
|
|
|
|
ABVD | 0 | 4 |
|
| RT | 1 | 1 |
|
|
ABVD+RT | 2 | 6 |
|
| Advanced stage |
|
|
|
|
ABVD/ABVd | 11 | 20 |
|
|
ABVD+RT | 0 | 1 |
|
|
C-MOPP | 0 | 1 |
|
|
BDi | 0 | 1 |
|
| No
treatmentj | 2 | 0 |
|
| Complete remission
rate | 11/13
(84.6%)k | 31/32 (96.9%) | 0.196A |
| Treatment
completion rate (advanced stage) | 9/10
(90%)l | 15/20 (75.0%) | 0.326A |
| Rate of patients
with ABVD/ABVd dose reduction | 2/10m | 0/20 | 0.103A |
| Auto PBSCT | 0 | 1 |
|
Response was assessed according to the International
Workshop Criteria for non-Hodgkin's lymphoma (15). Overall survival (OS) was defined as
the interval from HL diagnosis to death from any cause.
Progression-free survival (PFS) was defined as the interval from HL
diagnosis and the date on which disease progresses or the date on
which the patient dies from any causes. Two HIVpositive
HL patients diagnosed by autopsy were excluded from prognostic
analysis. International Prognostic Score (IPS) was evaluated
according to a previous report (16).
Treatment completion was defined as completing the induction
therapy without discontinuance. Dose reduction was defined as a 10%
or more reduction in the optimal dose calculated according to body
surface area. One HIVpositive HL patient was being
treated with doxorubicin/bleomycin/vinblastine/dacarbazine (ABVD)
at the time of this study and was therefore excluded from the
analysis of treatment completion and dose reduction. Two
HIVnegative HL patients without evaluable clinical
response were excluded from the analysis of the response rate and
treatment completion (Table I).
Statistical analysis
Age difference according to HIV status was assessed
using the Wilcoxon signed rank test. The difference in clinical
parameters according to the HIV status was assessed using the
chi-square test or Fisher's exact test, when appropriate. Overall
survival and PFS between groups divided by the HIV status were
compared using the log-rank (Mantel-Cox) test. GraphPad Prism
software (version 5c for Macintosh; GraphPad Software Inc., La
Jolla, CA, USA) was used for the statistical analysis, and P-values
<0.05 were considered to indicate a statistically significant
difference.
Results
Details of EBV-positivity
Among 19 HIVpositive HL patients in the
previous study, there were 16 EBV-positive patients (EBER and/or
LMP-1 positive 16, negative 2, unknown 1). Among the newly obtained
123 HIVnegative HL patients, there were 34 EBV-positive
patients (EBER and/or LMP-1 positive 34, negative 43, unknown 5,
not operated 42).
Characteristics of EBV+
HIVpositive Hodgkin lymphoma patients
The clinicopathologic features of 50 EBV+
HL patients, consisting of 16 HIVpositive and 34
HIVnegative patients, are summarized in Table I. All HIVpositive patients,
but one, had HL diagnosis in the cART era (i.e., after 1997); 14 of
the 16 HIVpositive patients developed HL during the HIV
follow-up at 40 (median) months (range, 6–84) after HIV diagnosis,
and the remaining two were initially found to have HIV infection at
the time of HL diagnosis. The HIVpositive HL patients
were significantly younger in terms of median age than the
HIVnegative HL patients (45 years old vs. 60.5 years
old: P=0.0158). The median CD4+ cell count
(CD4+ count) at HL diagnosis was 231/µl (range,
1–567/µl) in HIV-positive cases.
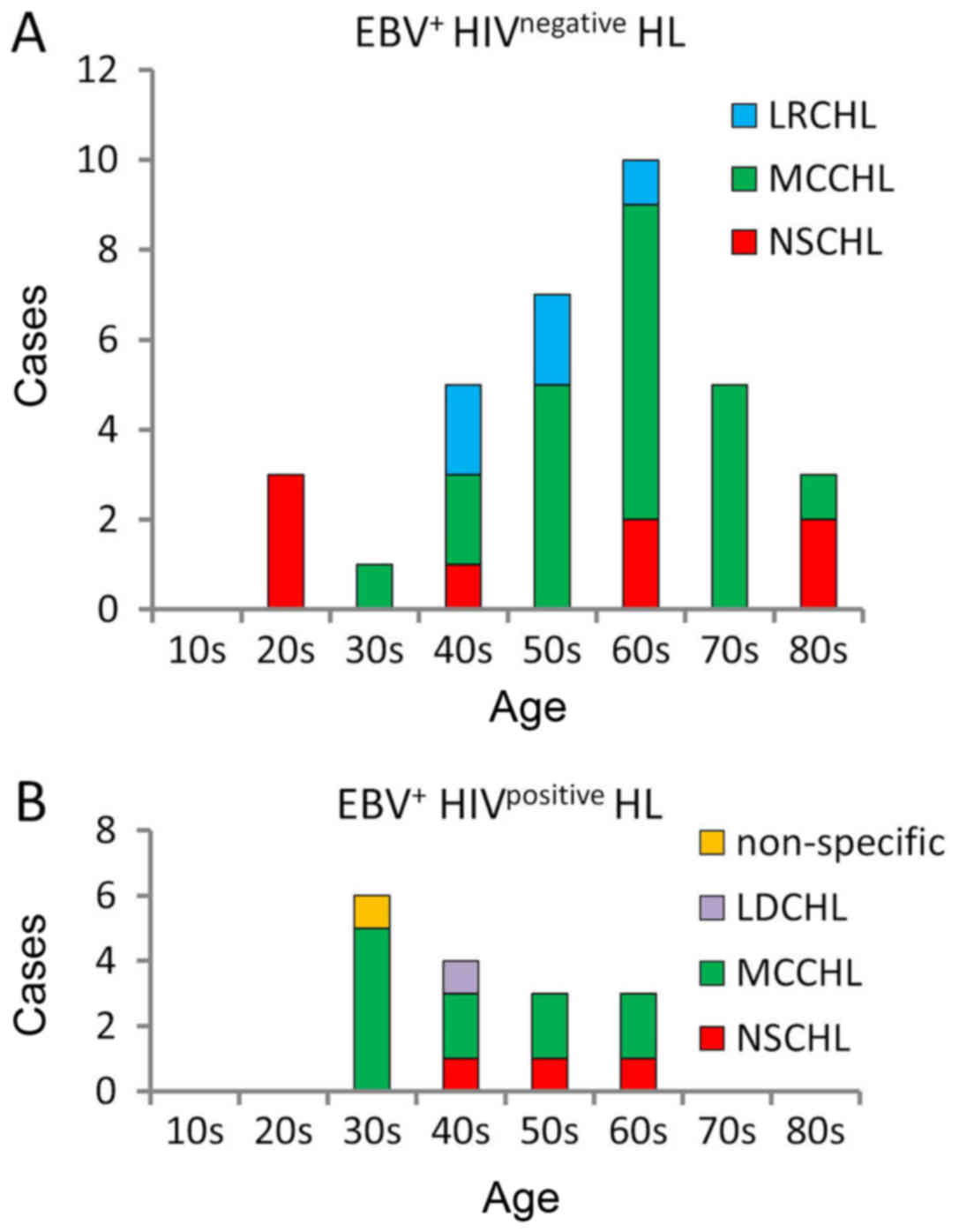
The most common subtype of HL was MCCHL in the
EBV+ HIVpositive group and EBV+
HIVnegative group (68.8% vs. 61.8%, respectively). The
patient's peak age of MCCHL incidence in the EBV+
HIVpositive group was in their 30s, whereas that in the
EBV+ HIVnegative group was in their 60s
(Fig. 1A and B). Patients in their
20s were not observed in the HIVpositive group; patients
in the EBV+ HIVnegative group showed a peak
incidence of nodular sclerosis classical Hodgkin lymphoma (NSCHL)
in their 20s and of MCCHL in their 60s similarly to previous
reports (17,18) (Fig. 1A).
By contrast, no case of NSCHL was encountered in the
EBV+ HIVpositive group particularly in the
patients who were below their 40s (Fig.
1B).
There were no significant differences in the
incidence of advanced stage between the HIVpositive
group and the HIVnegative group (81.3% vs. 67.6%:
P=0.258), or in the presence of B symptom (50% vs. 41.2%: P=0.388).
In contrast, significantly higher incidences of extranodal
involvement (56.3% vs. 20.6%: P=0.0150) and BM involvement by
itself (47.8% vs. 2.9%: P=0.000748) were observed in the
EBV+ HIVpositive group (Table I).
Treatment response and survival
The complete remission (CR) rate of the
HIVpositive HL patients was not significantly different
from that of the HIVnegative HL patients (84.6% vs.
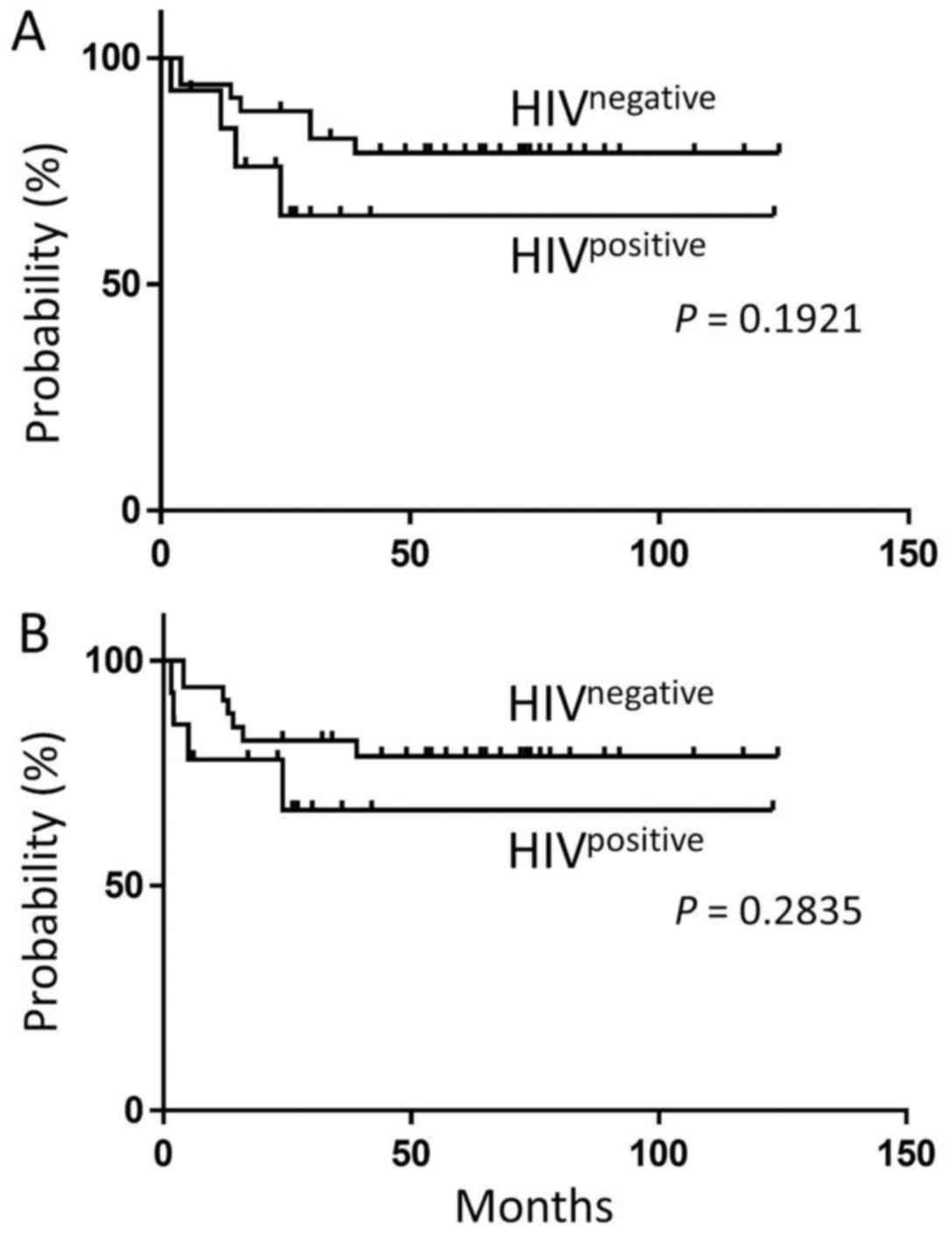
96.9%: P=0.196) (Table I). The OS of
the EBV+ HIVpositive HL patients, including
one patient under treatment, (median observational period, 23.5
months) was not significantly different from that of the
EBV+ HIVnegative HL patients (median
observational period, 64.5 months) (5-year OS probability: 65.1%
vs. 79.0%; P=0.1921) (Fig. 2A). There
was no significant difference in the PFS between EBV+
HIVpositive HL and EBV+
HIVnegative HL (5-year PFS probability: 66.8% vs. 78.7%;
P=0.2835) (Fig. 2B).
The treatment completion rate of the advanced-stage
patients treated with ABVD/ABVd was 90.0% in the
HIVpositive HL patients and 75.0% in the
HIVnegative HL patients (P=0.326) (Table I). The rate of the patients with
ABVD/ABVd dose reduction was 2/10 in the HIVpositive HL
patients and 0/20 in the HIVnegative HL patients
(P=0.103). Three of the 11 HIVpositive HL patients who
received ABVD/ABVd in the advanced stage expired due to disease
progression.
The CR rate of the advanced-stage
HIVpositive HL patients treated with ABVD/ABVd was 80.0%
(8/10), whereas that of the HIVnegative HL patients
treated with ABVD/ABVd was 94.7% (18/19). Among the advanced-stage
patients treated with ABVD/ABVd, the 5-year OS rate of the
EBV+ HIVpositive HL patients was not
significantly different from that of the EBV+
HIVnegative HL patients (56.6% vs. 75.0%; P=0.2063), as
well as the 5-year PFS rate (57.3% vs. 73.7%; P=0.2636). Of the 30
EBV+ advanced-stage HL patients, no significant
difference in OS was found in the low IPS group (0–2) (P=0.696) or high IPS (≥3) group (P=0.177)
by the log-rank test (data not shown).
Discussion
EBV infection is associated with an increased risk
of EBV-positive HL. EBV may play a role in the pathogenesis of
EBV-positive HL (5,6), but this aspect has not yet been fully
clarified. There have been comparisons between HL with EBV positive
and negative patients. A recent report by Koh et aldescribes
the impact of EBV-positivity on HL in Korea (12). There have also been comparisons
between HIV positive HL and HIV negative HL. However, there have
been no comparison data on EBV-positive HL between HIV positive and
negative patients. To find out the difference, we matched the
condition of EBV-positivity and made a comparison between the HIV
positive and negative groups. As EBV-positive HL and EBV-negative
HL act differently, it is essential to divide EBV-positive HL from
EBV-negative HL to elucidate the facts about the impact of HIV
infection.
The frequency of HL in HIV-infected individuals has
increased two-folds in the cART era (19). It is known that HIVpositive
HL patients have distinct clinicopathological features such as a
high rate of EBV positivity, advanced-stage disease (20–22), and
unfavorable histological subtypes, including MCCHL and
lymphocyte-depleted classical HL (4).
On the other hand, MCCHL is more likely to be EBV-positive across
all age groups, particularly in young adults (23). In the current study, the peak age of
the patients at EBV+ HIVpositive HL diagnosis
was during their 30s; this age distribution was quite different
from that observed in general HL showing biphasic peaks. Notably,
we never found NSCHL in the patients who were below their 40s in
the EBV+ HIVpositive HL group, whereas NSCHL
was generally found in younger HL patients likely in the
EBV+ HIVnegative HL group (Fig. 1A and B). This dissociation of
pathological subtypes in the young generation in HL regarding
HIVpositive HL, particularly for NSCHL, should be
confirmed in other ethnic cohorts as EBV-positive lymphoma is
frequently encountered in Asia.
We included two patients diagnosed by autopsy, since
the data of diagnosis and stage did not affect the clinical
results, including outcome (Table I).
They were excluded from prognostic analysis.
We found that EBV+ HIVpositive
HL showed a high frequency of extranodal involvement compared with
EBV+ HIVnegative HL, in accordance with
previous studies (20–22). In particular, BM involvement was
notable (47.8%) in HIV-positive patients. Ann Arbor staging
(P=0.5012) or IPS (P=0.7489) was not significantly different
whether the condition was HIV-positive or not, and the response to
therapy was comparative.
We cannot simply conclude that the comparative
clinical outcome between EBV+ HIVpositive HL
and EBV+ HIVnegative HL in this survey is
linked to the different aged populations with different
pathological subtypes. Nevertheless, even with the high frequency
of BM involvement in EBV+ HIVpositive HL,
there were no significant differences in the CR rate, OS
probability, and PFS compared with EBV+
HIVnegative HL. The current study demonstrated that the
standard chemotherapy for EBV+ HIVnegative HL
was acceptable for EBV+ HIVpositive HL in the
cART era, and we obtained almost even results in response to
chemotherapy as well as outcome between these two groups. These
results further suggest that Ann Arbor staging or IPS might be
helpful for planning therapeutic strategies for HL patients,
including EBV+ HIVpositive HL patients.
Introduction of new agents such as brentuximab
vedotin for CD30 blockade or nivolumab for programmed death (PD)-1
blockade (24) for relapsed HL
patients, and their combination with hematopoietic stem cell
transplantation for younger patients are currently major topics.
PD-1 is a regulator of the survival of virus-specific
CD8+ T cells in HIV infection (25), and it also plays a wide role in HIV
pathogenesis (26). Thus, PD-1 has
emerged as an attractive potential therapeutic target. The clinical
effects of humanized monoclonal antibodies for PD-1, including
nivolumab, for EBV+ HIVpositive HL are still
unknown. Therefore, we should pay more attention to the outcome of
EBV+ HIVpositive HL when treated with new
agents. As most EBV+ HIVpositive HL patients
are younger than 60 years, and more than 60% of them show MCCHL,
the therapeutic strategy for such patients is an important issue to
resolve.
The limitations of this study include the
retrospective nature of the analysis among different institutions
and terms. Although we performed a nationwide survey in Japan, the
number of patients is still small because of the low incidence of
HL in Japan [5% of malignant lymphoma (27)]. Nevertheless, there have been
apparently no data available regarding EBV+ HL patients
with comparison based on the HIV status.
In conclusion, we found that EBV+
HIVpositive HL preferentially occurred in a younger
population with no NSCHL, particularly in patients aged less than
40 years. In patients with the advanced stage of EBV+
HIVpositive HL, 80% of them did not require
dose-reduction and most of them completed chemotherapy. Standard
chemotherapy is effective and tolerable for EBV+ HL,
regardless of HIV infection.
HIV positivity may not have a negative impact on the
outcome in Japanese EBV+ HL. Thus, further evaluation of
different ethnic cohorts is needed to provide additional
information for delineating EBV+ HIVpositive
HL in the cART era.
Acknowledgements
The authors would like to thank Dr Isomura at the
Institute of Medical Science of Tokyo Medical University for his
suggestions in the statistical analysis. The authors also would
like to thank Dr Edward Barroga (http://orcid.org/0000-0002-8920-2607), Associate
Professor and Senior Medical Editor from the Department of
International Medical Communications of Tokyo Medical University
for reviewing and editing the manuscript.
Funding
This work was supported by the Research Program on
HIV/AIDS (grant nos. 16fk0410108h0001 and 15Afk0410004h0003) from
the Japan Agency for Medical Research and Development (AMED).
Availability of data and materials
The datasets used and analyzed during the current
study are available from the corresponding author on reasonable
request.
Author's contributions
MY, YI, and KO analyzed and interpreted the patient
data and were major contributors in writing the manuscript. SH
contributed to the nationwide data collection of HIV-positive
patients. YT and HN contributed for data collection of HIV-negative
patients. YO contributed to the pathological examination. SO
contributed to designing the study. AA, TU and JT contributed for
offering data from their affiliations.
Ethics approval and consent to
participate
This study was conducted in accordance with the
Declaration of Helsinki and local ethical legislation. This study
was approved by the Ethics Committee of Tokyo Medical University
Hospital (no. 2610; February 4, 2014), Cancer Institute Hospital of
JFCR, and National Hospital Organization Nagoya Medical Center.
Instead of obtaining informed consent from each patient,
participants were given the opportunity to opt-out.
Consent for publication
Not applicable.
Competing interests
MY declare that they have no competing interests. NH
received grants and personal fees from Chugai Pharmaceutical Co.,
grants and personal fees from Mundi Pharma, grants from Janssen
Pharmaceutical K.K, Celgene Corporation, Bayer Yakuhin Ltd., Abbvie
G.K., Takeda Pharmaceutical Co., Ltd., Bristol-Myers Squibb, and
personal fees from Sanofi K.K and Esai Co., Ltd. outside the
submitted work. KO received grants from Toyama Kagaku K.K., Nippon
Shinyaku K.K., Pfizer, Bristol-Myers Squibb, Alexion Pharma K.K.,
Taiho Yakuhin, Asahikasei, Chugai Pharma K.K., and Jansen Pharma
K.K, and personal fees from Celegen K.K., Novartis Pharma K.K., and
Dainippon-Sumitomo Pharma outside the submitted work.
References
|
1
|
Brugnaro P, Morelli E, Cattelan F,
Petrucci A, Panese S, Eseme F, Cavinato F, Barelli A and Raise E:
Non-acquired immunodeficiency syndrome defining malignancies among
human immunodeficiency virus-positive subjects: Epidemiology and
outcome after two decades of HAART era. World J Virol. 4:209–218.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Robbins HA, Pfeiffer RM, Shiels MS, Li J,
Hall HI and Engels EA: Excess cancers among HIV-infected people in
the United States. J Natl Cancer Inst. 107:dju5032015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Rubinstein PG, Aboulafia DM and Zloza A:
Malignancies in HIV/AIDS: From epidemiology to therapeutic
challenges. AIDS. 28:453–465. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Yotsumoto M, Hagiwara S, Ajisawa A, Tanuma
J, Uehira T, Nagai H, Fujikawa Y, Maeda S, Kitano K, Arima N, et
al: Clinical characteristics of human immunodeficiency
virus-associated Hodgkin lymphoma patients in Japan. Int J Hematol.
96:247–253. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Said JW: Immunodeficiency-related Hodgkin
lymphoma and its mimics. Adv Anat Pathol. 14:189–194. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Flavell KJ and Murray PG: Hodgkin's
disease and the Epstein-Barr virus. Mol Pathol. 53:262–269. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Dolcetti R, Boiocchi M, Gloghini A and
Carbone A: Pathogenetic and histogenetic features of HIV-associated
Hodgkin's disease. Eur J Cancer. 37:1276–1287. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Carbone A, Gloghini A, Serraino D and
Spina M: HIV-associated Hodgkin lymphoma. Curr Opin HIV AIDS.
4:3–10. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Lee JH, Kim Y, Choi JW and Kim YS:
Prevalence and prognostic significance of Epstein-Barr virus
infection an classical Hodgkin's lymphoma: A meta-analysis. Arch
Med Res. 45:417–431. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Keegan TH, Glaser SL, Clarke CA, Gulley
ML, Craig FE, Digiuseppe JA, Dorfman RF, Mann RB and Ambinder RF:
Epstein-Barr virus as a marker of survival after Hodgkin's
lymphoma: A population-based study. J Clin Oncol. 23:7604–7613.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Jarret RF, Stark GL, White J, Angus B,
Alexander FE, Krajewski AS, Freeland J, Taylor GM and Taylor PR:
Scotland and Newcastle Epidemiology of Hodgkin Disease Study Group:
Scotland and newcastle epidemiology of hodgkin disease study group.
Impact of tumor Epstein-Barr virus status on presenting features
and outcome in age-defined subgroups of patients with classic
Hodgkin lymphoma: A population-based study. Blood. 106:2444–2451.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Koh YW, Yoon DH, Suh C and Huh J: Impact
of the Epstein-Barr virus positivity on Hodgkin's lymphoma in a
large cohort from a single institute in Korea. Ann Hematol.
9:1403–1412. 2012. View Article : Google Scholar
|
|
13
|
Sorigué M, García O, Tapia G, Baptista MJ,
Moreno M, Mate JL, Sancho JM, Feliu E, Ribera JM and Navarro JT:
HIV-infection has no prognostic impact on advanced-stage Hodgkin
lymphoma. AIDS. 31:1445–1449. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Hagiwara S, Yotsumoto M, Odawara T,
Ajisawa A, Uehira T, Nagai H, Tanuma J and Okada S:
Non-AIDS-defining hematological malignancies in HIV-infected
patients: An epidemiological study in Japan. AIDS. 27:279–283.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Cheson BD, Horning SJ, Coiffer B, Shipp
MA, Fisher RI, Connors JM, Lister TA, Vose J, Grillo-López A,
Hagenbeek A, et al: Report of an international workshop to
standardize response criteria for non-Hodgkin's lymphomas. NCI
Sponsored International Working Group. J Clin Oncol. 17:12441999.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Hasenclever D and Diehl V: A prognostic
score for advanced Hodgkin's disease. International prognostic
factors project on advanced hodgkin's disease. N Engl J Med.
339:1506–1514. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Morton LM, Wang SS, Devesa SS, Hartge P,
Weisenburger DD and Linet MS: Lymphoma incidence patterns by WHO
subtype in the United States, 1992–2001. Blood. 107:265–276. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Shimabukuro-Vornhagen A, Haver H, Engert
A, Balleisen L, Majunke P, Heil G, Eich HT, Stein H, Diehl V and
Josting A: Lymphocyte-rich classical Hodgkin's lymphoma: Clinical
presentation and treatment outcome in 100 patients treated within
German Hodgkin's Study Group trials. J Clin Oncol. 23:5739–3745.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Shiels MS, Pfeiffer RM, Gail MH, Hall HI,
Li J, Chaturvedi AK, Bhatia K, Uldrick TS, Yarchoan R, Goedert JJ
and Engels EA: Cancer burden in the HIV-infected population in the
United States. J Natl Cancer Inst. 103:753–762. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Hoffman C, Chow KU, Wolf E, Faetkenheuer
G, Stellbrink HJ, van Lunzen J, Jaeger H, Stoehr A, Plettenberg A,
Wasmuth JC, et al: Strong impact of highly active antiretroviral
therapy on survival in patients with human immunodeficiency
virus-associated Hodgkin's disease. Br J Haematol. 125:455–462.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Nguyen ML, Farrell KJ and Gunthel CJ:
Non-AIDS-defining malignancies in patients with HIV infection in
the HAART era. Curr Infect Dis Rep. 12:46–55. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Spina M, Carbone A, Gloghini A, Serraino
D, Berretta M and Tirelli U: Hodgkin's disease in patients with HIV
infection. Adv Hematol. 2011:4026822011. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Glaser SL, Lin RJ, Stewart SL, Ambinder
RF, Jarrett RF, Brousset P, Pallesen G, Gulley ML, Khan G, O'Grady
J, et al: Epstein-Barr virus-associated Hodgkin's disease:
Epidemiologic characteristics in international data. Int J Cancer.
70:375–382. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Ansell SM, Lesokhin AM, Borrello I,
Halwani A, Scott EC, Gutierrez M, Schuster SJ, Millenson MM, Cattry
D, Freeman GJ, et al: PD-1 blockade with nivolumab in relapsed or
refractory Hodgkin's lymphoma. N Engl J Med. 372:311–319. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Petrovas C, Casazza JP, Brenchley JM,
Price DA, Gostick E, Adams WC, Precopio ML, Schacker T, Roederer M,
Douek DC and Koup RA: PD-1 is a regulator of virus-specific
CD8+ T cell survival in HIV infection. J Exp Med.
203:2281–2292. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Porochis F and Kaufmann DE: Role of PD-1
in HIV pathogenesis and as target for therapy. Curr HIV/AIDS Rep.
9:81–90. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
The World Health Organization
classification of malignant lymphomas in Japan: Incidence of
recently recognized entities. Lymphoma Study Group of Japanese
Pathologists. Pathl Int. 50:696–702. 2000. View Article : Google Scholar
|