Introduction
Colorectal carcinoma (CRC) is one of the most common
digestive tract malignancies in humans, particularly in
economically transitioning countries, such as China. Chinese
Traditional Medicine arsenic (As2O3) is a
toxic substance, used in ancient China to treat psoriasis,
rheumatism, cold, heat stroke and some other diseases, but its
applicability is limited by its high toxicity. With increasing
research, the treatment of As2O3 for
gastrointestinal tumors has attracted more attention (1–3). Several
studies have demonstrated that As2O3 can
induce tumor cell differentiation, promote tumor cell apoptosis and
inhibit tumor cell proliferation (4,5).
Aldehyde dehydrogenase 1 (ALDH1) is a detoxifying
enzyme responsible for the oxidation of intracellular aldehydes
(6,7).
Through its role in oxidizing retinol to retinoic acid, ALDH1 plays
an important role in the early differentiation of stem cells
(8). It has been demonstrated that
murine, human hematopoietic, neural stem and progenitor cells
exhibited high ALDH1 activity (9).
ALDH1 activity may represent a common marker for normal and
malignant stem cells (10). As a
marker of cancer stem cells (CSCs) in various malignancies, the
expression of ALDH1 is associated with chemoresistance and
increased malignant potential (10,11). Thus,
ALDH1 may play an important role in CRC carcinogenesis and it may
be used as a special marker for CSCs. Detecting the ALDH1 content
may help differentiate between colorectal carcinogenesis and normal
colorectal tissues (12).
In primary cell culture, we observed the colorectal
CSCs marked with ALDH1 under different concentrations of
As2O3. Notably, the primary culture of CRC
cells is associated with a high risk of bacterial contamination
(13). The aim of the present study
was to elucidate whether different concentrations of
As2O3 can affect colorectal CSCs and CRC
cells.
Materials and methods
Ethics statement
All experiments and the study protocol were approved
by the Ethics Committee of the First Hospital of Qiqihar (Qiqihar,
China).
Patients and tissue specimens
A total of 10 fresh CRC tissue specimens were
obtained from the Department of Pathology of the First Hospital of
Qiqihar between January 2015 and October 2015. Prior to surgery,
the patients included in the present study had received no therapy,
such as radiation therapy or chemotherapy. The tumor specimens were
moderately differentiated adenocarcinomas; 5 samples were rejected
due to bacterial overgrowth in the primary cell culture, and the
remaining 5 cases were included in the following experiments until
the completion of the present study.
Preparation of culture medium
Dulbecco's modified Eagle's medium (DMEM) and L-15
were added into a 2-l sterilized clean narrow-mouth bottle and the
bottle was filled with triple-distilled water to 920 ml, then
heated and gently stirred; 2.5 g sodium bicarbonate was added and
dissolved. After autoclaving, we added 20,000 IU/ml epidermal
growth factor (EGF), 10 mg basic fibroblast growth factor (bFGF),
10 mg leukemia inhibitory factor (LIF), 1 mol/l sodium hydroxide
solution to adjust the pH to 7.2, and triple-distilled water to 1
l. Finally, 111.11 ml fetal bovine serum (FBS) was added to a total
concentration of 10%. After sterilization, the medium was divided
into 200-ml bottles, sealed and stored in a refrigerator at 4°C.
After preparation of the culture medium, 2 ml medium was added into
a 12-well culture plate, then cultured for 3 days and placed in an
incubator at 37°C and 5% CO2 to prepare culture medium
I. As2O3 0.1 mol/l was diluted 50-fold with
culture medium I to a final concentration of 2.0 µM, which was used
as the culture medium II. As2O3 0.1 mol/l was
then diluted 25-fold with culture medium I to a final concentration
of 4.0 µM (culture medium III). Similarly, 0.1 mol/l
As2O3 was diluted 12.5 times with culture
medium I to a final concentration of 8.0 µM (culture medium
IV).
Primary cell culture steps
In brief, tissues were washed several times in
serum-free DMEM supplemented with antibiotic-antimycotic agents
(Sigma-Aldrich; Merck KGaA, Darmstadt, Germany). Then, the
specimens were minced into 1–2 mm3 pieces followed by
incubation in collagenase type IV (0.05 mg/ml; Sigma-Aldrich; Merck
KGaA) and hyaluronidase (2 µg/ml; Sigma-Aldrich; Merck KGaA) at
37°C for 1 h. Single-cell suspension was obtained by mixing every
15 min and filtration through a 70-µm cell strainer (BD
Biosciences, Franklin Lakes, NJ, USA). The primary CRC cell culture
was maintained in serum-free stem cell medium containing DMEM-F12
with B-27 (1×) and N-2 (1×) supplements (Invitrogen; Thermo Fisher
Scientific, Inc., Waltham, MA, USA), bovine serum albumin (4 mg/ml;
Roth, Qiqihar, China), non-essential amino acids (1×;
Sigma-Aldrich; Merck KGaA), glucose 0.15% (Sigma-Aldrich; Merck
KGaA), insulin (4 U/l; Sigma-Aldrich; Merck KGaA), heparin (4
µg/ml), N-acetylcystein (1 mm; Sigma-Aldrich; Merck KGaA),
EGF (20 ng/ml; Biomol), bFGF (20 ng/ml; Miltenyi Biotec, Inc.,
Cambridge, MA, USA), and penicillin/streptomycin (1×; China
National Pharmaceutical Group, Ltd., Beijing, China).
According to the culture medium used (I, II, III or
IV), 24-well plates were labeled as Groups A-D, respectively. In
the B-D Groups 20 µl As2O3 was added to a
final concentration in the cell culture of 2.0, 4.0 and 8.0 µM,
respectively (M60, China National Pharmaceutical Group, Ltd.). A
total of 2,000 cells were plated in each well of the 24-well
plates, and 0.4 ml medium was added to each well. After mixing, the
plates were placed in a 5% CO2 incubator at 37°C. The
cell culture medium was changed every 24 h using the same amount of
fluid and the same ratio of resuspended culture cells, while
recording the absolute cell count and mean cell density.
From the following day, every 24 h a well was
removed from each group, placed on an anti-off slide and fixed with
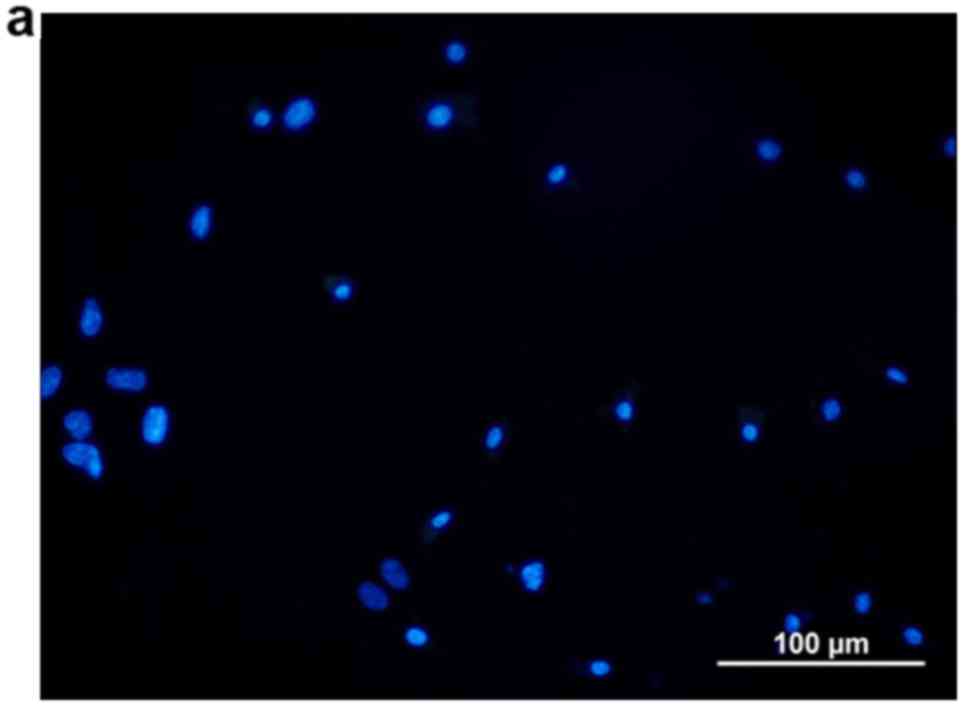
neutral formalin. After drying, fluorescent specific staining was
performed and the percentage of ALDH1-positive cells was detected
and recorded (HZ3487111; EarthOx LLC, San Francisco, CA, USA). In
addition, 4′,6′-diamidino-2-phenyl-indole (DAPI; D9564;
Sigma-Aldrich, St. Louis, MO, USA) was used to non-specifically
stain the nuclei of cancer cells and CSCs with blue color.
Statistical analysis
All data were analyzed by SPSS software version 11.0
(SPSS, Inc., Chicago, IL, USA). Test data are presented as the mean
± standard deviation of 4 independent experiments. Two groups of
data were compared using an independent samples t-test. Multiple
groups of quantitative data were compared using one-way analysis of
variance with Student-Newman-Keuls post hoc test if the data was
homogenous and Dunnett's T3 post hoc test if the data was not.
P<0.05 was considered to indicate statistically significant
differences. When drawing the cell survival curves, the vertical
axis represents the number of cells and the horizontal axis
represents the time of primary cell culture.
Results
As2O3 effect on
CSCs
The results revealed that different concentrations
of As2O3 exerted different effects.
Particularly in Group D (As2O3 8.0 µM), after
6 days the density of DAPI-labeled tumor cells was significantly
lower as observed through an inverted biological microscope, in
contrast with the density of ALDH1-labeled CSCs that was
significantly higher.
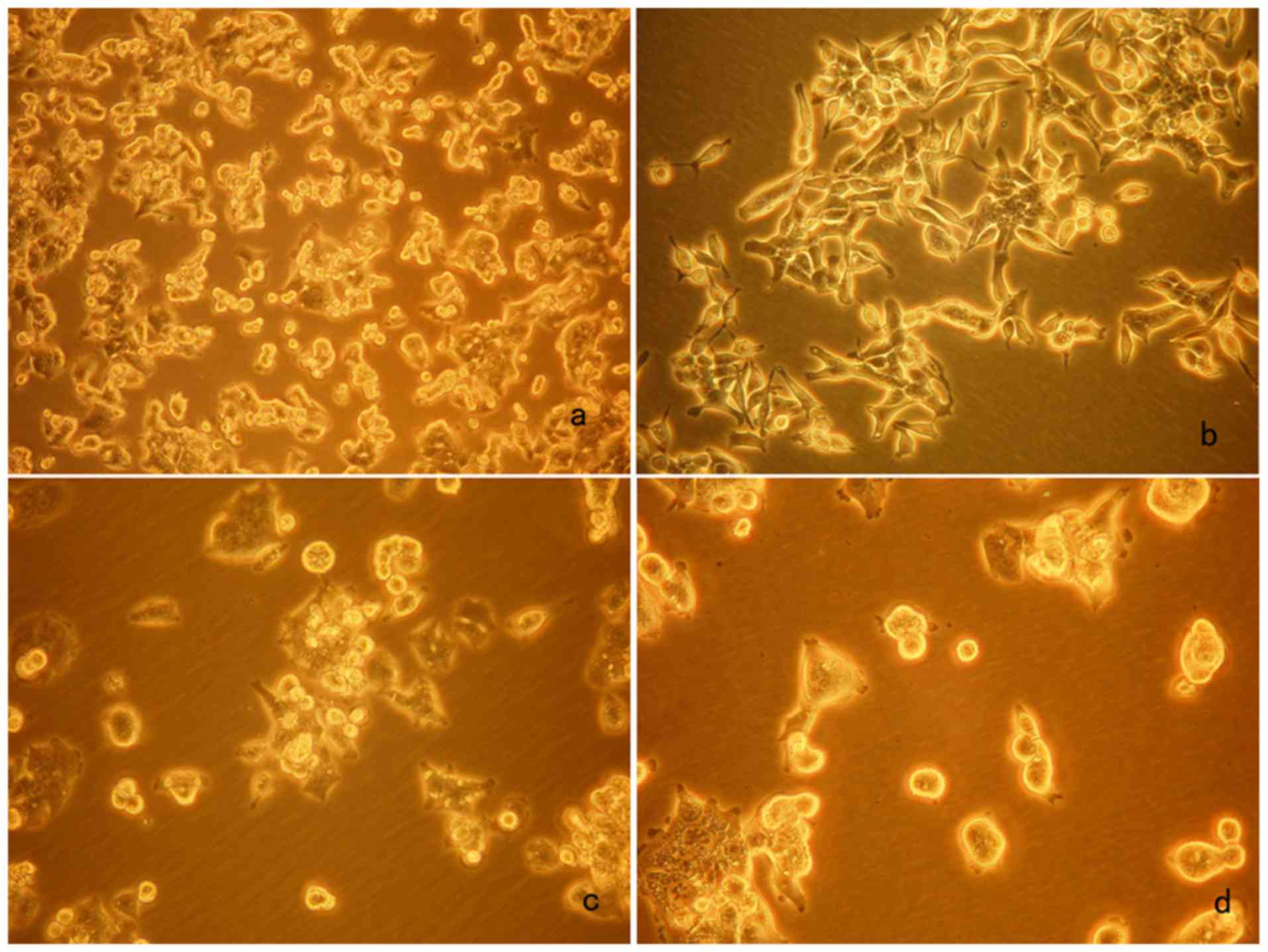
Evaluation of cell morphology
In the blank control (Group A), cell proliferation
was observed, and a proportion of the cells adhered to the wall
after 24 h as observed using an inverted biological microscope.
After 72 h, cell growth was dense and cell morphology was diverse,
with active cell proliferation covering the bottom of the well by
>75%. CSCs stained by ALDH1 were not identified in Group A.
Continuing this culture would deviate from the purpose of the
experiment; therefore, data were not recorded after this period.
After 72 h, the number of cells was lower in Group B
(As2O3 2.0 µM) and proliferating cells could
not be identified. The fluorescence staining of ALDH1 detected very
few cells. In Group C (As2O3 4.0 µM), a
proportion of the cells adhered to the wall, meantime cell mass
reduced and the number of CSCs was low at 72 h. In Group D
(As2O3 8.0 µM), after 72 h a proportion of
the cells adhered to the wall, the shape of cells was spherical and
cell mass reduced, at which time visible fluorescent staining for
ALDH1 appeared. On the following day, the number of cells was
significantly lower, the shape of cells was spherical and most
cells were suspended, with very few cells adhering to the wall
(Tables I–VI). In Groups B, C and D, the respective
concentrations of As2O3 were applied 3 times
for each experiment. With increasing concentration of
As2O3, the viability of CRC cells decreased
significantly (P<0.05), indicating a dose-dependent inhibitory
effect of As2O3 on cancer cell growth
(Table VII). Primary cell culture
images at 72 h are shown in Fig.
1a-d. Fluorescent ALDH1 cytoplasmic red staining and DAPI
nuclear blue staining at 72 h in Group D may be seen in Fig. 2a-c.
 | Table I.ALDH1 expressed in a 5% CO2
incubator after 24 h at 37°C. |
Table I.
ALDH1 expressed in a 5% CO2
incubator after 24 h at 37°C.
| Groups | Mean cell density
(107/ml) | ALDH1 positive rate
(%) |
|---|
| A | 2.5674±0.7895 | 0.1532±0.0032 |
| B | 1.5478±0.2812 | 0.2013±0.0013 |
| C | 1.0038±0.3786 | 0.3341±0.0031 |
| D | 0.5113±0.0879 | 4.6673±0.5076 |
 | Table VI.ALDH1 expressed in a 5%
CO2 incubator after 6 days at 37°C. |
Table VI.
ALDH1 expressed in a 5%
CO2 incubator after 6 days at 37°C.
| Groups | Mean cell density
(107/ml) | ALDH1 positive rate
(%) |
|---|
| A | – | – |
| B | – | – |
| C | 0.0030±2.3245 | – |
| D | 0.0088±0.0019 | 87.6302±1.6723 |
 | Table VII.Analysis of colorectal carcinoma cell
viability. |
Table VII.
Analysis of colorectal carcinoma cell
viability.
| Groups | 24 h | 72 h | 96 h |
|---|
| A | 2.5674±0.7895 | 20.8231±0.8011 |
124.6730±3.0027 |
| B |
1.5478±0.2812a |
1.0138±0.8996a |
0.6431±0.4832a |
| C |
1.0038±0.3786a |
0.9235±0.5644a |
0.1506±0.0513a |
| D |
0.5113±0.0879a |
0.0223±0.0013a |
0.0063±0.0016a |
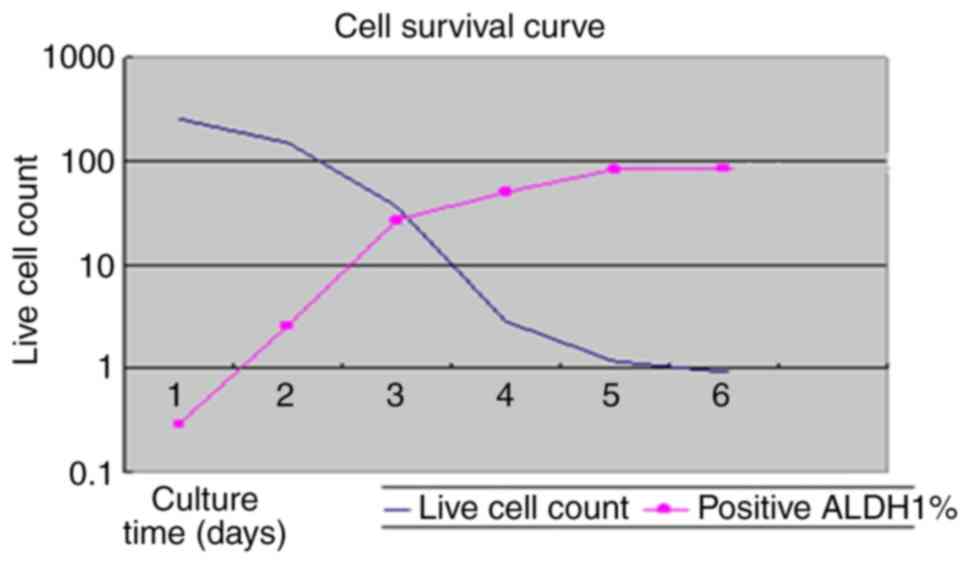
Cell survival curve
The abscissa represents the culture time of the
primary cells and the ordinate represents the live cell count in
the cell survival curve. The curve demonstrated that ALDH1-labeled
CSCs were significantly increased, while the number of CRC cells
was significantly reduced under the effect of
As2O3 8.0 µM (Fig.
3). Data were normalized to control (Group A).
These results demonstrated that
As2O3 inhabited CRC cell growth. However,
As2O3 could not induce CSCs death, but
instead increased the CSC population.
Discussion
CRC is one of the most common digestive tract
malignancies in humans and one of the major causes of
cancer-related mortality (14).
Primary cell culture is valuable as it may closely replicate the
real living environment of the cells. Due to the complex intestinal
bacterial environment in CRC, the final stage of the culture often
fails due to bacterial contamination; however, the survival stage
of the primary cell culture may still be used for valuable
research.
ALDH1 is a detoxifying enzyme that oxidizes
intracellular aldehydes, thereby conferring resistance to
alkylating agents (15,16). ALDH1 is a known common expression
marker in CSCs and stem cells. Compared with normal colorectal
tissues, tumor tissues express high levels of ALDH1 (17). In addition, some studies demonstrated
that ALDH1 may be associated with chemoresistance and increased
malignant potential (10,11). ALDH1 was first used in combination
with CD133 to detect CSCs in CRC; however, the results revealed
that they both stained the cytoplasm of CSCs, which would interfere
with the observation. Upon staining for each antibody individually,
the results were similar. Thus, ALDH1 was used to mark the CSCs of
CRC, and DAPI was used to non-specifically stain the nuclei of the
tumor cells, which confirmed the theory that CSCs are derived from
normal stem cell mutations and may help in investigating the effect
of As2O3 on CSCs (6).
The pharmacological effects of
As2O3 attracted more attention following
promising results as treatment for acute promyelocytic leukemia
(APL). The main antitumor mechanism of action of
As2O3 is through inducing tumor cell
differentiation and apoptosis, inhibiting tumor cell proliferation
and affecting the tumor neoangiogenesis (4,5,18). Our research demonstrated that
As2O3 inhibited the growth of tumor cells,
the conversion from colorectal CSCs to CRC cells, and it increased
the density of CSCs. The reason may be related to the expression of
the Bcrp1/ABCG2 gene on the cell surface of CSCs. This gene is a
highly efficient membrane transporter that transfers
As2O3 from the intracellular to the
extracellular environment. Furthermore, 0.1 mol/l
As2O3 was diluted to obtain a concentration
of >8.0 µM, and we found that this concentration of
As2O3 destroyed all the cells in the culture
dish. Therefore, this high toxicity would damage all normal cells
and deviated from the purpose of the experiment.
In conclusion, appropriate concentration of
As2O3 may increase the CSC population, which
may help with the research regarding the tumorigenic ability and
drug resistance of CSCs.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
All data and materials generated or analyzed in the
present study are included in this manuscript.
Authors' contributions
KN, FZ were responsible for study conception and
design. WN, XN and XW performed data collection and assembly. All
authors approved the final version of this manuscript.
Ethics approval and consent to
participate
All experiments and the study protocol were approved
by the Ethics Committee of the First Hospital of Qiqihar (Qiqihar,
China). Informed consent was obtained from all patients prior to
their inclusion within the study.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
CRC
|
colorectal carcinoma
|
|
CSCs
|
cancer stem cells
|
|
As2O3
|
Chinese Traditional Medicine
arsenic
|
|
ALDH1
|
aldehyde dehydrogenase 1
|
|
DAPI
|
4′,6′-diamidino-2-phenyl-indole
|
|
APL
|
acute promyelocytic leukemia
|
|
DMEM
|
Dulbecco's modified Eagle's medium
|
References
|
1
|
Shao QS, Ye ZY, Ling ZQ and Ke JJ: Cell
cycle arrest and apoptotic cell death in cultured human gastric
carcinoma cells mediated by arsenic trioxide. World J
Gastroenterol. 11:3451–3456. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Liu H, Zhang Z, Chi X, Zhao Z, Huang D,
Jin J and Gao J: Arsenite-loaded nanoparticles inhibit PARP-1 to
overcome multidrug resistance in hepatocellular carcinomacells. Sci
Rep. 6:310092016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Yu G, Chen X, Chen S, Ye W, Hou K and
Liang M: Arsenic trioxide reduces chemo-resistance to
5-fluorouracil and cisplatin in HBx-HepG2 cells via complex
mechanisms. Cancer Cell Int. 15:1162015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Chan JY, Siu KP and Fung KP: Effect of
arsenic trioxide on multidrug resistant hepatocellular carcinoma
cells. Cancer Lett. 236:250–258. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Ji H, Li Y, Jiang F, Wang X, Zhang J, Shen
J and Yang X: Inhibition of transforming growth factor beta/SMAD
signal by MiR-155 is involved in arsenic trioxide-induced
anti-angiogenesis in prostate cancer. Cancer Sci. 105:1541–1549.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Rahadiani N, Ikeda J, Mamat S, Matsuzaki
S, Ueda Y, Umehara R, Tian T, Wang Y, Enomoto T, Kimura T, et al:
Expression of aldehyde dehydrogenase 1 (ALDH1) in endometrioid
adenocarcinoma and its clinical implications. Cancer Sci.
102:903–908. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Rassouli FB, Matin MM and Saeinasab M:
Cancer stem cells in human digestive tract malignancies. Tumour
Biol. 37:7–21. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Chute JP, Muramoto GG, Whitesides J,
Colvin M, Safi R, Chao NJ and McDonnell DP: Inhibition of aldehyde
dehydrogenase and retinoid signaling induces the expansion of human
hematopoietic stem cells. Proc Natl Acad Sci USA. 103:11707–11712.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Matsui W, Huff CA, Wang Q, Malehorn MT,
Barber J, Tanhehco Y, Smith BD, Civin CI and Jones RJ:
Characterization of clonogenic multiple myeloma cells. Blood.
103:2332–2336. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Li T, Su Y, Mei Y, Leng Q, Leng B, Liu Z,
Stass SA and Jiang F: ALDH1A1 is a marker for malignant prostate
stem cells and predictor of prostate cancer patients' outcome. Lab
Invest. 90:234–244. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Li K, Guo X, Wang Z, Li X, Bu Y, Bai X,
Zheng L and Huang Y: The prognostic roles of ALDH1 isoenzymes in
gastric cancer. Onco Targets Ther. 9:3405–3414. 2016.PubMed/NCBI
|
|
12
|
Zhou F, Mu YD, Liang J, Liu ZX, Zhou D,
Ning WL, Li YZ, Ding D and Zhang JF: Aldehyde dehydrogenase 1: A
specific cancer stem cell marker for human colorectal carcinoma.
Mol Med Rep. 11:3894–3899. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Qureshi-Baig K, Ullmann P, Rodriguez F,
Frasquilho S, Nazarov PV, Haan S and Letellier E: What do we learn
from spheroid culture systems? Insights from tumorspheres derived
from primary colon cancer tissue. PLoS One. 11:e01460522016.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Center MM, Jemal A and Ward E:
International trends in colorectal cancer incidence rates. Cancer
Epidemiol Biomarkers Prev. 18:1688–1694. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Duester G: Families of retinoid
dehydrogenases regulating vitamin A function: Production of visual
pigment and retinoic acid. Eur J Biochem. 267:4315–4324. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Sophos NA and Vasiliou V: Aldehyde
dehydrogenase gene superfamily: The 2002 update. Chem Biol
Interact. 143–144:5–22. 2003. View Article : Google Scholar
|
|
17
|
Zhou F, Mu YD, Liang J, Liu ZX, Chen HS
and Zhang JF: Expression and prognostic value of tumor stem cell
markers ALDH1 and CD133 in colorectal carcinoma. Oncol Lett.
7:507–512. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Lang M, Wang X, Wang H, Dong J, Lan C, Hao
J, Huang C, Li X, Yu M, Yang Y, et al: Arsenic trioxide plus PX-478
achieves effective treatment in pancreatic ductal adenocarcinoma.
Cancer Lett. 378:87–96. 2016. View Article : Google Scholar : PubMed/NCBI
|