Introduction
Basal cell carcinoma (BCC) is the most common type
of skin malignancy in the Caucasian population, with a continuously
increasing incidence rate (1).
Metastatic BCC is rare, but the tumor may occasionally grow
aggressively, causing extensive tissue destruction, and the high
incidence of this cancer represents a major public health concern
(2). BCC most commonly occurs in
adults, particularly in the elderly. However, in recent years it
has become increasingly more common among younger adults,
particularly women (3). BCC has
several subtypes with distinct clinical and histological
characteristics, with the nodular (nBCC) and superficial subtypes
being the most common. nBCC, which accounts for 40–60% of BCCs,
usually occurs in sun-exposed body areas, most commonly on the
head, neck and upper extremities (4).
Despite extensive scientific research, the etiology
of BCC remains unknown, but appears to have a multifactorial
origin, which is the result of intrinsic and extrinsic factors
(5). The development of BCC is
clearly associated with sunburns, exposure to arsenic acid,
pesticides, ionizing radiation and X-rays (5,6). In
addition, intrinsic constitutional factors, including gender, age
and genetic predisposition, as well as pigment-associated traits,
including fair skin, blond or red hair, light eye color, tendency
to sunburn and poor tanning ability (Fitzpatricks skin phototype
I), have all been associated with a higher risk of BCC (7). However, the primary established risk
factor in the development of BCC, which is primarily responsible
for cutaneous damage, is considered to be ultraviolet radiation
(UVR), particularly short-wavelength UVB (8,9). UVB
radiation damages DNA and its repair system, which contributes to
immune system alterations and resulting in genetic changes that may
eventually lead to cancer formation (10). Furthermore, changes in the expression
profile of specific proteins may be associated with skin
carcinogenesis (11–16).
Transforming growth factor (TGF)-β is a
multifunctional polypeptide, the cytostatic and apoptotic functions
of which restrain tissue growth. Abnormalities or complete loss of
these functions lead to hyperproliferative disorders that are
common in cancer (11). Furthermore,
tumor cells may overproduce TGF-β to create a local
immunosuppressive environment that contributes to tumor growth
(11). Thus, interest has been
focused on elucidating the association between BCC and the
expression of the proteins involved in the TGF-β signaling
pathway.
Matrix metalloproteinases (MMPs) are a family of
proteolytic enzymes that have the ability to degrade collagen,
elastin fibers and other proteins of connective tissue. Diseases
characterized by degradation of extracellular matrix (ECM)
proteins, including cancer, are also associated with upregulation
of MMP expression, often previously induced by UVR (12,13). It
has not been clearly determined which of these molecules serve
essential roles in the development and progression of BCC.
Cathepsin-K is a cysteine protease from the papain
family with collagenolytic and elastinolytic properties. It was
recently demonstrated that cathepsin-K is involved in the
degradation of ECM in a number of organs, including the skin, which
is an important step in tumor invasion and metastasis (14,15). The
role of this protein in cutaneous tumors has not been fully
elucidated. Recent data indicate that overexpression of cathepsin-K
is associated with the invasive and metastatic tendency of
malignant melanoma. Its expression was also detected in squamous
cell carcinoma (SCC) (14,16). However, the expression of cathepsin-K
in BCC has been reported in only a few studies (14,15).
The mutant laminin A protein, also known as
progerin, was identified in Hutchinson-Gilford syndrome, but
elevated expression of this protein was also detected in naturally
aging skin (17). To the best of our
knowledge, there is no current information in the available
literature regarding the expression of progerin in skin with
BCC.
The aim of the present study was to assess the
expression profile of selected proteins (TGF-β, Smad2, MMP-1, −3,
−8 and −9, cathepsin-K and progerin) in samples with diagnosed BCC
in comparison with control skin samples collected from matched
areas using western blotting and reverse transcription-quantitative
polymerase chain reaction (RT-qPCR) analysis.
Materials and methods
Patients
The study group consisted of 22 patients (10 men and
12 women; mean age, 59 years; range, 44–82 years) with
histopathologically confirmed nBCC, and 22 healthy volunteers (10
men and 12 women; mean age, 59 years; range, 43–78 years) as a
control group. All patients with nBCC were diagnosed and treated at
the Department of Dermatology and Venereology at the Medical
University of Łódź (Łódź, Poland) between January 2013 and December
2015. All subjects signed written informed consent forms prior to
enrolment to the study. The study design was approved by the local
Ethics Committee (Komisja Bioetyczna przy Uniwersytecie Medycznym w
Łodzi) and was conducted in accordance with the principles outlined
in the Declaration of Helsinki. None of the examined subjects were
HIV-positive, transplant recipients, suffering from other
immunodeficiency disorders or receiving immunosuppressive
treatment. Subjects in the healthy volunteer group who used indoor
tanning or were overexposed to the sun during the last 2 months
were excluded from the present study. Skin specimens in the control
group were collected from the same areas as in the BCC group using
the punch biopsy method (18). The
tissues from all participants were immediately frozen in liquid
nitrogen and stored at −80°C until analysis.
Western blotting
For western blotting, skin specimens from all the
participants were lysed in 0.25% Triton X-100 lysis buffer
(Sigma-Aldrich; Merck KGaA, Darmstadt, Germany). Protein
concentrations were measured using a bicinchoninic acid Protein
Assay kit (Thermo Fisher Scientific, Inc., Waltham, MA, USA). A
total of 50 µg crude cell lysate protein per lane was separated via
SDS-PAGE (10% gel) and transfered to polyvinylidene difluoride
membranes (EMD Millipore, Billerica, MA, USA). The membranes were
incubated with 5% skimmed milk with 0.1% Tween 20 (Sigma-Aldrich;
Merck KGaA) overnight at 4°C with gentle agitation. Subsequently,
the membranes were incubated for 1 h, at room temperature, with
gentle agitation with specific protein-conjugated primary
antibodies purchased from Santa Cruz Biotechnology, Inc. (Dallas,
TX, USA): Rabbit anti-TGF-β2 (V, sc-90), goat anti-Smad (C-17,
sc-6030), mouse anti MMP-1 (3B6, sc-21731), goat anti-MMP-3 (C-19,
sc-6839), goat anti-MMP-8 (M-20, sc-8848), mouse anti-MMP-9 (2C3,
sc-21733), mouse anti-cathepsin K (E-7), sc-48353) and pmouse
anti-progerin (13A4D4, sc-81611), all at 1:200 dilution. Following
three washes, the membranes were incubated for 1 h at room
temperature, with gentle agitation, with horseradish
peroxidase-conjugated secondary antibodies: Mouse anti-rabbit IgG
(sc-2357), mouse anti-goat (sc-2354) and donkey anti-mouse IgG
(sc-2318), all at 1:20.000 dilution (Santa Cruz Biotechnology,
Inc.), and developed for 5 min in darkness using an enhanced
chemiluminesence plus Western Blotting Detection kit (GE
Healthcare, Chicago, IL, USA) and visualized with the ChemiImager
system (ProteinSimple, San Jose, CA, USA). The bands corresponding
to the proteins were digitalized using ChemiImager software Alpha
Innotech 4400 (ProteinSimple). Quantitative densitometry analysis
was performed using AlphaEaseFC Version 3.3.0. (ProteinSimple, San
Jose, CA, USA).
RT-qPCR
To determine the TGF-β, Smad2, MMP-1, MMP-3, MMP-8,
MMP-9, CTSK (cathepsin-K) and LMNA (progerin) mRNA levels, RT-qPCR
was employed. Total RNA was isolated using RNeasy Mini kit (Qiagen,
Inc., Valencia, CA, USA). The RNA was treated with DNase I to
remove contaminated genomic DNA and 2 µg RNA was
reverse-transcribed using the High-Capacity cDNA Reverse
Transcription kit according to the manufacturers protocol (Applied
Biosystems; Thermo Fisher Scientific, Inc.). The expression of
target gene mRNA, as well as the expression of the endogenous Actb
(β-actin) mRNA, was assessed using TaqMan® probes dyed
with FAM and TaqMan® Gene Expression Master mix (Applied
Biosystems; Thermo Fisher Scientific, Inc.). TaqMan®
probes TGF-β (Hs00234244_m1), Smad2 (Hs00183425), MMP-1
(Hs00899658_m1), MMP-3 (Hs00968305_m1), MMP-8 (Hs01029057_m1),
MMP-9 (Hs00234579_m1), CTSK (Hs00166156_m1), LMNA (Hs00153462_m1)
and hActb (Hs99999903_m1) detect only genomic DNA and span an exon
junction. Gene-specific PCR products were measured using the 7900HT
Fast Real-Time PCR system (Applied Biosystems; Thermo Fisher
Scientific, Inc.) continuously during 40 cycles. The amplification
conditions were as follows: Initial denaturation at 95°C for 20
sec, followed by 40 cycles of amplification at 95°C for 30 sec and
60°C for 30 sec. Target gene expression was normalized to the
endogenous expression of β-actin in each cDNA sample. Quantitative
analysis of data was performed according to the ∆∆Cq method
(19).
Statistical analysis
Statistical analysis was performed using Statistica
12 (StatSoft, Inc., Tulsa, OK, USA) and GraphPad Prism 6 (GraphPad
Software, Inc., La Jolla, CA, USA) software. The results were
analyzed using the Mann-Whitney U test and P<0.05 was considered
to indicate a statistically significant difference. Results are
presented as the medians + interquartile range of integrated
density values (IDV) for protein expression and mean + standard
deviations for mRNA expression.
Results
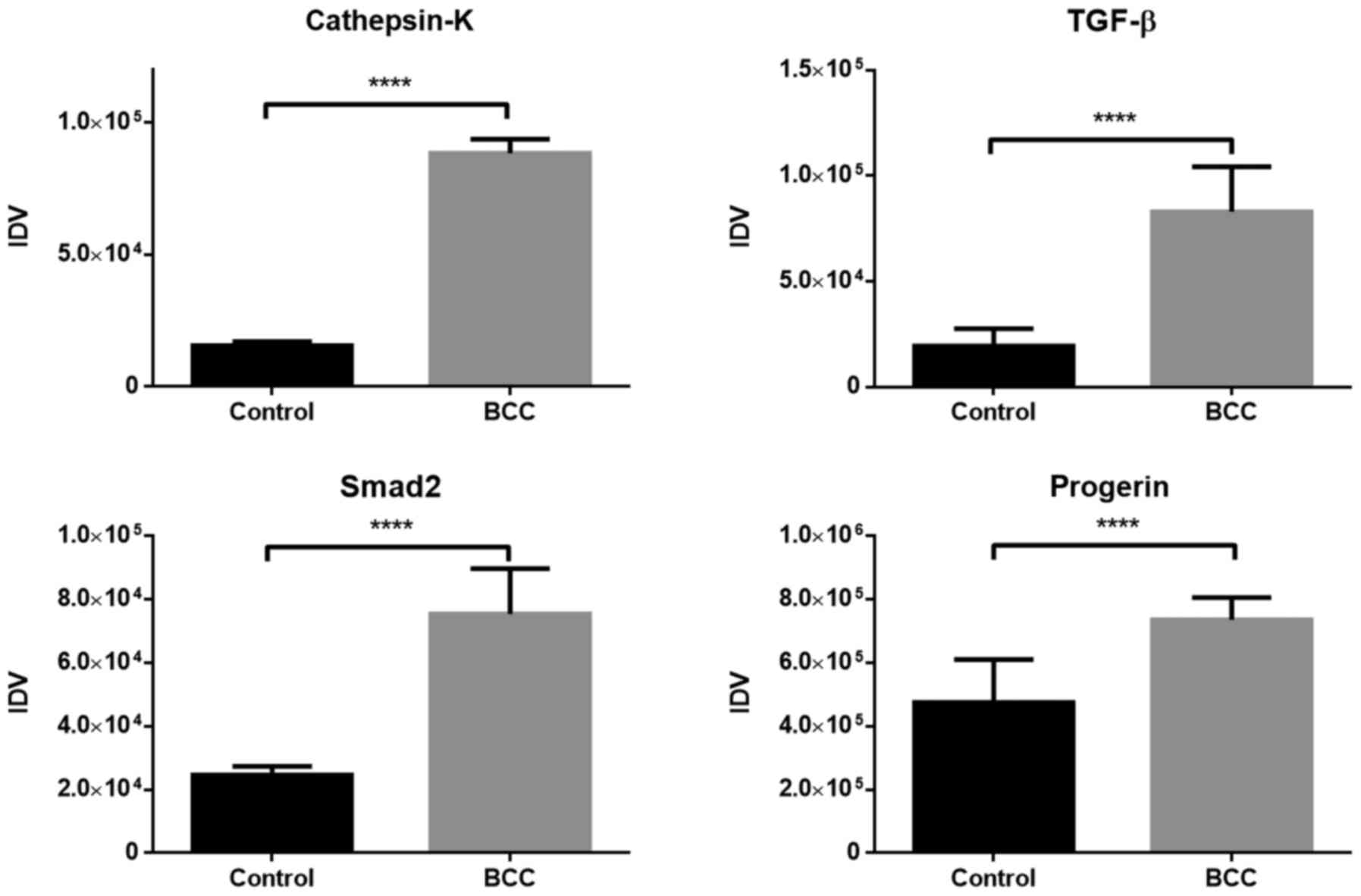
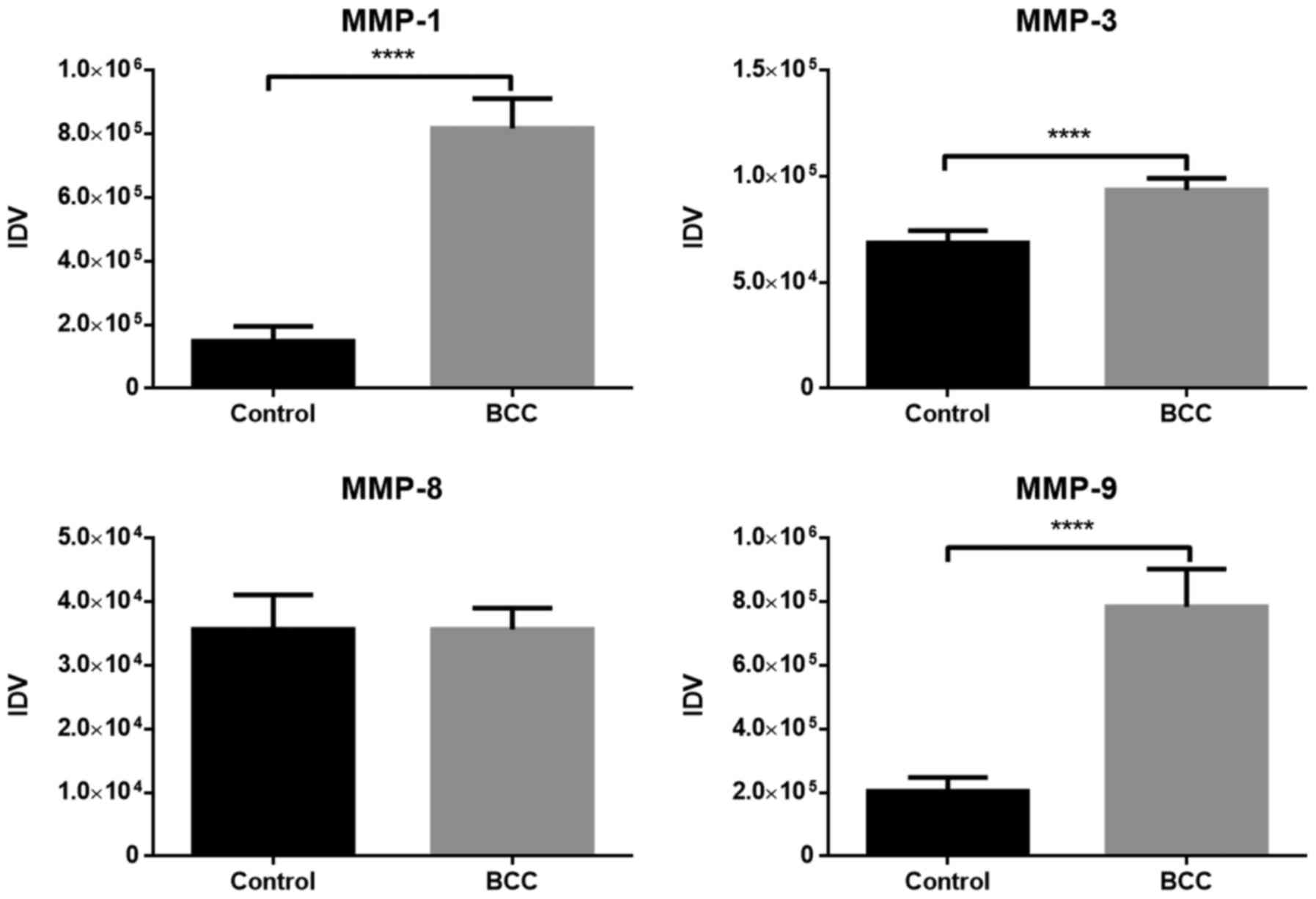
The expression of TGF-β, Smad2, MMP-1, −3, −8 and
−9, cathepsin-K and progerin was assessed in all skin specimens.
The expression levels of the analyzed proteins in skin biopsies
with diagnosed BCC compared with the healthy control group are
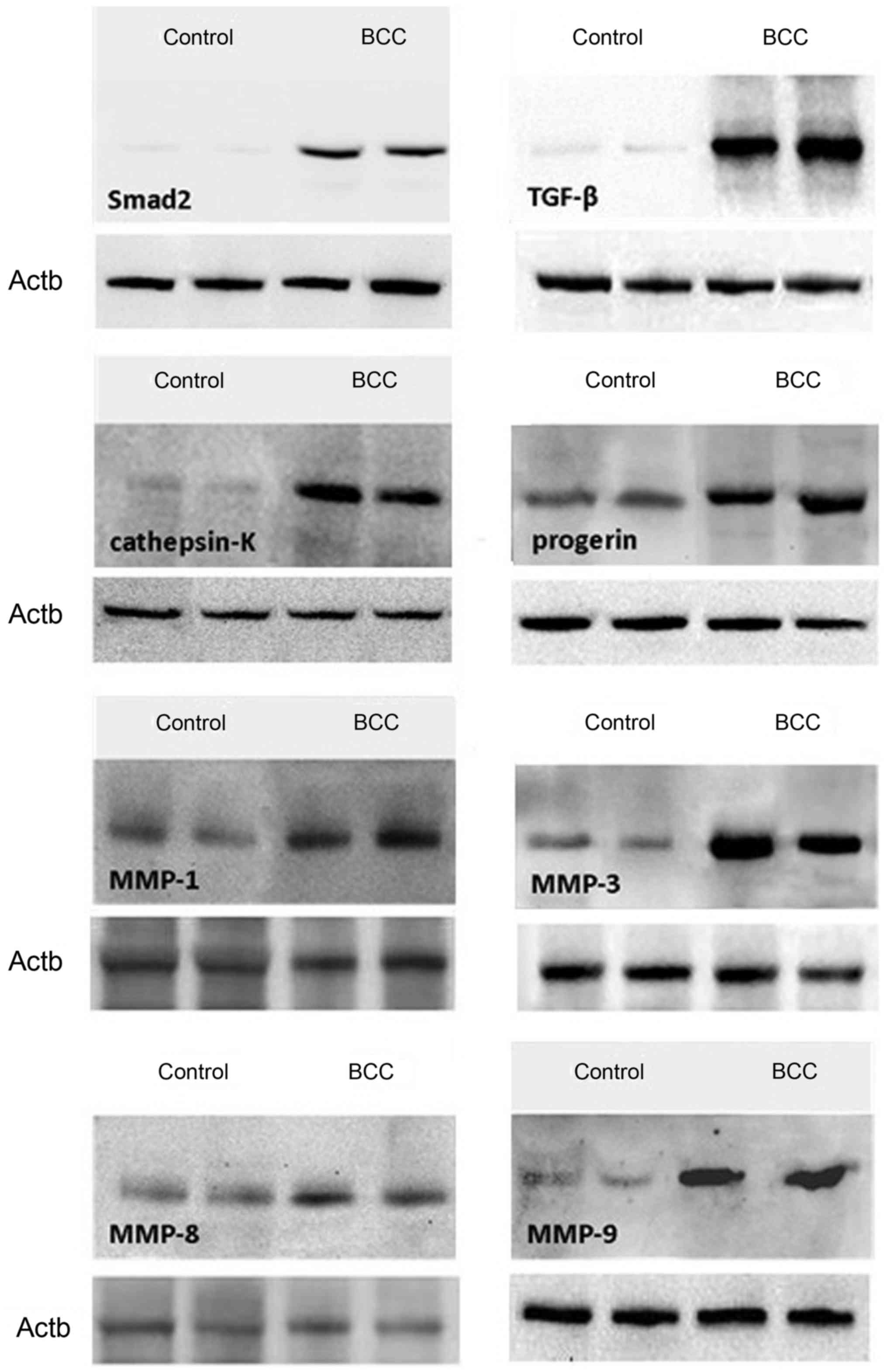
presented in Figs. 1 and 2, while representative western blots of the
protein expression levels are presented in Fig. 3. All values in the figures are shown
as the medians of IDV. The expression of all proteins evaluated
with western blotting was demonstrated to be significantly higher
in samples with diagnosed BCC compared with those from the control
group (P<0.05), apart from MMP-8, for which the difference in
expression between the two groups was insignificant (P=0.59).
The control group exhibited the highest expression
level for progerin compared with other proteins. The median value
of progerin expression was 4.75×105 IDV, while the value
of expression in tumor tissues was 7.37×105 IDV, and
those changes were statistically significant (P<0.05). MMP-1
exhibited the highest difference in protein expression between
healthy and affected tissues.
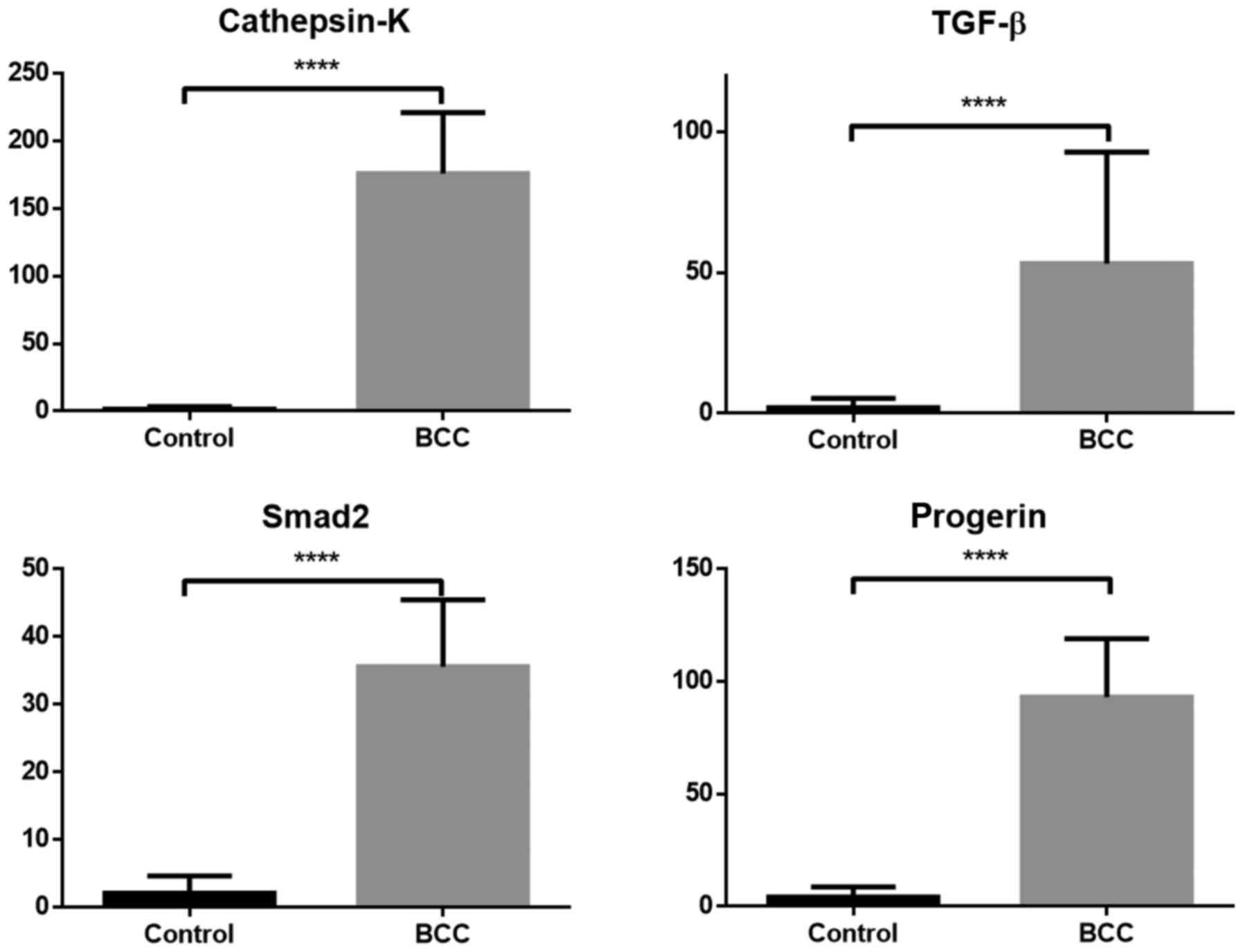
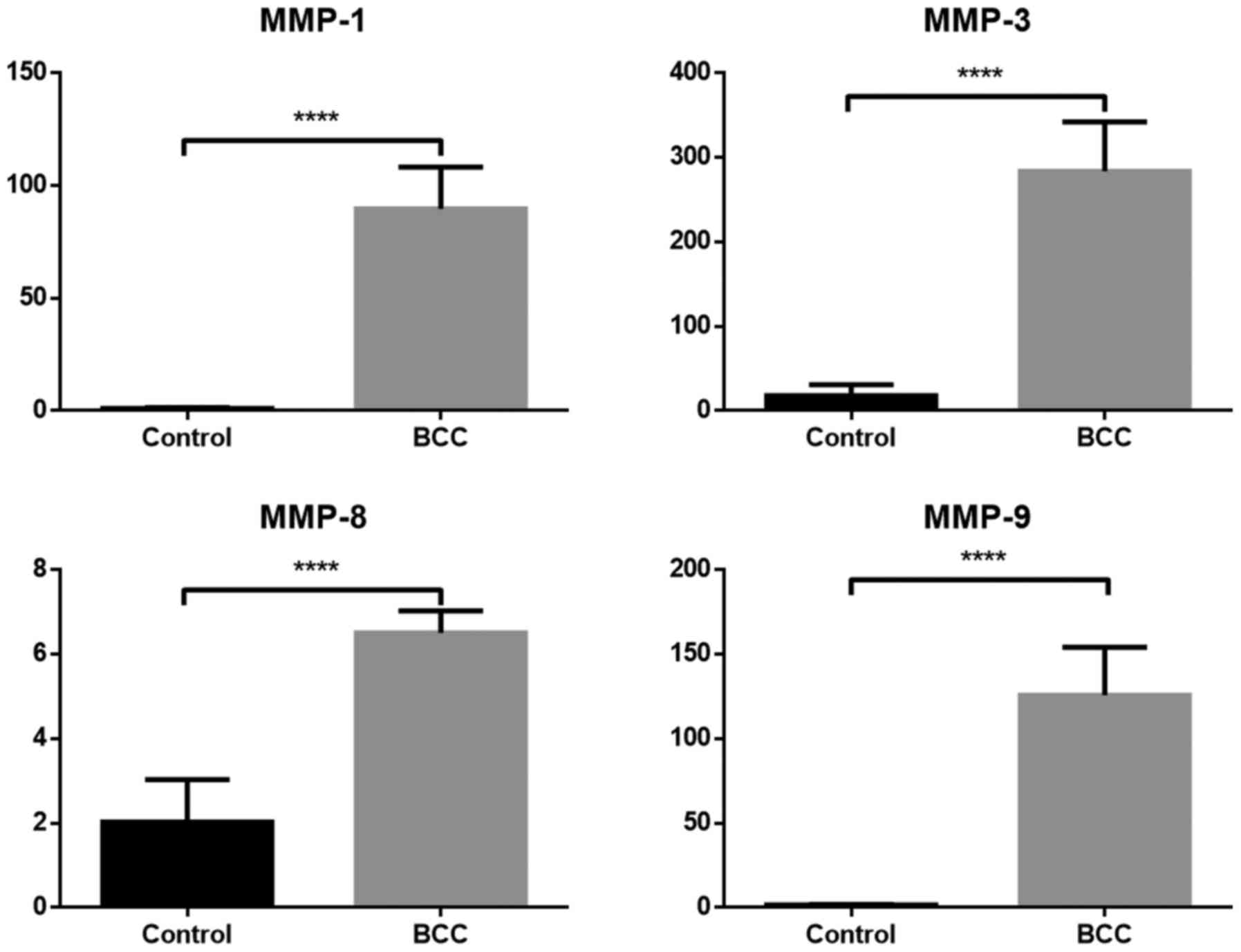
A comparison of mRNA expression between the BCC and
control group for analyzed samples that were evaluated by RT-qPCR
is presented in Figs. 4 and 5. The mRNA level for all samples (TGF-β,
Smad2, cathepsin-K, progerin and MMP-1, −3, −8 and −9) was
significantly higher in samples with diagnosed BCC compared with
the healthy control group (P<0.05). MMP-3 exhibited the highest
difference in mRNA expression between BCC and control cases.
Discussion
BCC remains the most common skin malignancy, with a
rapidly increasing incidence rate worldwide (20). The exact mechanism underlying the
pathogenesis of BCC has not been clearly determined. A possible,
but unsupported explanation is the role of proteins that are
involved in skin aging, as well as proteins that are responsible
for the regulation of cell division in the skin, the dysregulation
of which may lead to carcinogenesis. In the present study, the
contribution of eight proteins to the development of nBCC was
examined.
The role of TGF-β in the development of multiple
carcinomas has been previously confirmed. Available data regarding
dysregulation of the TGF-β/Smad pathway indicate its role in the
development of pancreatic or breast cancer, as well as various skin
cancer types, including melanoma and SCC (21). However, the role of this cytokine and
its effector proteins in BCC development remains unclear (21).
Cui et al (22)
confirmed that the role of TGF-β changes as the carcinogenic
process evolves. TGF-β in normal tissues inhibits cell
proliferation and is able to block the cell cycle in the
G1 phase, whereas in tumor tissues it is involved in the
progression of carcinogenesis and metastatic spread (23–26). This
phenomenon is referred to as the TGF-β paradox (24,25).
Schmid et al (27) compared the mRNA expression of all
three isoforms of TGF-β (TGF-β1, TGF-β2 and TGF-β3) and TGF-β type
II receptor (TβR II) in normal human skin and BCC tissue, and
observed increased TGF-β1 and TβR II. Tumor tissues of all BCCs
exhibited reduced TGF-β3 mRNA and protein expression, compared with
normal interfollicular epidermis; whereas, the expression of TGF-β1
mRNA was notably reduced in tumor tissues and normal skin epithelia
(27). The data were confirmed by
research performed by Gambichler et al (28) and Furue et al (29).
Gambichler et al (28) revealed that the mRNA levels of
TGF-β/Smad observed in healthy control skin did not significantly
differ from the TGF-β/Smad levels detected in non-lesioned skin of
patients with previously diagnosed BCC. As opposed to previous
studies, significant mRNA overexpression of TGF-β1, Smad3 and Smad7
was observed in BCC compared with non-lesional skin. These data on
TGF-β are consistent with those of the present study, which
revealed a significant increase of TGF-β expression at the mRNA and
protein levels in skin biopsies with diagnosed BCC compared with
healthy skin biopsies collected from the control group. Similar
results were obtained in the results of the present study regarding
Smad2. Thus, this data indicates a possible role of TGF-β/Smad
signaling in the pathogenesis of BCC.
MMPs are produced by healthy cells, as well as
cancer cells. Overexpression of certain MMPs in pancreatic,
gastric, lung and breast cancer is associated with a more
aggressive disease course and higher propensity for invasion and
metastasis (30). Altered MMP
activity in cancer cells leads to excessive activation of
extracellular proteolysis. MMP-9 serves the most essential role in
BCC progression. This protein degrades collagen and elastin fibers,
enhancing the ability of cancer cells to migrate beyond the tumor
(31). Increased expression of MMP-9
is associated with the extent of tumor progression (32–34). MMP-1
and MMP-3 are also involved in the degradation of collagens,
promoting rapid tumor development. The present study revealed
significant differences in the level of mRNA and protein expression
of MMP-1, −3 and −9 between healthy skin biopsies and in skin
biopsies with diagnosed BCC.
Wyględowska-Kania et al (35) reported a significant increase in the
number of MMP-1 gene copies in all BCC samples. The available
literature confirms that the expression of MMP-9 was significantly
higher in BCC samples compared with that in unaffected skin.
Monhian et al (36) stated
that the expression levels of MMP-1 and MMP-9 were consistently
elevated in the peritumoral tissue compared with skin from more
distal sites. Varani et al (37) analyzed tissues from 54 histologically
confirmed BCCs of the skin using immunochemical procedures. In
normal skin, the activity levels of MMP-1 and MMP-9 were low to
undetectable. By contrast, the expression of these proteins was
increased in tumor tissues.
The results of the current study demonstrated
detectable expression of MMPs in skin samples with diagnosed BCC,
as well as healthy skin. Tumor-affected tissues exhibited
significantly increased expression of MMP-1, −3 and −9 at the mRNA
and protein levels compared with unaffected tissues. These data
confirm the important role of this protein in carcinogenesis, and
these changes in expression may be useful in the diagnosis of
tumor-affected tissues.
Previous analysis of MMP-8 in breast cancer patients
indicated that expression of this MMP by breast tumors is
associated with a lower incidence of lymph node metastasis
(38). On this basis, MMP-8 may be a
tumor protective factor, which also has the ability to reduce the
metastatic potential of malignant cells in humans (38). In the present study, significant
overexpression of MMP-8 mRNA was observed in BCC, which has
previously been observed in human SCC (39).
However, the western blot studies in the present
study revealed only a slight increase in MMP-8 protein in the tumor
tissue. This discrepancy in MMP-8 expression at the mRNA and
protein levels may be explained by the fact that RT-qPCR is a
significantly more sensitive technique that is able to document the
slightest evidence of gene expression. However, western blot
analysis is important, since it reveals whether the transcript is
translated and functionally available. Furthermore, the protein may
be translated at normal levels as observed in the control group,
but degraded at an abnormal level as observed in the BCC group,
which may also be a plausible explanation. To the best of our
knowledge, these results were the first to demonstrate the
expression of MMP-8 in BCC tissues by applying RT-PCR and western
blot analysis.
Cathepsin-K serves an important role in homeostasis
of dermal ECM by maintaining the balance between protein synthesis
and proteolysis (40,41). It was previously confirmed that
cathepsin-K is involved in various processes associated with
photodestruction, scarring and carcinogenesis (40). However, the expression of cathepsin-K
in BCC has not been well characterized. To the best of our
knowledge, there are only few published studies that have
investigated the expression of cathepsin-K in BCC (14,15).
In a study performed by Quintanilla-Dieck et
al (14), it was demonstrated
that cathepsin-K immunostaining has distinct staining patterns for
melanoma and non-melanoma skin cancer (NMSCs). Unlike melanoma,
SCCs and BCCs do not or only focally express this protein. Weak
focal expression of cathepsin-K within tumor cells was detected in
only 2/3 cases of diagnosed BCC, while in the remaining BCC samples
no staining was observed. However, in all samples with BCC, there
was strong expression in the peritumoral stroma, which was markedly
more intense compared with that in the peritumoral stroma
surrounding certain melanomas. It was suggested that stromal
cathepsin-K expression may promote invasion of NMSCs and contribute
to bone invasion by activation of osteoclasts at the invasion
front, whereas when these tumor cells encounter an invasion barrier
in which cathepsin-K is not induced, they may fail to invade and
produce metastasis. This may explain why NMSCs, particularly BCC,
are less prone to metastasis compared with melanoma.
Ishida et al (15) assessed cathepsin-K expression by
immunohistochemical methods in a group of 50 consecutive operative
cases of BCC and compared the results with the expression of this
protein in healthy skin. In normal skin, cathepsin-K expression was
observed in the stratum corneum, mature sebaceous cells and outer
root sheath of the hair follicles. Cathepsin-K was expressed in the
tumor cells of all BCC cases. Increased levels of cathepsin-K
expression may contribute to tumor invasion of BCC, similar to
malignant melanoma. Data from these trials obtained using
immunohistochemistry indicate that the expression of cathepsin-K
protein is altered in BCC (14,15). The
present study aimed to investigate the mRNA as well as protein
expression levels of cathepsin-K in patients with BCC and healthy
controls. The expression of cathepsin-K was detected in all
analyzed samples. This expression was the weakest in healthy skin
and was significantly higher in BCC compared with unaffected
skin.
Lamins are components of nuclear proteins that
affect essential processes in the nucleus, including transcription
and replication. In previous studies, reduced or no expression of
the normal form of lamin A was identified to be associated with a
rapid growth rate of the tumor (42–44). By
contrast, another study demonstrated the presence of substantial
amounts of lamin A in proliferating BCC cells (45). In recent years, increasing efforts
have been focused on evaluating the role of progerin (mutated form
of lamin A) in photoaging and mechanisms leading to carcinogenesis.
Scaffidi and Misteli (46) revealed a
contribution of progerin to the photoaging process. This protein is
resistant to degradation induced by MMPs. McClintock et al
(17) analyzed the expression of
progerin in skin biopsies from unaffected individuals with a wide
age range (newborn to 97 years old). They revealed that progerin
mRNA may be detected at uniform levels by RT-PCR analysis in all
tissue samples, but the protein appears to accumulate with
increasing age. Their findings revealed that progerin expression is
a biomarker of normal cellular aging, and may be associated with
terminal differentiation and senescence in elderly individuals.
However, although the role of progerin expression in relation to
skin aging has been investigated, to the best of our knowledge, the
present study is the first systematic study to investigate the
expression of progerin protein in human nBCC using RT-qPCR as well
as western blotting.
In the present study, all analyzed skin biopsies
exhibited progerin expression at the mRNA and protein level.
Furthermore, a significant increase in expression was observed in
biopsies with diagnosed BCC compared with healthy skin samples. The
accumulation of progerin may contribute to the dysregulation of
cell proliferation that results in carcinogenesis, which may
explain the overexpression of progerin in BCC tissues.
In summary, the results of the present study were
the first to demonstrate enhanced expression of progerin in BCC
tissue. Furthermore, the comparison of the expression of TGF-β,
Smad2, cathepsin-K and MMP-1, −3, −8 and −9 between BCC lesions and
healthy control skin samples confirms the important role of these
proteins in skin carcinogenesis. These findings may enable a better
understanding of the mechanisms underlying BCC development and
provide new insight into the development of novel therapeutic
strategies for BCC. Thus far, therapy with inhibitor proteins has
been successfully used in the treatment of bone loss associated
with osteoporosis, as well as in an experimental study on breast
cancer cell invasion (38,47,48). The
present study investigated the protein expression pattern in BCC,
and assessed its usefulness as a potential diagnostic marker and
molecular therapy target for the treatment of common skin cancers,
including BCC. Further studies are required to verify the
effectiveness of topical administration of protein inhibitors as a
treatment for BCC. However, the validity of the present data may be
limited, as only the nBCC subtype was examined. Further studies are
required to investigate the effect of various proteins on other BCC
subtypes.
Acknowledgements
Not applicable.
Funding
The present study was funded by the National Centre
of Science (grant no. 2012/05/B/NZ5/01885) and the Medical
University of Łódź (grant nos. 503/5-064-01/503-01 and
503/1-152-01/503-11-002).
Availability of data and materials
The datasets used and analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors contributions
PK, JN and AW conceived and designed the
experiments. KW, MS, AL and MC performed the experiments. MC wrote
the manuscript with support from MS. IAB assisted in interpreting
the results and worked on the manuscript. AL supervised the
project. All authors discussed the results and commented on the
manuscript. All authors read and approved the final manuscript.
Ethics approval and consent to
participate
All subjects signed written informed consent forms
prior to enrolment to the study. The study design was approved by
the local Ethics Committee (Komisja Bioetyczna przy Uniwersytecie
Medycznym w Łodzi) and was conducted in accordance with the
principles outlined in the Declaration of Helsinki.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Lesiak A, Slowik-Rylska M, Rogowski-Tylman
M, Sysa-Jedrzejowska A, Norval M and Narbutt J: Risk factors in
Central Poland for the development of superficial and nodular basal
cell carcinomas. Arch Med Sci. 6:270–275. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Tilli CM, Van Steensel MA, Krekels GA,
Neumann HA and Ramaekers FC: Molecular aetiology and pathogenesis
of basal cell carcinoma. Br J Dermatol. 152:1108–1124. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Miller DL and Weinstock MA: Nonmelanoma
skin cancer in the United States: Incidence. J Am Acad Dermatol.
30:774–778. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Daya-Grosjean L and Couvé-Privat S: Sonic
hedgehog signaling in basal cell carcinomas. Cancer Lett.
225:181–192. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Dessinioti C, Antoniou C, Katsambas A and
Stratigos AJ: Basal cell carcinoma: Whats new under the sun.
Photochem Photobiol. 86:481–491. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Corona R, Dogliotti E, DErrico M, Sera F,
Iavarone I, Baliva G, Chinni LM, Gobello T, Mazzanti C, Puddu P and
Pasquini P: Risk factors for basal cell carcinoma in a
Mediterranean population: Role of recreational sun exposure early
in life. Arch Dermatol. 137:1162–1168. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Green A, Battistutta D, Hart V, Leslie D
and Weedon D: Skin cancer in a subtropical Australian population:
Incidence and lack of association with occupation. The Nambour
Study Group. Am J Epidemiol. 144:1034–1040. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Gallagher RP and Lee TK: Adverse effects
of ultraviolet radiation: A brief review. Prog Biophys Mol Biol.
92:119–131. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Oberyszyn TM: Non-melanoma skin cancer:
Importance of gender, immunosuppressive status and vitamin D.
Cancer Lett. 261:127–136. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Lupi O: Correlations between the Sonic
Hedgehog Pathway and basal cell carcinoma. Int J Dermatol.
46:1113–1117. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Siegel PM and Massagué J: Cytostatic and
apoptotic actions of TGF-beta in homeostasis and cancer. Nat Rev
Cancer. 3:807–820. 2003. View
Article : Google Scholar : PubMed/NCBI
|
|
12
|
Yucel T, Mutnal A, Fay K, Fligiel SE, Wang
T, Johnson T, Baker SR and Varani J: Matrix metalloproteinase
expression in basal cell carcinoma: Relationship between enzyme
profile and collagen fragmentation pattern. Exp Mol Pathol.
79:151–160. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Poswar FO, Fraga CA, Farias LC,
Feltenberger JD, Cruz VP, Santos SH, Silveira CM, de Paula AM and
Guimarães AL: Immunohistochemical analysis of TIMP-3 and MMP-9 in
actinic keratosis, squamous cell carcinoma of the skin, and basal
cell carcinoma. Pathol Res Pract. 209:705–709. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Quintanilla-Dieck MJ, Codriansky K, Keady
M, Bhawan J and Rünger TM: Cathepsin K in melanoma invasion. J
Invest Dermatol. 128:2281–2288. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Ishida M, Kojima F and Okabe H:
Cathepsin-K expression in basal cell carcinoma. J Eur Acad
Dermatology Venereol. 27:e128–e130. 2013. View Article : Google Scholar
|
|
16
|
Yan X, Takahara M, Xie L, Oda Y, Nakahara
T, Uchi H, Takeuchi S, Tu Y, Moroi Y and Furue M: Stromal
expression of cathepsin-K in squamous cell carcinoma. J Eur Acad
Dermatology Venereol. 25:362–365. 2011. View Article : Google Scholar
|
|
17
|
McClintock D, Ratner D, Lokuge M, Owens
DM, Gordon LB, Collins FS and Djabali K: The mutant form of lamin A
that causes Hutchinson-Gilford progeria is a biomarker of cellular
aging in human skin. PLoS One. 2:e12692007. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zuber TJ: Punch biopsy of the skin. Am Fam
Physician. 65(1155–1158): 1161–1162, 1164. 2002.
|
|
19
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Ting PT, Kasper R and Arlette JP:
Metastatic basal cell carcinoma: Report of two cases and literature
review. J Cutan Med Surg. 9:10–15. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Han G and Wang XJ: Roles of TGFβ signaling
Smads in squamous cell carcinoma. Cell Biosci. 2011(1): 412011.
View Article : Google Scholar
|
|
22
|
Cui W, Fowlis DJ, Bryson S, Duffie E,
Ireland H, Balmain A and Akhurst RJ: TGFbeta1 inhibits the
formation of benign skin tumors, but enhances progression to
invasive spindle carcinomas in transgenic mice. Cell. 86:531–542.
1996. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Stalińska L and Ferenc T: The role of
TGF-beta in cell cycle regulation. Postepy Hig Med Dosw (Online).
59:441–449. 2005.(In Polish). PubMed/NCBI
|
|
24
|
Bierie B and Moses HL: Tumour
microenvironment: TGFbeta: The molecular Jekyll and Hyde of cancer.
Nat Rev Cancer. 6:506–520. 2006. View
Article : Google Scholar : PubMed/NCBI
|
|
25
|
Hata A, Shi Y and Massagué J: TGF-beta
signaling and cancer: Structural and functional consequences of
mutations in Smads. Mol Med Today. 4:257–262. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Li AG, Lu SL, Han G, Kulesz-Martin M and
Wang XJ: Current view of the role of transforming growth factor
beta 1 in skin carcinogenesis. J Investig Dermatol Symp Proc.
10:110–117. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Schmid P, Itin P and Rufli T: In situ
analysis of transforming growth factors-beta (TGF-beta 1, TGF-beta
2, TGF-beta 3) and TGF-beta type II receptor expression in basal
cell carcinomas. Br J Dermatol. 134:1044–1051. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Gambichler T, Skrygan M, Kaczmarczyk JM,
Hyun J, Tomi NS, Sommer A, Bechara FG, Boms S, Brockmeyer NH,
Altmeyer P and Kreuter A: Increased expression of TGF-beta/Smad
proteins in basal cell carcinoma. Eur J Med Res. 12:509–514.
2007.PubMed/NCBI
|
|
29
|
Furue M, Kato M, Nakamura K, Nashiro K,
Kikuchi K, Okochi H, Miyazono K and Tamaki K: Dysregulated
expression of transforming growth factor beta and its type-I and
type-II receptors in basal-cell carcinoma. Int J Cancer.
71:505–509. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Witek K, Goździalska A and Jaśkiewicz J:
Metaloproteinazy jako nowy wskaźnik diagnostyczny nowotworów skóry.
Stan skóry wykładnikiem stanu zdrowia. (red.) Anna Goździalska,
Jerzy Jaśkiewicz. Kraków: Oficyna Wydawnicza AFM. 19–29. 2012.
|
|
31
|
Śliwowska I and Kopczyński Z:
Metaloproteinazy macierzy zewnątrzkomórkowej-charakterystyka
biochemiczna i kliniczna wartość oznaczania u chorych na raka
piersi. Wsp Onkol. 9:327–335. 2005.(In Polish).
|
|
32
|
Lukaszewicz-Zajac M, Mroczko B and
Szmitkowski M: The significance of metalloproteinases and their
inhibitors in gastric cancer. Postepy Hig Med Dosw (Online).
63:258–265. 2009.(In Polish). PubMed/NCBI
|
|
33
|
Groblewska M, Mroczko B and Szmitkowski M:
The role of selected matrix metalloproteinases and their inhibitors
in colorectal cancer development. Postepy Hig Med Dosw (Online).
64:22–30. 2010.(In Polish). PubMed/NCBI
|
|
34
|
Kwiatkowski P: The role of matrix
metalloproteinases in tumour invasion. Pol Ann Med. 43–50.
2008.
|
|
35
|
Wyględowska-Kania M, Gola J, Fila-Daniłow
A, Wcisło-Dziadecka D, Brzezińska-Wcisło L and Mazurek U: Molecular
studies of non-melanoma skin cancers. Borgis-Postępy Nauk Med.
752–757. 2012.
|
|
36
|
Monhian N, Jewett BS, Baker SR and Varani
J: Matrix metalloproteinase expression in normal skin associated
with basal cell carcinoma and in distal skin from the same
patients. Arch Facial Plast Surg. 7:238–243. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Varani J, Hattori Y, Chi Y, Schmidt T,
Perone P, Zeigler ME, Fader DJ and Johnson TM: Collagenolytic and
gelatinolytic matrix metalloproteinases and their inhibitors in
basal cell carcinoma of skin: Comparison with normal skin. Br J
Cancer. 82:657–665. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Gutiérrez-Fernández A, Fueyo A, Folgueras
AR, Garabaya C, Pennington CJ, Pilgrim S, Edwards DR, Holliday DL,
Jones JL, Span PN, et al: Matrix metalloproteinase-8 functions as a
metastasis suppressor through modulation of tumor cell adhesion and
invasion. Cancer Res. 68:2755–2763. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Goździalska Anna, Gawędzka Anna, Brzewski
Paweł and Anna Wojas-Pelc JJ: Skin cancer diagnosis based on the
determination of the expression metalloproteinases.
Interdescyplinarne aspekty Nauk o zdrowiu. 41–56. 2010.
|
|
40
|
Rao Q, Wang Y, Xia QY, Shi SS, Shen Q, Tu
P, Shi QL, Zhou XJ and Wu B: Cathepsin-K in the immunohistochemical
diagnosis of melanocytic lesions. Int J Clin Exp Pathol.
7:1132–1139. 2014.PubMed/NCBI
|
|
41
|
Zheng Y, Chen H, Lai W, Xu Q, Liu C, Wu L
and Maibach HI: Cathepsin D repairing role in photodamaged skin
barrier. Skin Pharmacol Physiol. 28:97–102. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Prokocimer M, Davidovich M, Nissim-Rafinia
M, Wiesel-Motiuk N, Bar DZ, Barkan R, Meshorer E and Gruenbaum Y:
Nuclear lamins: Key regulators of nuclear structure and activities.
J Cell Mol Med. 13:1059–1085. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Venables RS, McLean S, Luny D, Moteleb E,
Morley S, Quinlan RA, Lane EB and Hutchison CJ: Expression of
individual lamins in basal cell carcinomas of the skin. Br J
Cancer. 84:512–519. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Oguchi M, Sagara J, Matsumoto K, Saida T
and Taniguchi S: Expression of lamins depends on epidermal
differentiation and transformation. Br J Dermatol. 147:853–858.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Tilli CM, Ramaekers FC, Broers JL,
Hutchison CJ and Neumann HA: Lamin expression in normal human skin,
actinic keratosis, squamous cell carcinoma and basal cell
carcinoma. Br J Dermatol. 148:102–109. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Scaffidi P and Misteli T: Lamin
A-dependent nuclear defects in human aging. Science. 312:1059–1063.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Lin W, Huang J, Yuan Z, Feng S, Xie Y and
Ma W: Protein kinase C inhibitor chelerythrine selectively inhibits
proliferation of triple-negative breast cancer cells. Sci Rep.
7:20222017. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Baudhuin M, Lamoureux F, Jacques C,
Calleja Rodriguez L, Quillard T, Charrier C, Amiaud J, Berreur M,
Brounais-LeRoyer B, Owen R, et al: Inhibition of BET proteins and
epigenetic signaling as a potential treatment for osteoporosis.
Bone. 94:10–21. 2017. View Article : Google Scholar : PubMed/NCBI
|