Gliomas are histologically divided into four main
grades based on World Health Organisation (WHO) criteria. Grade II
astrocytomas are known as diffuse astrocytomas, while grade III are
anaplastic astrocytomas (AA) and are considerably more
proliferative and infiltrative than grade II gliomas (1). Yet, grade IV astrocytomas or
glioblastoma, despite being histologically similar to AA, are
notably more proliferative, invasive and angiogenic (2). Glioblastoma is the most common and
aggressive tumour of the central nervous system, and it represents
17% of all primary intracranial tumours (3). The current standard of treatment for
patients with glioblastoma is maximal surgical resection,
concurrent and adjuvant temozolomide and radiotherapy (known as the
Stupp protocol) (4). Despite this
standard of care treatment, the median survival is less than 15
months, median progression-free survival is less than 7 months and
5-year survival rate of treated patients is about 10–20% (4,5).
The tumour microenvironment plays numerous key roles
in cancer progression including the release of cytokines and other
effectors that mediate responses and events that aid in tumour
growth (6,7). These cytokines promote factors that are
central to cancer formation and progression, incorporating
sustained growth, cell migration, inhibition of apoptosis and
differentiation of tumour cells. It is well established that the
IL-6-STAT3 signalling pathway, along with other inflammatory
cytokines such as IL1β, IL-23 and TNFα has been implicated in
cancer progression in many tumour types including glioblastoma
contributing to tumour resistance and recurrence of glioblastoma
(8).
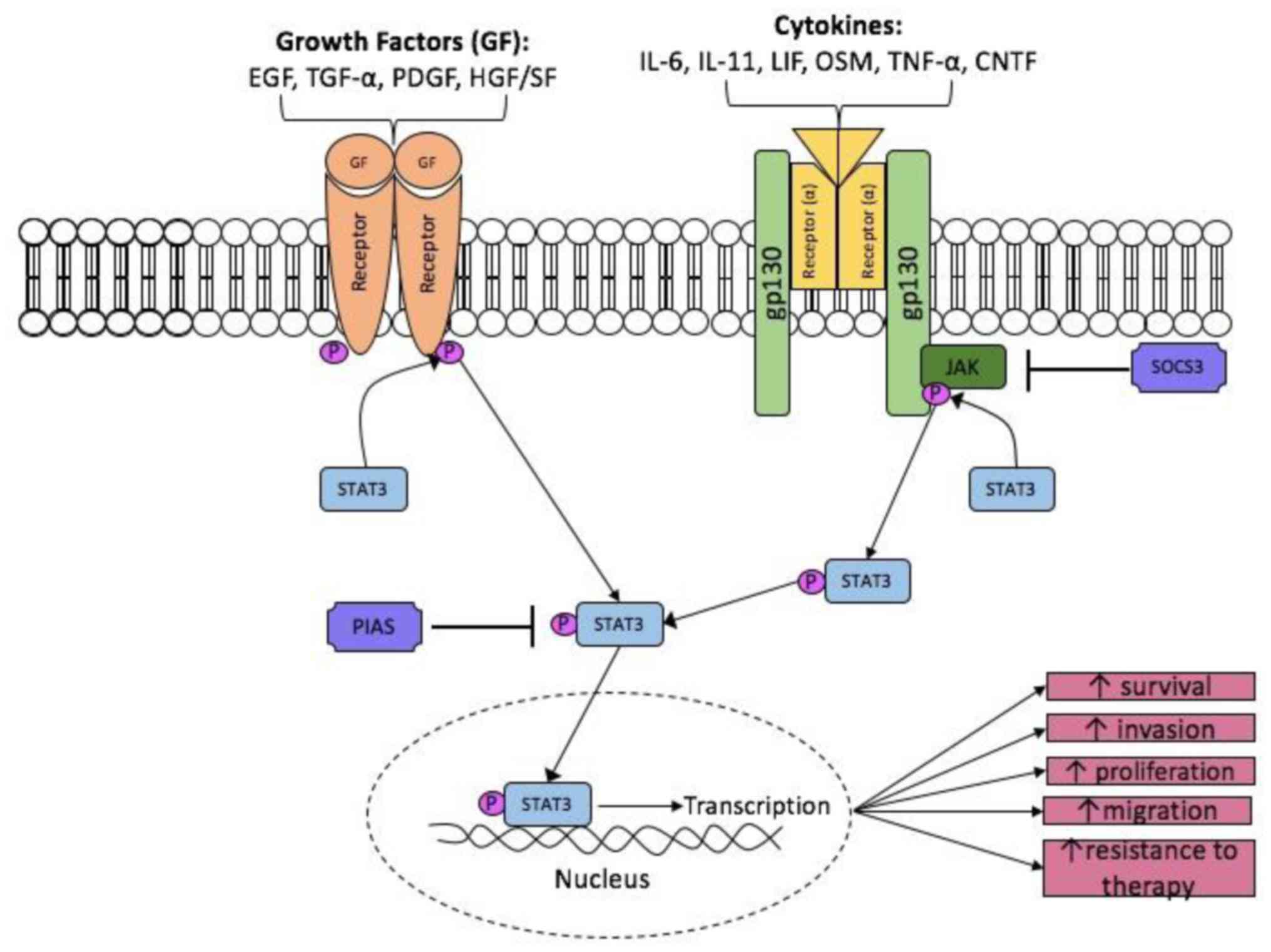
IL-6 is part of the IL-6 cytokine family, which
consist of IL-11, IL-27, leukaemia inhibitor factor (LIF) and
oncostatin M (OSM) (18). They share
the common co-receptor (gp130 β subunit), which is expressed in
almost every tissue and organ in the body. It is this subunit that
is responsible for the transmission of signalling into the cell by
activating associated cytoplasmic tyrosine kinases (e.g. JAK),
resulting in the phosphorylation of various transcription factors
including STAT3 (18,19). Among the family of IL-6 cytokines,
attention has focussed on IL-6 itself, as its expression levels is
highly up-regulated and has been shown to be correlated with poor
survival in many cancers.
IL-6 binds and signals through its own distinct
receptor alpha subunit (IL-6Rα; 80 kDa) on the plasma membrane
(20). This IL-6/IL-6Rα complex then
associates with the shared IL-6 family co-receptor gp130, resulting
in an activated hexameric complex (two molecules of IL-6, two
molecules of IL-6Ra and two molecules of gp130). There are two
types of IL-6 mediated signalling: Classical and trans-signalling.
Classical IL-6 signalling is the major form of IL-6 signalling and
the membrane-bound IL-6R (mIL-6Rα) which is only expressed in
certain tissues such as hepatocytes, some epithelial cells and
leukocytes (21). Alternatively, the
trans-signalling mechanism allows IL-6 signalling to occur in more
cell types, as gp130 is expressed ubiquitously. This occurs due to
trans-signalling of soluble IL-6Rα (sIL-6Rα), which lacks the
trans-membrane domain, through the interaction with gp130. sIL-6Rα
is then generated by the alternative splicing of the IL-6Rα mRNA by
cytoplasm-bound metalloproteinases (ADAM10 and ADAM17) (18,22–24). As a
result, the presence of sIL-6Rα permits IL-6 signalling to occur in
adjacent cells. Both signalling pathways lead to the activation of
JAK proteins, which are responsible for intracellular signalling
and the subsequent phosphorylation of STAT transcription factors,
in particular STAT3 (25).
Several studies demonstrate a correlation with IL-6
expression with glioma tumour grade and overall patient survival.
IL-6 mRNA expression was found to be significantly greater in
glioblastoma patient samples compared to those with lower
histopathological grade (including Grade II and III astrocytomas
and Grade I pilocytic astrocytomas; Table
I) (26). In addition, no IL-6
gene amplification was detected in low-grade or anaplastic tumors
(0/17), whereas amplification was found in 15 out of 36 (42%)
glioblastoma sections (27).
Importantly, this IL-6 gene amplification correlated with
significantly shorter survival compared to glioblastoma patients
without amplification (27). In
addition, immunohistochemistry analysis identified the IL-6
receptor expression in 6/6 (100%) patient glioblastoma samples
compared to 0/7 (0%) in normal brain tissue (28). IL-6 has also been detected in the
cerebrospinal fluid of 11/13 (85%) and the tumor cyst fluid of 5/5
(100%) glioblastoma patients (29).
In contrast, only 3/16 (19%) cerebrospinal Fluid (CSF) samples
obtained from control patients had detectable IL-6 levels (29).
Once IL-6 has bound, the β subunit (gp130)
homodimerises and the receptor-associated Janus Kinase (JAK1, JAK2
and Tyk2) become activated (18).
Activated JAKs act as a platform for phosphorylation of STAT3. Two
STAT3 monomers can form a dimer and up-regulate STAT3 target genes
in the nucleus (Fig. 1). The STAT
protein family is a group of transcription factors that play
crucial roles in the transmission of extracellular signals into the
nucleus for the transcription of a variety of genes (30,31). In
cancer research, there is particular focus on STAT3 because of its
oncogenic abilities. STAT3 up-regulates genes that can facilitate
tumour survival, angiogenesis, resistance to cell death and cell
cycle progression. Target genes of STAT3 include vascular
endothelial growth factor (VEGF), Bcl-2, Bcl-xL, cyclin D1, human
telomerase reverse transcriptase and c-myc (30).
Involvement of STAT3 in tumourigenesis was first
described in fibroblasts and epithelial cells that were transformed
by Src tyrosine kinase, where constitutive STAT3 activity was first
observed (32,33). This was further supported in
transgenic mice (transformed with v-src), where they
developed astrocytoma that led to secondary glioblastoma (34). It only became clear that STAT3 itself
could be implicated in cancer when it was found that
dominant-negative STAT3 decreased tumourigenesis behaviour of
src-transformed cells, whereas constitutively active STAT3
enhanced tumourigenesis (32,35,36).
Interestingly, mutations within the STAT3 gene are rare, indicating
that constitutive activation of STAT3 is usually due to abnormal
signalling from upstream regulators, such as IL-6 (33).
Interestingly, another study reported that the
down-modulation of pSTAT3-Y705 resulted in resistance to
Temozolomide, as it was able to minimise
O6-methylguanine DNA methyltransferase (MGMT) expression
post-transcriptionally in recurrent glioblastoma (41). It was observed in this study that
STAT3 inhibition reduced MGMT levels, which led to the
re-sensitisation to Temozolomide treatment (41). Potential therapies utilising STAT3 as
a target are currently being investigated and clinical trials are
underway for pSTAT3-Y705 inhibitors (42).
It is well established that IL-6 signalling plays a
crucial role in a variety of cancers, whereby expression levels of
IL-6 mRNA and protein are significantly increased in colorectal
(43), prostate (44), breast (45), ovarian (46), pancreatic (46,47), lung
(48) and cervical cancer (49). Weissenberger and colleagues showed the
importance of IL-6 signalling in glioma and its impact on the
tumorigenicity in glioblastoma, using transgenic mice (50). Mice expressing the
GFAP-v-src+/− transgene develop spontaneous astrocytomas
with a penetrance of around 21% (12 out of 56). However, only 1 out
of 35 transgenic mice (3%) that lack IL-6 developed astrocytic
tumour formation (50).
IL-6 signalling promotes a variety of activities
that support gliomagenesis including cell invasion and migration,
contributing to the invasive nature of glioblastoma, resulting in
reduced treatment efficacy and high rates of recurrence. STAT3
activation induced by IL-6 signalling has been shown to promote
cell invasion and migration in U251 and T98G glioblastoma cells
(51). In a study by Liu et al
(51), increased levels of IL-6
positively correlated with the levels of matrix metalloproteinase-9
(MMP-9) expression. MMPs belong to a family of proteases
responsible for degrading extracellular matrix proteins and play a
major role in migration (both adhesion and dispersion) as well as
cell proliferation. Similarly, a study by Li et al (52), demonstrated IL-6 stimulation of U87MG
glioblastoma cells resulted in increased MMP-2 expression and
secretion and enhanced cell invasion.
Furthermore, IL-6 signalling correlates with
increased fascin-1 expression. Fascin-1 is involved in cell
invasion by the formation of actin-based protrusions known as
invadopodia and the subsequent degradation of the extracellular
matrix to promote migration and invasion (52). Immunofluorescence staining of fascin-1
revealed that the number of protrusions increased and fascin-1
protein was localised to the peripheries of the cell when
glioblastoma cells were treated with IL-6. This study demonstrated
that IL-6 signalling influences the distribution of fascin-1 and
alters the structural aspects of the cell to become a more invasive
phenotype in glioblastoma cells (51).
Tumour angiogenesis is another crucial process that
is required for the growth and invasion of tumours. Angiogenesis
requires migration of vascular endothelial cells into the tumour
bulk (51). This process involves the
release of mediators such as TNF-β, TNF-α, MMP-9, MMP-2 and VEGF
(53). The STAT3 transcription factor
is known to up-regulates the expression of VEGF-2 and its receptor,
VEGFR-2, contributing to invasion through the action of IL-6
signalling and subsequent JAK-STAT3 activation (54–56). IL-6
secretion into neighbouring cells further induces this behaviour,
thereby promoting cell migration through these vascular endothelial
cells (51).
VEGF is an important mediator in tumour-induced
angiogenesis. It has been observed that high expression of VEGF
correlates with higher tumour grade and shorter survival (57,58), while
one of the mechanisms of progression in glioblastoma is postulated
to involve tumour resistance to anti-angiogenic treatments
(59,60). This may be due to these angiogenic
switches and up-regulation of different angiogenic pathways
(57,61–63) as
well as mesenchymal cell transition (64).
Another essential pro-angiogenic factor is
fibroblastic growth factor-2 (FGF-2), which is a heparin-binding
protein that has a range of roles, including serving an important
pathway in tumorigenesis (65).
Recent research has indicated that both VEGF and FGF2 work together
to influence the process of angiogenesis. It has been shown that
when both factors are expressed in mouse models, there is enhanced
tumour growth coupled with the presence of high density vessels.
When either FGF2 or VEGF signalling is inhibited, the rate of the
tumour growth decreases significantly (65–67).
Although an early study (20) showed
that IL-6 acts as a growth factor in glioblastoma, a more recent
study did not reach this conclusion and demonstrated that IL-6 did
not have a proliferative effect in GBM cell lines, but instead
promoted a more invasive phenotype by increasing the migration
ability of cells (51).
In addition to evading growth suppression signaling
through the loss of TP53 function, tumours such as glioblastoma are
also associated with an increase in the expression of
anti-apoptotic regulators or down-regulation of pro-apoptotic
factors. IL-6 signalling disrupts the balance between anti- and
pro-apoptotic protein expression by favouring anti-apoptotic
signalling through JAK-STAT3 activation, as well as NF-kB
signalling, which activates the expression of many anti-apoptotic
proteins such as Bcl-2, Bcl-xL and Mcl-1 (51,68). These
anti-apoptotic proteins are also important in cell proliferation as
they are direct targets of STAT3 (69). Other than increased Bcl-2 and Bcl-xL
expression, IL-6 also promotes the expression of survivin through
JAK-STAT3 activation (69,70). It has been found that the
downregulation of survivin by inhibiting STAT3 induces apoptosis in
tumour cells (70).
Cancer stem cells (CSCs) are a group of tumour cells
that have stem cell-like properties and are capable of
self-renewal. Studies have shown that CSCs are critical in the
progression of cancer and resistance to various therapies in
different types of solid tumours, including brain tumours (71–76). In
recent years the cancer stem cell (CSC) hypothesis has grown in
popularity. This states that a small group of cells can maintain
the cancer growth and survival, whilst providing resistance to
therapy. It has been established that gliomas are one of the
tumours where cancer stem cells, or glioma stem cells (GSCs) has
been established (77,78). Subsequently, GSCs have been intensely
studied for understanding their capacity for self-renewal and
importantly, their contribution to therapeutic resistance as well
as tumour recurrence (79). GSCs are
thought to play important roles in gliomagenesis, recurrence and
aggressiveness (80,81).
In other cancers, IL-6 is also implicated in
promoting STAT3 mediated CSC expansion, such as in prostate and
breast CSCs (82–84). Through the activation of JAK-STAT3
signalling (mainly from IL-6 signalling), hypoxic conditions
activate these CSCs and promote self-renewal (85). It was found that upon STAT3
inhibition, GSCs lose their stem-cell phenotype permanently,
suggesting that STAT3 is required for the self-renewal and growth
of stem cells within glioblastoma (40,86). The
contribution of CSCs to the difficulty of cancer treatment and
therapy is crucial, and targeting the JAK-STAT3 pathway may
therefore become a potential mechanism to overcome CSC-mediated
Temozolomide resistance in glioblastoma and other solid
tumours.
Several studies have found that GSCs express the
IL-6 receptor and ligand, which indicates that IL-6 could be a
potential cytokine to contribute to the tumourigenicity of these
GSCs. Wang et al (2009) (87)
found that GSCs co-expressed elevated levels of IL-6Rα and gp130 (β
subunit), as demonstrated by immunofluorescence staining, although
they also showed that IL-6 mRNA levels were lower in GSCs that
non-GSCs. Knockdown of IL-6 or IL-6Rα by shRNA led to reduced cell
growth, neurosphere formation and increased cell death in GSCs
isolated from D456MG human glioma xenografts. IL-6 or IL-6Rα
suppression also led to reduced tumour growth and increased
survival of mice bearing intracranial xenografts (87). IL-6 has also been shown to induces the
expression of glioma pathogenesis-related 1 (GPR1, also known as
RTVP1) via the STAT3 pathway (88).
GPR1 plays an important role in GSCs, contributing to migration,
resistance to therapy and tumour recurrence. GPR1 was originally
discovered in glioblastoma as one of the target genes of the tumour
suppressor gene p53, one of the most commonly mutated genes in
human cancer.
STAT3 has been shown to be highly expressed in GSCs,
and inhibition of STAT3 levels has led to decreases in the
proliferation of these GSCs (40).
Furthermore, inhibition of STAT3 also diminishes the multipotency
characteristic of these GSCs. STAT3 has been identified as a
potential target for CSC-mediated therapy as it is the convergence
point for many signaling pathways. It has been found that
inhibition of STAT3 using a shRNA approach prevented proliferation
and formation of neurospheres in GSCs (30,40,89).
Moreover, leukaemia inhibitory factor (LIF), a member of the IL-6
family, has been found to be responsible for the tumour development
of GSCs via the JAK-STAT pathway (90).
The importance of targeting the STAT3 signalling
pathway due to its function in recurrence, GSCs and resistance to
Temozolomide is well established (90). Therapeutic targets of STAT3 have been
investigated, but only a small number has been tested against
glioblastoma (summarised in Table
II). Furthermore, there has not been much success at preventing
downstream nuclear signalling (91).
Studies into STAT3 inhibitors should continue, as the importance of
this pathway is continually being demonstrated (92,93).
Furthermore, the inhibitors that have been investigated with
glioblastoma generally have had significant challenges in the
translation into clinical practice, mainly because of the form of
administration, toxicity, cell permeability and non-selective
activity (91).
The disruption of upstream receptor tyrosine kinases
is one of the potential methods for inhibiting STAT3 activity. By
inhibiting JAKs, growth factor receptors, receptor tyrosine kinases
(RTKS) and cytokine receptors, it would be possible to alter
downstream STAT3 activity. However, STAT3 is the convergence point
of several oncogenic signalling pathways, including EGFR,
heregulin-2/neuregulin receptor (Her2/Neu), Platelet Derived Growth
factor receptor, IL-6R/gp130, c-Met, Abelson Leukemia protein
(Abl), and Src tyrosine kinases. Therefore, it would be probable
that a compensatory pathway could override the initial inhibition
of STAT3 (94–98). On the other hand, preventing
homodimersation of STAT3 involves blocking the SH2 domains from
coming together after STAT3 phosphorylation, and ultimately
inhibiting STAT3′s transcription capabilities. Thus far, studies
have targeted the SH2 domain to prevent homodimerisation (99–102). Two
groups (100,101) have shown that blocking the
phosphorylated tyrosine peptide at position 705 was able to prevent
STAT3 from binding to DNA in the nucleus and furthermore, the SH2
domain of STAT3 has been shown to interact with upstream signalling
proteins to STAT3, including EGFR and IL-6 and its receptor.
Studies that have targeted this particular homodimerisation have
generated positive results, yet they have yet to be translated into
an in vivo setting (28,103–105).
There is a lack of evidence regarding the mechanism
of action of over-activation of STAT3 in recurrent glioblastoma.
The STAT3 signalling pathway has been extensively investigated in
primary glioblastoma, but it has not yet been shown why
phosphorylated STAT3 is overexpressed in recurrent glioblastoma
compared to primary glioblastoma (44). Furthermore, increased phosphorylation
of STAT3 has been correlated with increased grade of astrocytoma
(106,107). Based on this evidence, it is
possible that STAT3 could be considered as a prognostic factor. Tu
et al (107), have
demonstrated that JAK/STAT activation is correlated to higher grade
gliomas, and furthermore, it was shown that JAK/STAT activation is
a prognostic indicator of decreased survival. One potential mode of
action is through a mutated MSH6 gene, which has been correlated
with a methylated O6-methylguanine-DNA transferase (MGMT) promoter,
whose cellular levels are known to be regulated by STAT3 through
IL-6 (41). This potential mechanism
is outlined in the next section.
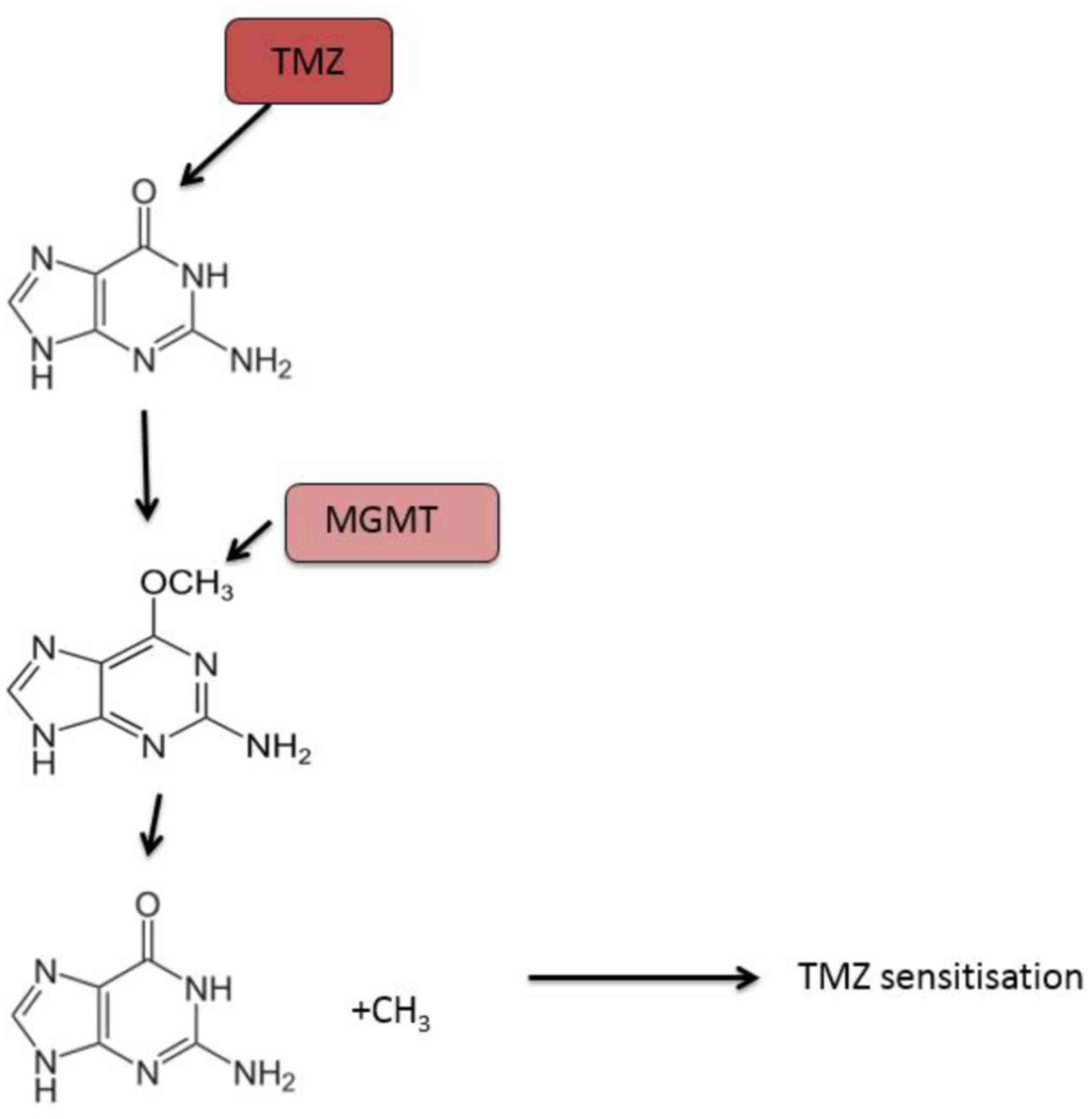
Currently, the DNA repair protein, MGMT, is the most
studied predictive biomarker of response to temozolomide treatment
in patients with glioblastoma. Temozolomide exerts its cytotoxic
activity by methylating specific DNA sites including the
O6 position of the nucleotide guanine, resulting in cell
death (108). MGMT directly inhibits
the cytotoxic effect of temozolomide by removing the methyl group
off the O6 position of guanine in DNA (109,110).
Thus, patients with glioblastoma that expressed reduced MGMT due to
epigenetic methylation of the MGMT promoter have better response
rates to temozolomide and are associated with longer overall
survival (Fig. 2) (111).
In support of this notion is the study from Koksaka
and colleagues who did indeed evaluate a potential correlation
between phosphorylated STAT3 and MGMT expression (41). In this paper, a significant
correlation was observed between pSTAT3 staining intensity by
immunohistochemistry with MGMT expression in 44 surgically resected
human glioma specimens. Koksaka and colleagues also generated a
U87MG sub-population that was temozolomide-refractory after 3 weeks
of continuous exposure to low-dose temozolomide. These resistant
cells designated U87R displayed greater expression of IL-6, STAT3
and MGMT compared to their sensitive parental counterparts.
Importantly, pharmacological inhibition of STAT3 or STAT3 knockdown
with shRNA resulted in reduced MGMT expression in the U87R cells
and other glioblastoma cell lines. STAT3 inhibition also
re-sensitized cells to temozolomide (41).
Taken together, these articles strongly link
IL-6-STAT3 signaling with MGMT expression and methylation and to
temozolomide sensitivity. However, further studies are required to
investigate the mechanisms of action for the epigenetic control of
tumour cell functions by IL-6. Overall, it would be advantageous to
extensively study the potential of IL-6/STAT3 inhibitors on MGMT
expression levels, and determine if this could potentially aid in
overcoming temozolomide resistance in glioblastoma considering that
MGMT requires STAT3 activation to modulate cellular levels of
MGMT.
Understanding how overexpressed or over-active
signalling pathways promote recurrent glioblastoma progression may
lead to the development of new therapies. In particular, targeting
the important IL-6-JAK/STAT3 signaling pathway should result in
reduced tumour proliferation and invasion and improved patient
outcomes. In addition, therapeutic targeting of STAT3 and its
components may provide a basis for sensitising these recurrent
tumours to the currently available treatments for glioma. For this
strategy to proceed, further preclinical research into how
IL-6-STAT3 promotes glioblastoma recurrence and progression is
still required.
Not applicable.
RBL is a mid-career fellowship holder with the
Victorian Cancer Agency (grant no. MCRF 15017).
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
AJW, VT and RBL contributed to the writing of this
manuscript, while HPTN, SSS, APM, AHK and RBL contributed the
conception design and editing of the manuscript.
Not applicable.
Not applicable.
The authors declare that there are no competing
interests.
|
1
|
Aldape K, Zadeh G, Mansouri S,
Reifenberger G and von Deimling A: Glioblastoma: Pathology,
molecular mechanisms and markers. Acta Neuropathol. 129:829–848.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Agnihotri S, Burrell KE, Wolf A, Jalali S,
Hawkins C, Rutka JT and Zadeh G: Glioblastoma, a brief review of
history, molecular genetics, animal models and novel therapeutic
strategies. Arch Immunol Ther Exp (Warsz). 61:25–41. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Wen PY and Kesari S: Malignant gliomas in
adults. N Engl J Med. 359:492–507. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Stupp R, Mason WP, van den Bent MJ, Weller
M, Fisher B, Taphoorn MJ, Belanger K, Brandes AA, Marosi C, Bogdahn
U, et al: Radiotherapy plus concomitant and adjuvant temozolomide
for glioblastoma. N Engl J Med. 352:987–996. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Tully PA, Gogos AJ, Love C, Liew D,
Drummond KJ and Morokoff AP: Reoperation for recurrent glioblastoma
and its association with survival benefit. Neurosurgery.
79:678–689. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Desbaillets I, Diserens AC, Tribolet N,
Hamou MF and Van Meir EG: Upregulation of interleukin 8 by
oxygen-deprived cells in glioblastoma suggests a role in leukocyte
activation, chemotaxis, and angiogenesis. J Exp Med. 186:1201–1212.
1997. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Shan Y, He X, Song W, Han D, Niu J and
Wang J: Role of IL-6 in the invasiveness and prognosis of glioma.
Int J Clin Exp Med. 8:9114–9120. 2015.PubMed/NCBI
|
|
8
|
Jarnicki A, Putoczki T and Ernst M: Stat3:
Linking inflammation to epithelial cancer-more than a ‘gut’
feeling? Cell Div. 5:142010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kumari N, Dwarakanath BS, Das A and Bhatt
AN: Role of interleukin-6 in cancer progression and therapeutic
resistance. Tumour Biol. 37:11553–11572. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Iliopoulos D, Hirsch HA and Struhl K: An
epigenetic switch involving NF-kappaB, Lin28, Let-7 MicroRNA, and
IL6 links inflammation to cell transformation. Cell. 139:693–706.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Akira S and Kishimoto T: IL-6 and NF-IL6
in acute-phase response and viral infection. Immunol Rev.
127:25–50. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Wu CT, Chen MF, Chen WC and Hsieh CC: The
role of IL-6 in the radiation response of prostate cancer. Radiat
Oncol. 8:1592013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Yang R, Lin Q, Gao HB and Zhang P:
Stress-related hormone norepinephrine induces interleukin-6
expression in GES-1 cells. Braz J Med Biol Res. 47:101–109. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Scheller J, Chalaris A, Schmidt-Arras D
and Rose-John S: The pro- and anti-inflammatory properties of the
cytokine interleukin-6. Biochim Biophys Acta. 1813:878–888. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Gruys E, Toussaint MJ, Niewold TA and
Koopmans SJ: Acute phase reaction and acute phase proteins. J
Zhejiang Univ Sci B. 6:1045–1056. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Tilg H, Trehu E, Atkins MB, Dinarello CA
and Mier JW: Interleukin-6 (IL-6) as an anti-inflammatory cytokine:
Induction of circulating IL-1 receptor antagonist and soluble tumor
necrosis factor receptor p55. Blood. 83:113–118. 1994.PubMed/NCBI
|
|
17
|
Aderka D, Le JM and Vilcek J: IL-6
inhibits lipopolysaccharide-induced tumor necrosis factor
production in cultured human monocytes, U937 cells, and in mice. J
Immunol. 143:3517–3523. 1989.PubMed/NCBI
|
|
18
|
Jones SA, Scheller J and Rose-John S:
Therapeutic strategies for the clinical blockade of IL-6/gp130
signaling. J Clin Invest. 121:3375–3383. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Mihara M, Hashizume M, Yoshida H, Suzuki M
and Shiina M: IL-6/IL-6 receptor system and its role in
physiological and pathological conditions. Clin Sci (Lond).
122:143–159. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Goswami S, Gupta A and Sharma SK:
Interleukin-6-mediated autocrine growth promotion in human
glioblastoma multiforme cell line U87MG. J Neurochem. 71:1837–1845.
1998. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Taga T and Kishimoto T: Gp130 and the
interleukin-6 family of cytokines. Annu Rev Immunol. 15:797–819.
1997. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Chalaris A, Garbers C, Rabe B, Rose-John S
and Scheller J: The soluble interleukin 6 receptor: Generation and
role in inflammation and cancer. Eur J Cell Biol. 90:484–494. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Jones SA, Horiuchi S, Topley N, Yamamoto N
and Fuller GM: The soluble interleukin 6 receptor: Mechanisms of
production and implications in disease. FASEB J. 15:43–58. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Yoshida K, Taga T, Saito M, Suematsu S,
Kumanogoh A, Tanaka T, Fujiwara H, Hirata M, Yamagami T, Nakahata
T, et al: Targeted disruption of gp130, a common signal transducer
for the interleukin 6 family of cytokines, leads to myocardial and
hematological disorders. Proc Natl Acad Sci USA. 93:pp. 407–411.
1996; View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Yeung YT, McDonald KL, Grewal T and Munoz
L: Interleukins in glioblastoma pathophysiology: Implications for
therapy. Br J Pharmacol. 168:591–606. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Rolhion C, Penault-Llorca F, Kémény JL,
Lemaire JJ, Jullien C, Labit-Bouvier C, Finat-Duclos F and Verrelle
P: Interleukin-6 overexpression as a marker of malignancy in human
gliomas. J Neurosurg. 94:97–101. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Tchirkov A, Khalil T, Chautard E, Mokhtari
K, Véronèse L, Irthum B, Vago P, Kémény JL and Verrelle P:
Interleukin-6 gene amplification and shortened survival in
glioblastoma patients. Br J Cancer. 96:474–476. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Kudo M, Jono H, Shinriki S, Yano S,
Nakamura H, Makino K, Hide T, Muta D, Ueda M, Ota K, et al:
Antitumor effect of humanized anti-interleukin-6 receptor antibody
(tocilizumab) on glioma cell proliferation. Laboratory
investigation. J Neurosurg. 111:219–225. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Van Meir E, Sawamura Y, Diserens AC, Hamou
MF and de Tribolet N: Human glioblastoma cells release interleukin
6 in vivo and in vitro. Cancer Res. 50:6683–6688. 1990.PubMed/NCBI
|
|
30
|
Ashizawa T, Miyata H, Iizuka A, Komiyama
M, Oshita C, Kume A, Nogami M, Yagoto M, Ito I, Oishi T, et al:
Effect of the STAT3 inhibitor STX-0119 on the proliferation of
cancer stem-like cells derived from recurrent glioblastoma. Int J
Oncol. 43:219–227. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Zhong Z, Wen Z and Darnell JE Jr: Stat3
and Stat4: Members of the family of signal transducers and
activators of transcription. Proc Natl Acad Sci USA. 91:pp.
4806–4810. 1994; View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Bromberg JF, Horvath CM, Besser D, Lathem
WW and Darnell JE Jr: Stat3 activation is required for cellular
transformation by v-src. Mol Cell Biol. 18:2553–2558. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Ouédraogo ZG, Biau J, Kemeny JL, Morel L,
Verrelle P and Chautard E: Role of STAT3 in genesis and progression
of human malignant gliomas. Mol Neurobiol. 54:5780–5797. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Smilowitz HM, Weissenberger J, Weis J,
Brown JD, O'Neill RJ and Laissue JA: Orthotopic transplantation of
v-src-expressing glioma cell lines into immunocompetent mice:
Establishment of a new transplantable in vivo model for malignant
glioma. J Neurosurg. 106:652–659. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Dechow TN, Pedranzini L, Leitch A, Leslie
K, Gerald WL, Linkov I and Bromberg JF: Requirement of matrix
metalloproteinase-9 for the transformation of human mammary
epithelial cells by Stat3-C. Proc Natl Acad Sci USA. 101:pp.
10602–10607. 2004; View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Turkson J, Bowman T, Garcia R, Caldenhoven
E, De Groot RP and Jove R: Stat3 activation by Src induces specific
gene regulation and is required for cell transformation. Mol Cell
Biol. 18:2545–2552. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Catlett-Falcone R, Landowski TH, Oshiro
MM, Turkson J, Levitzki A, Savino R, Ciliberto G, Moscinski L,
Fernández-Luna JL, Nuñez G, et al: Constitutive activation of Stat3
signaling confers resistance to apoptosis in human U266 myeloma
cells. Immunity. 10:105–115. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Grandis JR, Drenning SD, Chakraborty A,
Zhou MY, Zeng Q, Pitt AS and Tweardy DJ: Requirement of Stat3 but
not Stat1 activation for epidermal growth factor receptor- mediated
cell growth in vitro. J Clin Invest. 102:1385–1392. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Rahaman SO, Harbor PC, Chernova O, Barnett
GH, Vogelbaum MA and Haque SJ: Inhibition of constitutively active
Stat3 suppresses proliferation and induces apoptosis in
glioblastoma multiforme cells. Oncogene. 21:8404–8413. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Sherry MM, Reeves A, Wu JK and Cochran BH:
STAT3 is required for proliferation and maintenance of multipotency
in glioblastoma stem cells. Stem Cells. 27:2383–2392. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Kohsaka S, Wang L, Yachi K, Mahabir R,
Narita T, Itoh T, Tanino M, Kimura T, Nishihara H and Tanaka S:
STAT3 inhibition overcomes temozolomide resistance in glioblastoma
by downregulating MGMT expression. Mol Cancer Ther. 11:1289–1299.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Heimberger AB: The therapeutic potential
of inhibitors of the signal transducer and activator of
transcription 3 for central nervous system malignancies. Surg
Neurol Int. 2:1632011. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Waldner MJ, Foersch S and Neurath MF:
Interleukin-6-a key regulator of colorectal cancer development. Int
J Biol Sci. 8:1248–1253. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Culig Z and Puhr M: Interleukin-6: A
multifunctional targetable cytokine in human prostate cancer. Mol
Cell Endocrinol. 360:52–58. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Dethlefsen C, Højfeldt G and Hojman P: The
role of intratumoral and systemic IL-6 in breast cancer. Breast
Cancer Res Treat. 138:657–664. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Macciò A and Madeddu C: The role of
interleukin-6 in the evolution of ovarian cancer: Clinical and
prognostic implications-a review. J Mol Med (Berl). 91:1355–1368.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Miura T, Mitsunaga S, Ikeda M, Shimizu S,
Ohno I, Takahashi H, Furuse J, Inagaki M, Higashi S, Kato H, et al:
Characterization of patients with advanced pancreatic cancer and
high serum interleukin-6 levels. Pancreas. 44:756–763. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Chang CH, Hsiao CF, Yeh YM, Chang GC, Tsai
YH, Chen YM, Huang MS, Chen HL, Li YJ, Yang PC, et al: Circulating
interleukin-6 level is a prognostic marker for survival in advanced
nonsmall cell lung cancer patients treated with chemotherapy. Int J
Cancer. 132:1977–1985. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Wei LH, Kuo ML, Chen CA, Chou CH, Lai KB,
Lee CN and Hsieh CY: Interleukin-6 promotes cervical tumor growth
by VEGF-dependent angiogenesis via a STAT3 pathway. Oncogene.
22:1517–1527. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Weissenberger J, Loeffler S, Kappeler A,
Kopf M, Lukes A, Afanasieva TA, Aguzzi A and Weis J: IL-6 is
required for glioma development in a mouse model. Oncogene.
23:3308–3316. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Liu Q, Li G, Li R, Shen J, He Q, Deng L,
Zhang C and Zhang J: IL-6 promotion of glioblastoma cell invasion
and angiogenesis in U251 and T98G cell lines. J Neurooncol.
100:165–176. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Li R, Li G, Deng L, Liu Q, Dai J, Shen J
and Zhang J: IL-6 augments the invasiveness of U87MG human
glioblastoma multiforme cells via up-regulation of MMP-2 and
fascin-1. Oncol Rep. 23:1553–1559. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Anton K, Banerjee D and Glod J:
Macrophage-associated mesenchymal stem cells assume an activated,
migratory, pro-inflammatory phenotype with increased IL-6 and
CXCL10 secretion. PLoS One. 7:e350362012. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Carmeliet P: Angiogenesis in life, disease
and medicine. Nature. 438:932–936. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Garonna E, Botham KM, Birdsey GM, Randi
AM, Gonzalez-Perez RR and Wheeler-Jones CP: Vascular endothelial
growth factor receptor-2 couples cyclo-oxygenase-2 with
pro-angiogenic actions of leptin on human endothelial cells. PLoS
One. 6:e188232011. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Shibuya M: Vascular endothelial growth
factor and its receptor system: Physiological functions in
angiogenesis and pathological roles in various diseases. J Biochem.
153:13–19. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Labussière M, Cheneau C, Prahst C, Gállego
Pérez-Larraya J, Farina P, Lombardi G, Mokhtari K, Rahimian A,
Delattre JY, Eichmann A and Sanson M: Angiopoietin-2 may be
involved in the resistance to bevacizumab in recurrent
glioblastoma. Cancer Invest. 34:39–44. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Zhou YH, Tan F, Hess KR and Yung WK: The
expression of PAX6, PTEN, vascular endothelial growth factor, and
epidermal growth factor receptor in gliomas: relationship to tumor
grade and survival. Clin Cancer Res. 9:3369–3375. 2003.PubMed/NCBI
|
|
59
|
Bergers G and Hanahan D: Modes of
resistance to anti-angiogenic therapy. Nat Rev Cancer. 8:592–603.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Takano S: Glioblastoma angiogenesis: VEGF
resistance solutions and new strategies based on molecular
mechanisms of tumor vessel formation. Brain Tumor Pathol. 29:73–86.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Li JL, Sainson RC, Oon CE, Turley H, Leek
R, Sheldon H, Bridges E, Shi W, Snell C, Bowden ET, et al:
DLL4-Notch signaling mediates tumor resistance to anti-VEGF therapy
in vivo. Cancer Res. 71:6073–6083. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Peterson TE, Kirkpatrick ND, Huang Y,
Farrar CT, Marijt KA, Kloepper J, Datta M, Amoozgar Z, Seano G,
Jung K, et al: Dual inhibition of Ang-2 and VEGF receptors
normalizes tumor vasculature and prolongs survival in glioblastoma
by altering macrophages. Proc Natl Acad Sci USA. 113:pp. 4470–4475.
2016; View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Tabouret E, Denicolai E, Delfino C,
Graillon T, Boucard C, Nanni I, Padovani L, Figarella-Branger D and
Chinot O: Changes in PlGF and MET-HGF expressions in paired initial
and recurrent glioblastoma. J Neurooncol. 130:431–437. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Piao Y, Liang J, Holmes L, Henry V, Sulman
E and de Groot JF: Acquired resistance to anti-VEGF therapy in
glioblastoma is associated with a mesenchymal transition. Clin
Cancer Res. 19:4392–4403. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Wesche J, Haglund K and Haugsten EM:
Fibroblast growth factors and their receptors in cancer. Biochem J.
437:199–213. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Fu Z, Chen X, Guan S, Yan Y, Lin H and Hua
ZC: Curcumin inhibits angiogenesis and improves defective
hematopoiesis induced by tumor-derived VEGF in tumor model through
modulating VEGF-VEGFR2 signaling pathway. Oncotarget.
6:19469–19482. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Wu XY, Xu H, Wu ZF, Chen C, Liu JY, Wu GN,
Yao XQ, Liu FK, Li G and Shen L: Formononetin, a novel FGFR2
inhibitor, potently inhibits angiogenesis and tumor growth in
preclinical models. Oncotarget. 6:44563–44578. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Waxman AB and Kolliputi N: IL-6 protects
against hyperoxia-induced mitochondrial damage via Bcl-2-induced
Bak interactions with mitofusins. Am J Respir Cell Mol Biol.
41:385–396. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Hirano T, Ishihara K and Hibi M: Roles of
STAT3 in mediating the cell growth, differentiation and survival
signals relayed through the IL-6 family of cytokine receptors.
Oncogene. 19:2548–2556. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Gritsko T, Williams A, Turkson J, Kaneko
S, Bowman T, Huang M, Nam S, Eweis I, Diaz N, Sullivan D, et al:
Persistent activation of stat3 signaling induces survivin gene
expression and confers resistance to apoptosis in human breast
cancer cells. Clin Cancer Res. 12:11–19. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Bonnet D and Dick JE: Human acute myeloid
leukemia is organized as a hierarchy that originates from a
primitive hematopoietic cell. Nat Med. 3:730–737. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Clarke MF and Fuller M: Stem cells and
cancer: Two faces of eve. Cell. 124:1111–1115. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Lapidot T, Sirard C, Vormoor J, Murdoch B,
Hoang T, Caceres-Cortes J, Minden M, Paterson B, Caligiuri MA and
Dick JE: A cell initiating human acute myeloid leukaemia after
transplantation into SCID mice. Nature. 367:645–648. 1994.
View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Magee JA, Piskounova E and Morrison SJ:
Cancer stem cells: Impact, heterogeneity, and uncertainty. Cancer
Cell. 21:283–296. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Visvader JE and Lindeman GJ: Cancer stem
cells in solid tumours: Accumulating evidence and unresolved
questions. Nat Rev Cancer. 8:755–768. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Willyard C: Stem cells: Bad seeds. Nature.
498:S12–S13. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Galli R, Binda E, Orfanelli U, Cipelletti
B, Gritti A, De Vitis S, Fiocco R, Foroni C, Dimeco F and Vescovi
A: Isolation and characterization of tumorigenic, stem-like neural
precursors from human glioblastoma. Cancer Res. 64:7011–7021. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Singh SK, Clarke ID, Terasaki M, Bonn VE,
Hawkins C, Squire J and Dirks PB: Identification of a cancer stem
cell in human brain tumors. Cancer Res. 63:5821–5828.
2003.PubMed/NCBI
|
|
79
|
Hossain A, Gumin J, Gao F, Figueroa J,
Shinojima N, Takezaki T, Priebe W, Villarreal D, Kang SG, Joyce C,
et al: Mesenchymal stem cells isolated from human gliomas increase
proliferation and maintain stemness of glioma stem cells through
the IL-6/gp130/STAT3 pathway. Stem Cells. 33:2400–2415. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Jafri NF, Clarke JL, Weinberg V, Barani IJ
and Cha S: Relationship of glioblastoma multiforme to the
subventricular zone is associated with survival. Neuro Oncol.
15:91–96. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Young GS, Macklin EA, Setayesh K, Lawson
JD, Wen PY, Norden AD, Drappatz J and Kesari S: Longitudinal MRI
evidence for decreased survival among periventricular glioblastoma.
J Neurooncol. 104:261–269. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Kroon P, Berry PA, Stower MJ, Rodrigues G,
Mann VM, Simms M, Bhasin D, Chettiar S, Li C, Li PK, et al:
JAK-STAT blockade inhibits tumor initiation and clonogenic recovery
of prostate cancer stem-like cells. Cancer Res. 73:5288–5298. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Marotta LL, Almendro V, Marusyk A,
Shipitsin M, Schemme J, Walker SR, Bloushtain-Qimron N, Kim JJ,
Choudhury SA, Maruyama R, et al: The JAK2/STAT3 signaling pathway
is required for growth of CD44+CD24stem cell-like breast
cancer cells in human tumors. J Clin Invest. 121:2723–2735. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Schroeder A, Herrmann A, Cherryholmes G,
Kowolik C, Buettner R, Pal S, Yu H, Müller-Newen G and Jove R: Loss
of androgen receptor expression promotes a stem-like cell phenotype
in prostate cancer through STAT3 signaling. Cancer Res.
74:1227–1237. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Zhou B, Damrauer JS, Bailey ST, Hadzic T,
Jeong Y, Clark K, Fan C, Murphy L, Lee CY, Troester MA, et al:
Erythropoietin promotes breast tumorigenesis through
tumor-initiating cell self-renewal. J Clin Invest. 124:553–563.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Guo X, Qiu J, Tu T, Yang X, Deng L, Anders
RA, Zhou L and Fu YX: Induction of innate lymphoid cell-derived
interleukin-22 by the transcription factor STAT3 mediates
protection against intestinal infection. Immunity. 40:25–39. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Wang H, Lathia JD, Wu Q, Wang J, Li Z,
Heddleston JM, Eyler CE, Elderbroom J, Gallagher J, Schuschu J, et
al: Targeting interleukin 6 signaling suppresses glioma stem cell
survival and tumor growth. Stem Cells. 27:2393–2404. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Giladi ND, Ziv-Av A, Lee HK, Finniss S,
Cazacu S, Xiang C, Waldman Ben-Asher H, deCarvalho A, Mikkelsen T,
Poisson L and Brodie C: RTVP-1 promotes mesenchymal transformation
of glioma via a STAT-3/IL-6-dependent positive feedback loop.
Oncotarget. 6:22680–22697. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Li GH, Wei H, Lv SQ, Ji H and Wang DL:
Knockdown of STAT3 expression by RNAi suppresses growth and induces
apoptosis and differentiation in glioblastoma stem cells. Int J
Oncol. 37:103–110. 2010.PubMed/NCBI
|
|
90
|
Yu H, Lee H, Herrmann A, Buettner R and
Jove R: Revisiting STAT3 signalling in cancer: New and unexpected
biological functions. Nat Rev Cancer. 14:736–746. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Jackson C, Ruzevick J, Amin AG and Lim M:
Potential role for STAT3 inhibitors in glioblastoma. Neurosurg Clin
N Am. 23:379–389. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Chang Q, Bournazou E, Sansone P, Berishaj
M, Gao SP, Daly L, Wels J, Theilen T, Granitto S, Zhang X, et al:
The IL-6/JAK/Stat3 feed-forward loop drives tumorigenesis and
metastasis. Neoplasia. 15:848–862. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
93
|
Liang Q, Ma C, Zhao Y, Gao G and Ma J:
Inhibition of STAT3 reduces astrocytoma cell invasion and
constitutive activation of STAT3 predicts poor prognosis in human
astrocytoma. PLoS One. 8:e847232013. View Article : Google Scholar : PubMed/NCBI
|
|
94
|
Bowman T, Broome MA, Sinibaldi D, Wharton
W, Pledger WJ, Sedivy JM, Irby R, Yeatman T, Courtneidge SA and
Jove R: Stat3-mediated Myc expression is required for Src
transformation and PDGF-induced mitogenesis. Proc Natl Acad Sci
USA. 98:pp. 7319–7324. 2001; View Article : Google Scholar : PubMed/NCBI
|
|
95
|
Hodge DR, Hurt EM and Farrar WL: The role
of IL-6 and STAT3 in inflammation and cancer. Eur J Cancer.
41:2502–2512. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
96
|
Kortylewski M and Yu H: Stat3 as a
potential target for cancer immunotherapy. J Immunother.
30:131–139. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
97
|
Yu CL, Meyer DJ, Campbell GS, Larner AC,
Carter-Su C, Schwartz J and Jove R: Enhanced DNA-binding activity
of a Stat3-related protein in cells transformed by the Src
oncoprotein. Science. 269:81–83. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
98
|
Zhong Z, Wen Z and Darnell JE Jr: Stat3: A
STAT family member activated by tyrosine phosphorylation in
response to epidermal growth factor and interleukin-6. Science.
264:95–98. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
99
|
Elliott LH, Brooks WH and Roszman TL:
Inability of mitogen-activated lymphocytes obtained from patients
with malignant primary intracranial tumors to express high affinity
interleukin 2 receptors. J Clin Invest. 86:80–86. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
100
|
Fletcher S, Drewry JA, Shahani VM, Page BD
and Gunning PT: Molecular disruption of oncogenic signal transducer
and activator of transcription 3 (STAT3) protein. Biochem Cell
Biol. 87:825–833. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
101
|
Jing N and Tweardy DJ: Targeting Stat3 in
cancer therapy. Anticancer Drugs. 16:601–607. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
102
|
Leeman RJ, Lui VW and Grandis JR: STAT3 as
a therapeutic target in head and neck cancer. Expert Opin Biol
Ther. 6:231–241. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
103
|
Heinrich PC, Behrmann I, Müller-Newen G,
Schaper F and Graeve L: Interleukin-6-type cytokine signalling
through the gp130/Jak/STAT pathway. Biochem J. 334:297–314. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
104
|
Kim SR, Bae MK, Kim JY, Wee HJ, Yoo MA and
Bae SK: Aspirin induces apoptosis through the blockade of
IL-6-STAT3 signaling pathway in human glioblastoma A172 cells.
Biochem Biophys Res Commun. 387:342–347. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
105
|
Shao H, Cheng HY, Cook RG and Tweardy DJ:
Identification and characterization of signal transducer and
activator of transcription 3 recruitment sites within the epidermal
growth factor receptor. Cancer Res. 63:3923–3930. 2003.PubMed/NCBI
|
|
106
|
Abou-Ghazal M, Yang DS, Qiao W,
Reina-Ortiz C, Wei J, Kong LY, Fuller GN, Hiraoka N, Priebe W,
Sawaya R and Heimberger AB: The incidence, correlation with
tumor-infiltrating inflammation, and prognosis of phosphorylated
STAT3 expression in human gliomas. Clin Cancer Res. 14:8228–8235.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
107
|
Tu Y, Zhong Y, Fu J, Cao Y, Fu G, Tian X
and Wang B: Activation of JAK/STAT signal pathway predicts poor
prognosis of patients with gliomas. Med Oncol. 28:15–23. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
108
|
Minniti G, Muni R, Lanzetta G, Marchetti P
and Enrici RM: Chemotherapy for glioblastoma: Current treatment and
future perspectives for cytotoxic and targeted agents. Anticancer
Res. 29:5171–5184. 2009.PubMed/NCBI
|
|
109
|
Baer JC, Freeman AA, Newlands ES, Watson
AJ, Rafferty JA and Margison GP: Depletion of O6-alkylguanine-DNA
alkyltransferase correlates with potentiation of temozolomide and
CCNU toxicity in human tumour cells. Br J Cancer. 67:1299–1302.
1993. View Article : Google Scholar : PubMed/NCBI
|
|
110
|
Wang G, Weiss C, Sheng P and Bresnick E:
Retrovirus-mediated transfer of the human O6-methylguanine-DNA
methyltransferase gene into a murine hematopoietic stem cell line
and resistance to the toxic effects of certain alkylating agents.
Biochem Pharmacol. 51:1221–1218. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
111
|
Hegi ME, Diserens AC, Gorlia T, Hamou MF,
de Tribolet N, Weller M, Kros JM, Hainfellner JA, Mason W, Mariani
L, et al: MGMT gene silencing and benefit from temozolomide in
glioblastoma. N Engl J Med. 352:997–1003. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
112
|
Piperi C, Themistocleous MS, Papavassiliou
GA, Farmaki E, Levidou G, Korkolopoulou P, Adamopoulos C and
Papavassiliou AG: High incidence of MGMT and RARbeta promoter
methylation in primary glioblastomas: Association with
histopathological characteristics, inflammatory mediators and
clinical outcome. Mol Med. 16:1–9. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
113
|
Giometto B, Bozza F, Faresin F, Alessio L,
Mingrino S and Tavolato B: Immune infiltrates and cytokines in
gliomas. Acta Neurochir (Wien). 138:50–56. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
114
|
Chang CY, Li MC, Liao SL, Huang YL, Shen
CC and Pan HC: Prognostic and clinical implication of IL-6
expression in glioblastoma multiforme. J Clin Neurosci. 12:930–933.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
115
|
Sasaki A, Ishiuchi S, Kanda T, Hasegawa M
and Nakazato Y: Analysis of interleukin-6 gene expression in
primary human gliomas, glioblastoma xenografts, and glioblastoma
cell lines. Brain Tumor Pathol. 18:13–21. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
116
|
Hussain SF, Kong LY, Jordan J, Conrad C,
Madden T, Fokt I, Priebe W and Heimberger AB: A novel small
molecule inhibitor of signal transducers and activators of
transcription 3 reverses immune tolerance in malignant glioma
patients. Cancer Res. 67:9630–9636. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
117
|
Iwamaru A, Szymanski S, Iwado E, Aoki H,
Yokoyama T, Fokt I, Hess K, Conrad C, Madden T, Sawaya R, et al: A
novel inhibitor of the STAT3 pathway induces apoptosis in malignant
glioma cells both in vitro and in vivo. Oncogene. 26:2435–2444.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
118
|
Stechishin OD, Luchman HA, Ruan Y, Blough
MD, Nguyen SA, Kelly JJ, Cairncross JG and Weiss S: On-target
JAK2/STAT3 inhibition slows disease progression in orthotopic
xenografts of human glioblastoma brain tumor stem cells. Neuro
Oncol. 15:198–207. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
119
|
McFarland BC, Ma JY, Langford CP,
Gillespie GY, Yu H, Zheng Y, Nozell SE, Huszar D and Benveniste EN:
Therapeutic potential of AZD1480 for the treatment of human
glioblastoma. Mol Cancer Ther. 10:2384–2393. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
120
|
He K, Qi Q, Chan CB, Xiao G, Liu X,
Tucker-Burden C, Wang L, Mao H, Lu X, McDonald FE, et al: Blockade
of glioma proliferation through allosteric inhibition of JAK2. Sci
Signal. 6:ra552013. View Article : Google Scholar : PubMed/NCBI
|
|
121
|
Senft C, Priester M, Polacin M, Schröder
K, Seifert V, Kögel D and Weissenberger J: Inhibition of the
JAK-2/STAT3 signaling pathway impedes the migratory and invasive
potential of human glioblastoma cells. J Neurooncol. 101:393–403.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
122
|
Lo HW, Cao X, Zhu H and Ali-Osman F:
Constitutively activated STAT3 frequently coexpresses with
epidermal growth factor receptor in high-grade gliomas and
targeting STAT3 sensitizes them to Iressa and alkylators. Clin
Cancer Res. 14:6042–6054. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
123
|
Mukthavaram R, Ouyang X, Saklecha R, Jiang
P, Nomura N, Pingle SC, Guo F, Makale M and Kesari S: Effect of the
JAK2/STAT3 inhibitor SAR317461 on human glioblastoma tumorspheres.
J Transl Med. 13:2692015. View Article : Google Scholar : PubMed/NCBI
|
|
124
|
Fuh B, Sobo M, Cen L, Josiah D, Hutzen B,
Cisek K, Bhasin D, Regan N, Lin L, Chan C, et al: LLL-3 inhibits
STAT3 activity, suppresses glioblastoma cell growth and prolongs
survival in a mouse glioblastoma model. Br J Cancer. 100:106–112.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
125
|
Ball S, Li C, Li PK and Lin J: The small
molecule, LLL12, inhibits STAT3 phosphorylation and induces
apoptosis in medulloblastoma and glioblastoma cells. PLoS One.
6:e188202011. View Article : Google Scholar : PubMed/NCBI
|
|
126
|
Sai K, Wang S, Balasubramaniyan V, Conrad
C, Lang FF, Aldape K, Szymanski S, Fokt I, Dasgupta A, Madden T, et
al: Induction of cell-cycle arrest and apoptosis in glioblastoma
stem-like cells by WP1193, a novel small molecule inhibitor of the
JAK2/STAT3 pathway. J Neurooncol. 107:487–501. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
127
|
Han TJ, Cho BJ, Choi EJ, Kim DH, Song SH,
Paek SH and Kim IA: Inhibition of STAT3 enhances the
radiosensitizing effect of temozolomide in glioblastoma cells in
vitro and in vivo. J Neurooncol. 130:89–98. 2016. View Article : Google Scholar : PubMed/NCBI
|