Introduction
In the last decade, the incidence of thyroid
carcinoma has rapidly increased, and its worldwide incidence has
more than doubled, accounting for between 2.7 and 17% of all
thyroid tumors (1). Papillary thyroid
cancer (PTC) is the most common type of thyroid malignancy. The
rate of PTC is the fastest growing amongst thyroid malignancies in
women, and the ratio of incidence between men and women is 1:2.58
(2). The incidence of PTC has also
rapidly increased in China in recent years (2).
Epidermal growth factor receptor (EGFR), which is
located mainly in the cell membrane, is a member of the receptor
tyrosine kinases (3). Overexpression
of EGFR and/or its ligands is frequently observed in human cancer.
In addition, it has been demonstrated that activating mutations of
EGFR are direct determinants of oncogenic transformation in breast,
ovarian and non-small cell lung cancer (4–6). It has
been demonstrated that expression of EGFR is an independent
prognostic factor in thyroid cancer (7). Previous research has indicated that
endocytotic recycling of EGFR may be the underlying molecular
mechanism for its increase (8).
Caveolin-1 (CAV-1), Eps15 homology domain 1 (EHD1) and RAB11 family
interacting protein 3 (RAB11FIP3) serve an important function in
endocytotic recycling.
CAV-1, an essential protein constituent of the
caveolae gene family, participates in vesicular trafficking and
signal transduction, and serves an important function in cell
proliferation, differentiation, migration, apoptosis and
angiogenesis (9,10). EGFR interacts with CAV-1 through a
CAV-binding sequence motif located in its intracellular kinase
domain (7). Mutations in CAV-1 or
abnormal CAV-1 expression may increase the expression of EGFR
(11,12).
The C-terminal EGFR pathway substrate 15 homology
domain/receptor-mediated endocytosis-1 family is a novel group of
endosomal scaffolding molecules that are required for receptor
recycling. It is notable that the most typical role for EHD1 is the
recycling of transmembrane cargo from the endosomal-recycling
compartment (ERC) to the plasma membrane, which is mediated by
clathrin-dependent and -independent mechanisms (13,14).
RAB proteins (Ras-related protein) serve a major
function in vesicle budding, delivery, tethering and fusion; for
example, the RAB11 GTPase subfamily members are enriched on the ERC
and regulate membrane trafficking through the ERC (15,16).
RAB11FIPs or FIP3, which are common in the RAB11-positive ERC, are
the most prominent members of this subfamily (17,18). FIP3,
which is located on the ERC during interphase, is required to
maintain the structural integrity of the ERC; furthermore, it also
participates in the process of membrane transport from the ERC to
the site of membrane insertion during cell division (17–19).
Endocytotic recycling is an important means for the
transport of biological macromolecules and proteins, and serves an
important role in normal cell metabolism and material transport in
the human body. In normal cells, EGFR internalizes to activate
signaling and then enters the lysosome for degradation, or accesses
the ERC and returns to the cell membrane via EHD1- and
RAB11FIP3-mediated ‘slow recycling’ (20). We hypothesize that in tumor cells,
EHD1 expression increases and accelerates the recycling of EGFR,
and it may extend the signal duration and effect, which is
conducive for tumor development.
In the present study, the expression of EHD1, EGFR,
CAV-1 and RAB protein was measured in patients with PTC, and the
correlation between EHD1, EGFR, CAV-1 and RAB protein expression
was analyzed. The aim of the present study was to investigate
whether the proteins involved in endocytotic recycling are also
involved in EGFR overexpression and tumor evolution in PTC, and to
assess the underlying molecular mechanism of this function.
Materials and methods
Tissue samples
A total of 72 pairs of paraformaldehyde-fixed
paraffin-embedded specimens were collected from patients with PTC
(median age, 45 years; age range, 27–64 years; male: female ratio,
1:8) resection between January 2012 and December 2014. Tumor
specimens were divided into two parts. One was fixed in 4%
formaldehyde for 24 h at 4°C, and then routinely processed into
paraffin blocks; the other were snap frozen in liquid nitrogen, and
stored at −80°C until subsequent use further detection. None of the
patients in this study had received radiation or chemotherapy prior
to surgery. The clinicopathological characteristics of the patients
examined are summarized in Table I.
The clinicopathological features did not include tumor grade and
tumor stage, thus these features were not investigated. The study
was retrospective, and the cases, which represent a spectrum of
PTCs, were retrieved from the Third Affiliated Hospital of Harbin
Medical University (Heilongjiang, China).
 | Table I.Clinical and pathological
characteristics of 72 patients. |
Table I.
Clinical and pathological
characteristics of 72 patients.
| Characteristic | Tissues, n |
|---|
| Sex |
|
| Male | 8 |
|
Female | 64 |
| Male:female
ratio | 1:8 |
| Age, years |
|
|
<45 | 46 |
| ≥45 | 26 |
| Tumor size, cm |
|
|
<2 | 52 |
| ≥2 | 20 |
| Lymph node
metastasis |
|
| No | 49 |
| Yes | 23 |
Immunohistochemistry (IHC)
The aforementioned 72 PTC tissue sections were
deparaffinized with xylene and rehydrated in a descending alcohol
series (85, 95, 100%). Subsequently, the sections were soaked in
citrate (pH 6.0) and autoclaved at 120°C for 2 min for antigen
retrieval, then cooled for 30 min to room temperature.
H2O2 (3%) was used to quench the samples, and
5% goat serum (OriGene Technologies, Inc., Beijing, China) was used
to block the samples for 10 min at room temperature. Rabbit
monoclonal antibody against EHD1 (cat. no. ab109311; Abcam,
Cambridge, UK), rabbit monoclonal antibody against EGFR (cat. no.
4267; Cell Signaling Technology, Inc., Danvers, MA, USA), mouse
polyclonal antibody against CAV-1 (cat. no. 3238; Cell Signaling
Technology, Inc.) and rabbit polyclonal antibody against RAB11FIP3
(cat. no. LS-C120286; LifeSpan BioSciences, Inc., Seattle, WA, USA)
were used as primary antibodies. The sections were incubated
overnight at 4°C with these primary antibodies, which were diluted
at 1:100, 1:45, 1:80 and 1:80, respectively, followed by incubation
with biotinylated sheep anti-rabbot IgG or goat anti-mouse IgG
secondary antibodies (SPN-9001 or SPN-9002; Origenes Technclogies,
Inc.) at room temperature for 30 min. The sections were then
stained with DAB. Following hematoxylin staining (5 min in room
temperature) according to the standard procedure and gradient
dehydration, the sections were mounted. Double-blind analysis was
performed on all samples by two independent investigators
(Department of Pathology in Harbin Medical University, Harbin,
China) without any prior knowledge of the clinicopathological
data.
The results of IHC were scored using the following
standard: Staining intensity was divided into four grades according
to the percentage of stained cells relative to the total number of
cells. The samples were given a grade as follows based on the
number of the cells stained: -, 0–5%; +, 6–25%; ++, 26–50%; and
+++, 51–100%. Samples were sorted into two categories based on
their positive staining rate and a threshold of 5% was used for
EHD1, EGFR, CAV-1 and RAB11FIP3. ‘Positive’ indicates that the
percentage of stained cells was >5%, while ‘negative’ indicates
that the percentage of stained cells was ≤5%.
Western blot analysis
A total of 30 pairs of fresh PTC tissues obtained
from the afformentioned 72 tissues were lysed with
radioimmunoprecipitation assay lysis buffer (P0013B; Beyotime
Institute of Biotechnology, Haimen, China) in the presence of
protease inhibitors (04693159001; Roche Diagnostics, Basel,
Switzerland). Samples were heated for 5 min at 95°C. Protein
concentration were determined by a BCA assay and equal amounts of
protein lysate (100 µg) were resolved using SDS-PAGE (12% gel).
Polyvinylidene difluoride (PVDF) membranes (EMD Millipore,
Billerica, MA, USA) were cut according to the size of the gel and
used for electrotransfer. For immunoblotting, antibodies against
EGFR (Cell Signaling Technology, Inc.), EHD1 (Abcam), CAV-1 (Cell
Signaling Technology, Inc.) and GAPDH (Sigma-Aldrich; Merck KGaA,
Darmstadt, Germany) were used. GAPDH solution was diluted with TBS
with Tween-20 (TBST) at 1:5,000 and incubated with the PVDF
membrane overnight at 4°C. The other primary antibody solutions
were diluted at 1:1,000 and incubated with the PVDF membrane
overnight at 4°C. Subsequently, the membranes were washed 3 times
with TBST and then a 1:5,000 dilution of the horseradish
peroxidase-conjugated goat anti-mouse IgG or goat anti-rabbit IgG
secondary antibodies (ZB-2305 or ZB-2308 respectively; Origene
Technologies, Inc.), which was diluted with TBST, was incubated
with the membrane with agitation for 1 h at room temperature.
Quantity One 4.62 software (Bio-Rad Laboratories Inc., Hercules,
CA, USA) was used for denistometric analysis.
Statistical analysis
The SPSS statistical software package (version 17.0;
SPSS, Inc., Chicago, IL, USA) was used for statistical
calculations. The comparison between two or multiple rates was
performed using the χ2 test or Continuity Correction
test. Spearman's correlation coefficient test was used to evaluate
correlations between EHD1 and the other proteins. The data are
expressed as the mean ± standard deviation where applicable.
One-way analysis of variance was used to analyze the differences
between groups with Fisher's least significant difference post-hoc
test. An independent t-test was used for comparison of differences
in the mean value. P<0.05 was considered to indicate a
statistically significant difference.
Results
Expression level of EHD1, EGFR,
RAB11FIP3 and CAV-1 in human PTC tissues
Characteristics describing the 72 patients included
in the study are summarized in Table
I. To determine whether EHD1 serves a function in endocytotic
recycling of EGFR, the expression of EHD1, EGFR, RAB11FIP3 and
CAV-1 was investigated. A representative sample of immunostaining
with antibodies against EHD1, EGFR, RAB11FIP3 and CAV-1 is
presented in Fig. 1A. Using IHC, EHD1
expression was detected in the cell nucleus and EGFR, RAB11FIP3 and
CAV-1 expression in the cytoplasm of the cancer tissues. IHC
revealed that the nuclear expression of EHD1 was increased in PTC
tissues, with 73.6% of tumors being positive for EDH1 expression,
while only 31.9% were positive in tumor-adjacent normal tissues.
The frequency of positive staining was 69.4% for EGFR, 87.5% for
CAV-1 and 87.5% for RAB11FIP3 in the PTC samples (n=72). The
frequency of positive staining was 27.8% for EGFR, 37.5% for CAV-1
and 25% for RAB11FIP3 in normal tissues (n=72). As presented in
Fig. 1B and Table II, it was identified that compared
with adjacent normal tissues, cancer tissues expressed
significantly higher levels of EHD1 in the nucleus and
significantly higher levels of EGFR, CAV-1 and RAB11FIP3 in the
cytoplasm (all P<0.01).
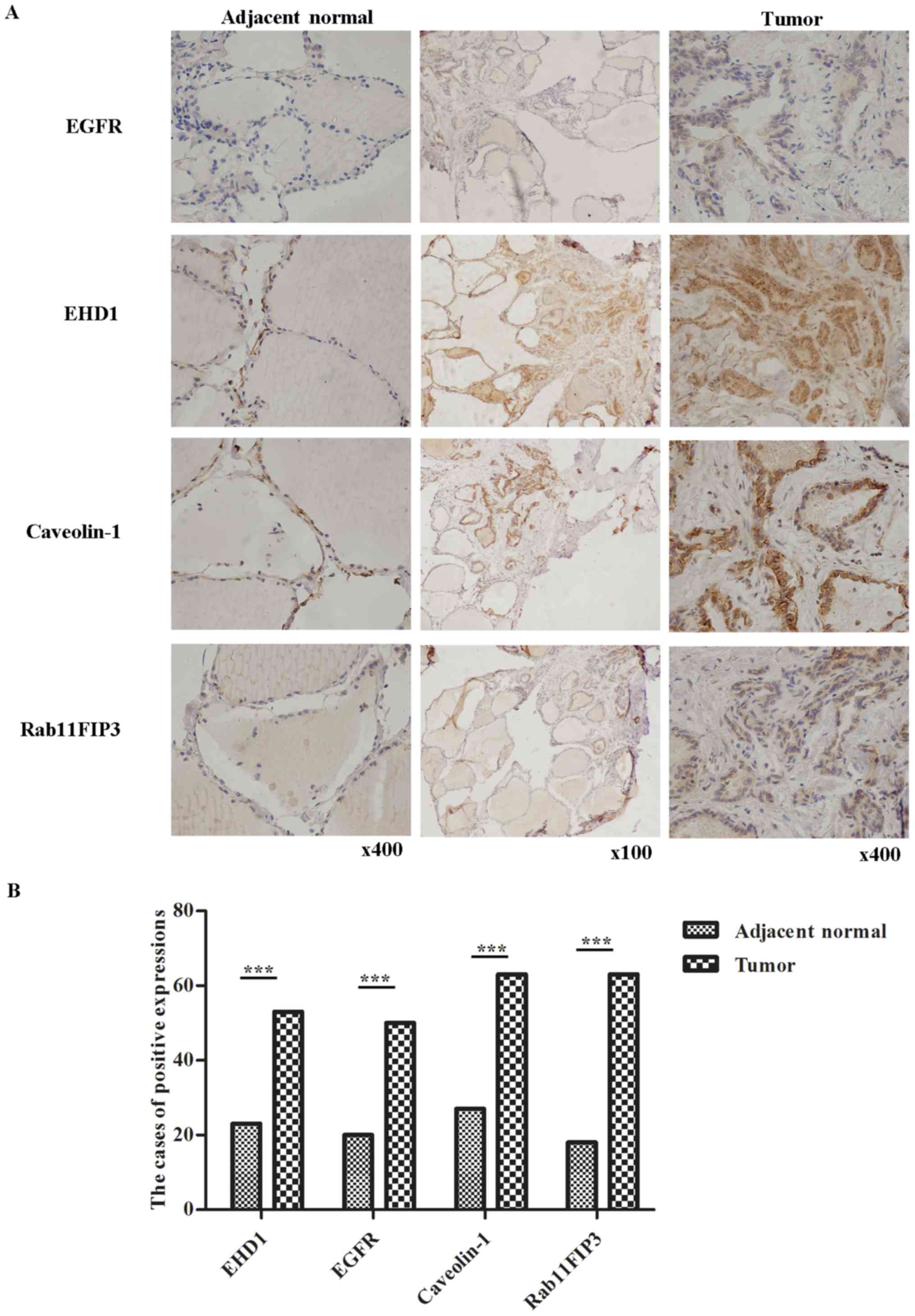 | Figure 1.Immunohistochemical analysis of
protein expression in adjacent normal tissues and tumor tissues of
papillary thyroid cancer (n=72). (A) Representative samples
expressing high EHD1, EGFR, RAB11FIP3 and CAV-1 in tumor tissues
relative to normal tissues. Representative samples of EHD1, EGFR,
RAB11FIP3 and CAV-1 expression (brown color staining), as detected
by immunohistochemistry in a pair of tumor tissues and adjacent
normal tissues. (B) Compared with adjacent normal tissues, tumor
tissues expressed significantly increased EHD1 (P<0.01), EGFR
(P<0.01), CAV-1 (P<0.01) and RAB11FIP3 (P<0.01)
expression. Fig. 1B was created from
the data of Table II. ***P<0.001.
EHD1, EH domain-containing 1; EGFR, epidermal growth factor
receptor; RAB11FIP3, RAB11 family interacting protein 3; CAV-1,
caveolin-1. |
 | Table II.Comparison of EHD1, EGFR, CAV-1 and
RAB11FIP3 expression between PTC and adjacent normal tissues
(n=144). |
Table II.
Comparison of EHD1, EGFR, CAV-1 and
RAB11FIP3 expression between PTC and adjacent normal tissues
(n=144).
|
| EHD1, n (%) |
| EGFR, n (%) |
| CAV-1, n (%) |
| RAB11FIP3, n (%) |
|
|---|
|
|
|
|
|
|
|
|
|
|
|---|
| Tissue | Positive | Negative | P-value | Positive | Negative | P-value | Positive | Negative | P-value | Positive | Negative | P-value |
|---|
| PTC | 53 (73.6) | 19 (26.4) |
| 50 (69.4) | 22 (30.6) |
| 63 (87.5) | 9 (12.5) |
| 63 (87.5) | 9 (12.5) |
|
| Adjacent normal
tissues | 23 (31.9) | 49 (68.1) |
<0.001a | 20 (27.8) | 52 (72.2) |
<0.001a | 27 (37.5) | 45 (62.5) |
<0.001a | 18 (25) | 54 (75) |
<0.001a |
The protein expression level of EHD1, EGFR and CAV-1
was also evaluated in 30 pairs of PTC and non-tumor samples by
western blotting. The RAB11FIP3 expression data were not associated
with the clinicopathological features of the patient samples by IHC
and therefore were not analyzed by western blotting.
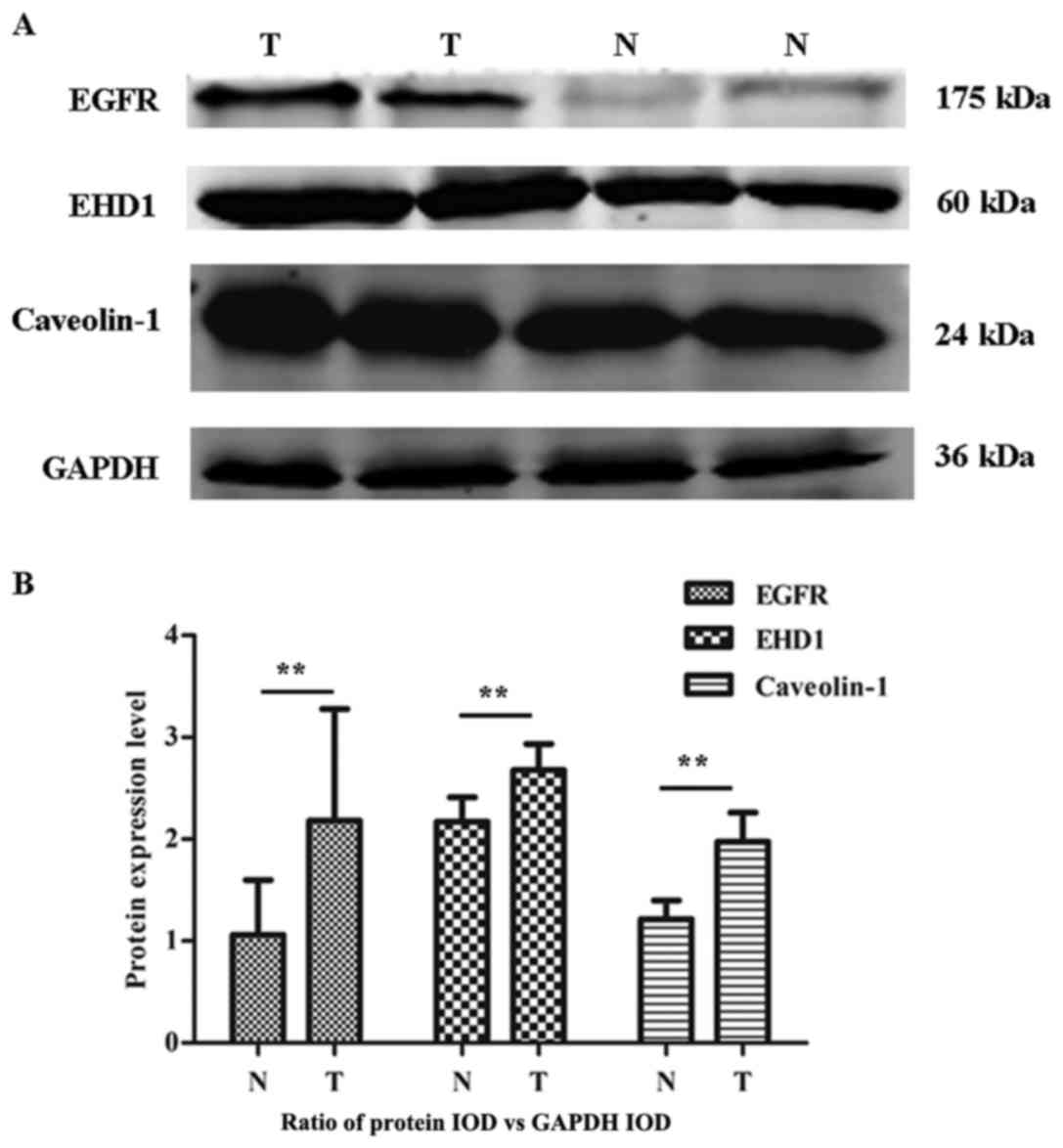
PTC exhibited higher levels of EGFR, EHD1 and CAV-1
compared with adjacent normal tissues (Fig. 2A). Furthermore, statistical analysis
revealed a significant difference in the mean expression levels of
EGFR, EHD1 and CAV-1 in the PTC group compared with the normal
tissue group (all P<0.01; Fig.
2B). These data, therefore, suggest that high expression of
EHD1 is associated with an increase in the endocytotic recycling of
EGFR, possibly contributing to the aggravation of EGFR recycling in
PTC.
Correlation of EHD1, EGFR, RAB11FIP3
and CAV-1 in human PTC tissues
A statistical correlation analysis (n=72), presented
in Table III, revealed that the
expression of EHD1 was positively correlated with the
overexpression of EGFR (r=0.564; P<0.05), CAV-1 (r=0.865;
P<0.01) and RAB11FIP3 (r=0.504; P<0.05). It was also
identified that expression of EGFR was positively correlated with
CAV-1 (r=0.595; P<0.05). These data suggested that high
expression of EHD1 and CAV-1 was positively correlated with EGFR,
which increased endocytotic recycling of EGFR in PTC.
 | Table III.Correlation between EHD1, EGFR, CAV-1
and RAB11FIP3 expression in human papillary thyroid carcinoma. |
Table III.
Correlation between EHD1, EGFR, CAV-1
and RAB11FIP3 expression in human papillary thyroid carcinoma.
| Protein | EHD1 | EGFR | CAV-1 |
|---|
| EHD1 |
|
|
|
|
r-value |
|
|
|
|
P-value |
|
|
|
| EGFR |
|
|
|
|
r-value | 0.564 |
|
|
|
P-value | 0.023a |
|
|
| CAV-1 |
|
|
|
|
r-value | 0.865 | 0.595 |
|
|
P-value |
<0.001b | 0.015a |
|
| RAB11FIP3 |
|
|
|
|
r-value | 0.504 | 0.227 | 0.487 |
|
P-value | 0.046a | 0.397 | 0.056 |
Clinical significance of EHD1, EGFR,
CAV-1 and RAB11FIP3
Protein expression data were analyzed for
associations with the clinicopathological features of the patient
samples (Table IV). The protein
levels of variables were associated with several known
clinicopathological characteristics of PTC. The results
demonstrated that expression of EHD1 was associated with tumor size
(P=0.011), lymph node metastasis (P=0.020) and EGFR expression
(P=0.003), but not with any other factors. Expression of CAV-1 was
associated with tumor size (P=0.013) and EGFR expression (P=0.003),
and EGFR expression was associated with lymph node metastasis
(P=0.027); however, RAB11FIP3 was not associated with any
clinicopathological characteristics. In Table V, western blotting results revealed
that upregulation of EGFR and CAV-1 was not significantly
associated with sex, age, tumor size or lymph node metastasis of
the patients. However, overexpression of EHD1 was positively
associated with tumor size (P=0.044).
 | Table IV.Clinicopathological characteristics
and expression of EHD1, EGFR, CAV-1 and RAB11FIP3 by
immunohistochemistry. |
Table IV.
Clinicopathological characteristics
and expression of EHD1, EGFR, CAV-1 and RAB11FIP3 by
immunohistochemistry.
|
|
| EGFR, n |
| EHD1, n |
| CAV-1, n |
| RAB11FIP3, n |
|
|---|
|
|
|
|
|
|
|
|
|
|
|
|---|
| Characteristic | n | Positive | Negative | P-value | Positive | Negative | P-value | Positive | Negative | P-value | Positive | Negative | P-value |
|---|
| Sex |
|
|
| NS |
|
| NS |
|
| NS |
|
| NS |
|
Male | 8 | 5 | 3 |
| 6 | 2 |
| 5 | 3 |
| 7 | 1 |
|
|
Female | 64 | 45 | 19 |
| 47 | 17 |
| 58 | 6 |
| 54 | 8 |
|
| Male:female
ratio | 1:8 |
|
|
|
|
|
|
|
|
|
|
|
|
| Age, years |
|
|
| NS |
|
| NS |
|
| NS |
|
| NS |
|
<45 | 46 | 30 | 16 |
| 37 | 9 |
| 40 | 6 |
| 42 | 4 |
|
|
≥45 | 26 | 20 | 6 |
| 16 | 10 |
| 23 | 3 |
| 21 | 5 |
|
| Tumor size, cm |
|
|
| NS |
|
| 0.011a |
|
| 0.013a |
|
| NS |
|
<2 | 52 | 37 | 15 |
| 34 | 18 |
| 49 | 3 |
| 44 | 8 |
|
| ≥2 | 20 | 13 | 7 |
| 19 | 1 |
| 13 | 6 |
| 19 | 1 |
|
| Lymph node
metastasis |
|
|
| 0.027a |
|
| 0.020a |
|
| NS |
|
| NS |
| No | 49 | 30 | 19 |
| 32 | 17 |
| 41 | 8 |
| 45 | 4 |
|
|
Yes | 23 | 20 | 3 |
| 21 | 2 |
| 22 | 1 |
| 18 | 5 |
|
| EGFR |
|
|
|
|
|
| 0.003b |
|
| 0.004b |
|
| NS |
|
Positive | 50 |
|
|
| 42 | 8 |
| 48 | 2 |
| 45 | 5 |
|
|
Negative | 22 |
|
|
| 11 | 11 |
| 15 | 7 |
| 18 | 4 |
|
 | Table V.Clinicopathological characteristics
and expression of EHD1, EGFR and CAV-1 by western blotting. |
Table V.
Clinicopathological characteristics
and expression of EHD1, EGFR and CAV-1 by western blotting.
|
|
| EGFR |
| EHD1 |
| CAV-1 |
|
|---|
|
|
|
|
|
|
|
|
|
|---|
| Characteristic | n | Mean score ±
SEM | P-value | Mean score ±
SEM | P-value | Mean score ±
SEM | P-value |
|---|
| Sex |
|
| NS |
| NS |
| NS |
|
Male | 5 | 1.24±2.07 |
| 2.26±0.52 |
| 2.70±1.75 |
|
|
Female | 25 | 2.54±6.79 |
| 2.71±1.56 |
| 1.91±1.58 |
|
| Age, years |
|
| NS |
| NS |
| NS |
|
<45 | 16 | 2.45±7.04 |
| 2.59±1.59 |
| 1.74±1.88 |
|
|
≥45 | 14 | 2.16±5.51 |
| 2.67±1.32 |
| 2.35±1.28 |
|
| Tumor size, cm |
|
| NS |
| 0.044a |
| NS |
|
<2 | 22 | 2.96±7.08 |
| 2.93±1.40 |
| 2.32±1.70 |
|
| ≥2 | 8 | 0.36±0.34 |
| 1.69±1.13 |
| 1.24±0.95 |
|
| Lymph node
metastasis |
|
| NS |
| NS |
| NS |
| No | 20 | 0.53±0.45 |
| 2.55±1.42 |
| 2.31±1.74 |
|
|
Yes | 10 | 0.51±0.56 |
| 2.8±1.52 |
| 1.50±1.19 |
|
Discussion
EGFR was a transporting signaling receptors;
however, as a key protein of slow recycling, the function of EHD1
is rarely reported in tumor tissues. However, in one previous
study, it was reported to be associated with endocytotic recycling
in non-small cell lung cancer (21).
Another previous study demonstrated that increased EHD1 and EGFR
was able to regulate breast cancer progression, and that the
combined expression of EHD1 and EGFR markers may aid physicians in
predicting the time to disease recurrence following surgery, and
subsequently judge patient prognosis (8). The results of the present study revealed
that EHD1 expression is significantly higher in PTC compared with
that in tumor-adjacent normal tissues. This is similar to the
results of a study by Gao et al (22) in non-small cell lung cancer. The
results of IHC and western blotting in the present study indicated
that increased EGFR expression was associated with increased
expression of EHD1 and that the two were positively associated with
PTC. The results of the present study demonstrated that in PTC,
increased expression of EHD1 may promote EGFR transport to the cell
membrane and then accelerate EGFR for use again, which can promote
cell proliferation and malignant transformation. EHD1 alters the
EGFR internalization and degradation pathway, which is beneficial
to the accumulation of EGFR on the cell surface, ultimately leading
to changes in EGFR signaling (23).
The present results demonstrated that expression of EHD1 and EGFR
was associated with tumor size and lymph node metastasis.
Therefore, combined EHD1 and EGFR markers may assist in
subsequently judging patient prognosis.
CAV-1, one of the main scaffold proteins in the cell
membrane, has a CAV-scaffold binding sequence motif in its
intracellular kinase domain, which may connect CAV-1 with EGFR to
regulate its activity (24), and this
interaction has been demonstrated to serve a function in regulating
tumorigenesis (11).
The present study confirmed that the expression of
EGFR and CAV-1 in PTC compared with that in tumor-adjacent normal
tissues was significantly different and that the expression was
associated with lymph node metastasis and tumor size, respectively.
These findings are consistent with findings by Lee and Lee
(25). Furthermore, elevated
expression of CAV-1 in PTC is consistent with the findings of
Paskas et al (26).
Additionally, studies have identified that overexpression of EGFR
is associated with high mortality in undifferentiated subtypes of
PTC (27,28). It was also identified that CAV-1
expression was positively associated with EGFR expression. The
findings in these studies, alongside the present findings, suggest
that expression of CAV-1 is able to promote EGFR signaling in
PTC.
In normal cells, EHD1 and RAB11 serve functions that
regulate recycling from the perinuclear ERC to the plasma membrane
(29). Furthermore, RAB11FIP3, a
RAB11 effector, is located upstream of EHD1 and participates in
endocytotic recycling (30). In the
present study, RAB11FIP3 expression was positively correlated with
EHD1 expression. Meanwhile, RAB11FIP3 expression was higher in
cancer tissues, and this increased expression was statistically
significant compared with that in tumor-adjacent normal tissues. We
hypothesized that RAB11FIP3 participates in endocytotic recycling
and promotes the transport of EGFR in PTC. The Rab11 GTPase
effector protein, FIP3, interacts with a part of the dynein motor
complex, and this molecular interaction contributes to driving
membrane transport at the periphery, and then sorting endosomes to
the ERC which are located in a central position (31). Meanwhile, this Rab11 effector, as an
interaction ligand of EHDs, coordinates with EHD1 to regulate the
exit of receptors from the ERC to the plasma membrane (30). This indicates that high expression of
RAB11FIP3 and EHD1 may increase the speed of the endocytotic
recycling of EGFR and increase EGFR expression on the cell surface,
promoting PTC cell proliferation and malignant transformation.
In conclusion, the high expression of CAV-1
increased the endocytosis of EGFR and EHD1 as the primary proteins
involved in recycling. Furthermore, EHD1 may speed up the recycling
process by connecting with the Rab11 effector, then promoting the
EGFR signal receptor to return to the cell membrane. This is a
novel way to increase the expression of EGFR, promoting cell growth
and proliferation in PTC. EHD1 is an important factor in promoting
tumor progression, and combined with EGFR, it may be more effective
than when used alone, at predicting the prognosis of a patient and
guiding evaluations of clinical significance.
Acknowledgements
No applicable.
Funding
The present study was supported by the National
Natural Science Foundation of China (grant nos. 81372611 and
81102081).
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
YLiu contributed to study design, experimental
procedures, performing experiments, analysis and interpretation of
data, and drafting of the manuscript. YLia performed experiments,
contributed to the data analysis, and drafted parts of the
manuscript. ML contributed to experimental procedures and
contributed to the data analysis. DL contributed to data collection
and data analysis. JT contributed to data analysis and drafted
parts of the manuscript. WY contributed to data collection and the
interpretation of clinical data. DT made substantial contributions
to establishing the histopathological diagnosis, and participated
in the interpretation of clinical data, conceptualization,
construction and drafting of the manuscript. XJ made substantial
contributions to the histopathological diagnosis,
conceptualization, construction and drafting of the manuscript.
Ethics approval and consent to
participate
The use of tissue samples in this study was approved
by the Hospital Ethics Committee for Ethical Review of Research
Involving Human Subjects at Harbin Medical University (Harbin,
China). Patient consent was obtained at the time of the original
tissue collection.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2017. CA Cancer J Clin. 67:7–30. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Mohammed AA and El-Shentenawy A: Advanced
thyroid cancers: New era of treatment. Med Oncol. 31:492014.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Herbst RS: Review of epidermal growth
factor receptor biology. Int J Radiat Oncol Biol Phys. 59 Suppl
2:S21–S26. 2004. View Article : Google Scholar
|
|
4
|
Hsu F, Nichol A, Toriumi T and De Caluwe
A: Miliary metastases are associated with epidermal growth factor
receptor mutations in non-small cell lung cancer: A
population-based study. Acta Oncol. 56:1175–1180. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Cao WM, Gao Y and Wang XJ: Lack of
epidermal growth factor receptor (EGFR)-activating mutations in
triple-negative breast cancer in china. Breast Cancer Res.
17:1152015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Tanaka Y, Terai Y, Tanabe A, Sasaki H,
Sekijima T, Fujiwara S, Yamashita Y, Kanemura M, Ueda M, Sugita M,
et al: Prognostic effect of epidermal growth factor receptor gene
mutations and the aberrant phosphorylation of Akt and ERK in
ovarian cancer. Cancer Boil Ther. 11:50–57. 2011. View Article : Google Scholar
|
|
7
|
Mallick UK and American Thyroid A: The
revised American Thyroid Association management guidelines 2009 for
patients with differentiated thyroid cancer: An evidence-based
risk-adapted approach. Clin Oncol (R Coll Radiol). 22:472–474.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Tong D, Liang YN, Stepanova AA, Liu Y, Li
X, Wang L, Zhang F and Vasilyeva NV: Increased Eps15 homology
domain 1 and RAB11FIP3 expression regulate breast cancer
progression via promoting epithelial growth factor receptor
recycling. Tumour Biol. 39:10104283176910102017. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Wang S, Wang N, Zheng Y, Zhang J, Zhang F
and Wang Z: Caveolin-1: An oxidative stress-related target for
cancer prevention. Oxid Med Cell Longev. 2017:74540312017.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Nwosu ZC, Ebert MP, Dooley S and Meyer C:
Caveolin-1 in the regulation of cell metabolism: A cancer
perspective. Mol Cancer. 15:712016. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Moreno-Caceres J, Caja L, Mainez J,
Mayoral R, Martín-Sanz P, Moreno-Vicente R, Del Pozo MÁ, Dooley S,
Egea G and Fabregat I: Caveolin-1 is required for TGF-β-induced
transactivation of the EGF receptor pathway in hepatocytes through
the activation of the metalloprotease TACE/ADAM17. Cell Death Dis.
5:e13262014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Xu L, Qu X, Li H, Li C, Liu J, Zheng H and
Liu Y: Src/caveolin- 1-regulated EGFR activation antagonizes
TRAIL-induced apoptosis in gastric cancer cells. Oncol Rep.
32:318–324. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zhang J, Naslavsky N and Caplan S: Rabs
and EHDs: Alternate modes for traffic control. Biosci Rep.
32:17–23. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Grant BD and Caplan S: Mechanisms of
EHD/RME-1 protein function in endocytic transport. Traffic.
9:2043–2052. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Butterworth MB, Edinger RS, Silvis MR,
Gallo LI, Liang X, Apodaca G, Frizzell RA and Johnson JP: Rab11b
regulates the trafficking and recycling of the epithelial sodium
channel (ENaC). Am J Physiol Renal Physiol. 302:F581–F590. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Bhuin T and Roy JK: Rab11 in disease
progression. Int J Mol Cell Med. 4:1–8. 2015.PubMed/NCBI
|
|
17
|
Vetter M, Stehle R, Basquin C and
Lorentzen E: Structure of Rab11-FIP3-Rabin8 reveals simultaneous
binding of FIP3 and Rabin8 effectors to Rab11. Nat Struc Mol Boil.
22:695–702. 2015. View Article : Google Scholar
|
|
18
|
Takahashi S, Takei T, Koga H, Takatsu H,
Shin HW and Nakayama K: Distinct roles of Rab11 and Arf6 in the
regulation of Rab11-FIP3/arfophilin-1 localization in mitotic
cells. Genes Cells. 16:938–950. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Collins LL, Simon G, Matheson J, Wu C,
Miller MC, Otani T, Yu X, Hayashi S, Prekeris R and Gould GW:
Rab11-FIP3 is a cell cycle-regulated phosphoprotein. BMC Cell Boil.
13:42012. View Article : Google Scholar
|
|
20
|
Iaea DB, Mao S, Lund FW and Maxfield FR:
Role of STARD4 in sterol transport between the endocytic recycling
compartment and the plasma membrane. Mol Biol Cell. 28:1111–1122.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Chung BM, Tom E, Zutshi N, Bielecki TA,
Band V and Band H: Nexus of signaling and endocytosis in
oncogenesis driven by non-small cell lung cancer-associated
epidermal growth factor receptor mutants. World J Clin Oncol.
5:806–823. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Gao Y, Wang Y, Sun L, Meng Q, Cai L and
Dong X: Expression of TGFβ-1 and EHD1 correlated with survival of
non-small cell lung cancer. Tumour Biol. 35:9371–9380. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Meng Q, Xing Y, Ren T, Lu H, Xi Y, Jiang
Z, Hu J, Li C, Sun L, Sun D and Cai L: Mammalian Eps15 homology
domain 1 promotes metastasis in non-small cell lung cancer by
inducing epithelial-mesenchymal transition. Oncotarget.
8:22433–22442. 2017.PubMed/NCBI
|
|
24
|
Hoop CL, Sivanandam VN, Kodali R, Srnec MN
and van der Wel PC: Structural characterization of the caveolin
scaffolding domain in association with cholesterol-rich membranes.
Biochemistry. 51:90–99. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Lee YM and Lee JB: Prognostic value of
epidermal growth factor receptor, p53 and galectin-3 expression in
papillary thyroid carcinoma. J Int Med Res. 41:825–834. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Paskas S, Jankovic J, Marecko I, Išić
Denčić T, Tatić S, Cvejić D and Savin S: Caveolin-1 expression in
papillary thyroid carcinoma: Correlation with clinicopathological
parameters and BRAF mutation status. Otolaryngol Head Neck Surg.
150:201–209. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Chen D, Qi W, Zhang P, Guan H and Wang L:
Expression of the estrogen receptor α, progesterone receptor and
epidermal growth factor receptor in papillary thyroid carcinoma
tissues. Oncol Lett. 10:317–320. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Tang C, Yang L, Wang N, Li L, Xu M, Chen
GG and Liu ZM: High expression of GPER1, EGFR and CXCR1 is
associated with lymph node metastasis in papillary thyroid
carcinoma. Int J Clin Exp Pathol. 7:3213–3223. 2014.PubMed/NCBI
|
|
29
|
Gidon A, Bardin S, Cinquin B, Boulanger J,
Waharte F, Heliot L, de la Salle H, Hanau D, Kervrann C, Goud B and
Salamero J: A Rab11A/myosin Vb/Rab11-FIP2 complex frames two late
recycling steps of langerin from the ERC to the plasma membrane.
Traffic. 13:815–833. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Naslavsky N, Rahajeng J, Sharma M, Jovic M
and Caplan S: Interactions between EHD proteins and Rab11-FIP2: A
role for EHD3 in early endosomal transport. Mol Boil Cell.
17:163–177. 2006. View Article : Google Scholar
|
|
31
|
Horgan CP, Oleksy A, Zhdanov AV, Lall PY,
White IJ, Khan AR, Futter CE, McCaffrey JG and McCaffrey MW:
Rab11-FIP3 is critical for the structural integrity of the
endosomal recycling compartment. Traffic. 8:414–430. 2007.
View Article : Google Scholar : PubMed/NCBI
|