Introduction
Gastric cancer is an intractable disease and due to
its poor prognosis and limited treatment options, it remains a
major clinical challenge worldwide. Ephrin-B2, a membrane-anchored
ligand that serves a key role in controlling angiogenic and
lymphangiogenic growth factors, is upregulated and involved in
tumor growth in various types of cancer (1). For example, ephrin-B2 protein
overexpression has been demonstrated to be associated with poor
overall survival rates in patients with head and neck squamous cell
carcinoma (2). Ephrin-B2 mRNA levels
have been reported to increase significantly with clinical stage,
and high ephrin-B2 protein expression predicted a high 24-month
survival rate of patients with ovarian cancer (3). Ephrin-B2 expression has also been
demonstrated to be upregulated in primary hepatocarcinoma,
glioblastoma and uterine cervical cancer (4–6). These
findings suggest that ephrin-B2 may be a prognostic indicator in
numerous types of cancer.
Ephrin-B2 is also a promising therapeutic target for
cancer treatment and mediates glioblastoma stem-like-cell
perivascular invasion (7).
Ephrin-B2-knockdown has been demonstrated to block tumor initiation
and treatment of established tumors via ephrin-B2 antibodies, which
suppress progression in human glioblastoma stem-like cell-derived
orthotopic xenografts (7). Systemic
administration of ephrin-B2 blocking antibody indicated a reduction
in the number of blood and lymphatic vessels in xenograft mice, and
a concomitant reduction in tumor growth (8). Silencing of ephrin-B2 by RNA
interference has been demonstrated to inhibit proliferation,
invasion, migration and angiogenesis and to induce apoptosis of
colorectal cancer cells; a possible result of vascular endothelial
growth factor (VEGF) and matric metallopeptidase 9 (MMP9)
regulation (9).
The association between ephrin-B2 and gastric cancer
prognosis and the potential of ephrin-B2 as a therapeutic target in
gastric cancer treatment remains unknown. The present study
investigated ephrin-B2 as a prognostic factor and therapeutic
target for gastric cancer.
Materials and methods
Collection of gastric cancer tissues
and serum samples
Twenty gastric cancer tissues along with the matched
healthy adjacent tissues were collected from patients at the
Qianfoshan Hospital of Shandong University (Shandong, China)
between April 2014 and April 2015. The serum samples were collected
upon physical examination of healthy personnel (age median, 58
years; age range from 32–78 years; male/female: 80/85; n=165) and
patients with gastric cancer (age median, 55 years; age range from
30–75 years; male/female: 78/84; n=162) from Qianfoshan Hospital of
Shandong University (Shandong, China) between March 2009 and April
2016. Subjects with any other types of cancer, diabetes,
cardiovascular and cerebrovascular diseases were excluded. Medical
records of patients with gastric cancer with clinical
Tumor-Node-Metastasis (TNM) staging and survival information were
collected. The basic demographic characteristics and clinical
features of the gastric cancer cases and heathy controls are
presented in Table I. Overall
survival (OS) is defined as the time from the date of pathological
diagnosis to the date of mortality from any cause. Progression-free
survival (PFS) is defined as the time from the date of pathological
diagnosis to the date of the disease progression or mortality, if
no progression was identified. All subjects provided written
informed consent to participate in the present study. This project
was approved by the Ethics Committee of The Qianfoshan Hospital,
Shandong University (Shandong, China).
 | Table I.Basic demographic characteristics and
clinical features of the patients with gastric cancer and healthy
controls. |
Table I.
Basic demographic characteristics and
clinical features of the patients with gastric cancer and healthy
controls.
| Characteristics | Gastric cancer tissue
(n=20) | Serum from patients
with gastric cancer (n=162) | Heathy control serum
(n=165) | P-value (patient vs.
healthy serum) |
|---|
| Age, years (mean ±
standard deviation) | 57.4±11.2 | 56.6±12.3 | 55.6±8.4 | 0.562 |
| Sex |
| Male | 12 | 78 | 80 | 0.951 |
|
Female | 8 | 84 | 85 |
|
| Tumor size |
| <5
cm | 7 | 78 |
|
|
| ≥5
cm | 13 | 84 |
|
|
| Distant
metastasis |
| No | 7 | 74 |
|
|
| Yes | 13 | 88 |
|
|
|
Tumor-Node-Metastasis |
| I–II | 5 | 69 |
|
|
|
III–IV | 15 | 93 |
|
|
Cell lines and cell transfection
The gastric cancer cell lines, HGC27 and MKN-45,
were obtained from the Cell Bank of the Chinese Academy of Sciences
(Shanghai, China). Cells were grown routinely in RPMI-1640 medium
(Invitrogen; Thermo Fisher Scientific, Inc., Waltham, MA, USA)
supplemented with 10% foetal bovine serum (Gibco; Thermo Fisher
Scientific, Inc.) and cultured in a 37°C with 5%
CO2.
Knockdown of ephrin-B2 was achieved by transfection
of lentiviruses containing ephrin-B2 small interfering RNA
(GeneCopoecia, Guangzhou, China; sequence:
CCGGTCTACATCAAATGGGTCTTTGCTCGAGCAAAGACCCATTTGATGTAGATTTTTG). The
cells transfected with empty lentivirus were used as control. Cells
were plated in 6 replicate wells in 96-well plates and cultured for
12 h. Cells were then transfected with the lentivirus (multiplicity
of infection=10) using Lipofectamine® 2000 (Invitrogen;
Thermo Fisher Scientific, Inc.) for 48 h according to the
manufacturer's protocol. Transfected cells were used in subsequent
assays.
Immunohistochemical (IHC)
staining
The human tissues were fixed in 4% paraformaldehyde
for 48 h at room temperature and paraffin-embedded. Tumor sections
were cut into 4-µm thick sections. The slides were regularly
deparaffinised and dehydrated. Slides were treated with citric acid
buffer (pH 6.0) in a microwave oven (500 W, 12 min). Subsequent to
cooling, the slices were blocked by normal goat serum (Wuhan Boster
Biological Technology, Ltd., Wuhan, China), and incubated with a
primary rabbit polyclonal anti-ephrin-B2 antibody (cat. no.
ab131536; 1:100 dilution; Abcam, Cambridge, UK) overnight at 4°C.
The sections were then washed with PBS and incubated with an Goat
Anti-Rabbit IgG (Biotin) (cat. no. ab6720; 1:2,000 dilution; Abcam)
for 2 h at 37°C and washed by PBS. The slides were stained by using
DAB Detection kit (Maxim, Xiamen, China) according to
manufacturer's protocol. Finally, the sections were counterstained
with 1% hematoxylin for 3 min at 45°C. The IHC images were obtained
at ×20 magnification under a light microscope (clipse Ni-E, Nikon,
Japan). The quantification of positive signalling was performed by
ImageJ (National Institutes of Health, Bethesda, MA, USA). The data
of ephrin-B2-positive staining was analyzed in 8 fields of view and
the data was averaged to produce a single value per subject.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
TRizol reagent (Invitrogen; Thermo Fisher
Scientific, Inc.) was used to extract total RNA from HGC27 and
MKN-45 cells following lentiviral transfection. ABScript II cDNA
First-Strand Synthesis kit (cat. no. RK20400; ABclonal
Biotechnology Co., Ltd, Wuhan, China) was used for reverse
transcription, according to the manufacturer's protocol. The
expression of ephrin-B2 was measured by SsoFast EvaGreen supermix
[cat. no. 1725201; Bio-Rad Laboratories (Shanghai) Co., Ltd.
Shanghai, China], according to the manufacturer's protocol.
Expression of β-actin was used as an endogenous control. The
following primers were used: Ephrin-B2, forward,
5′-GCTGGGGTGTTTTGATGGTTT-3′, reverse, 5′-AGTGCATCTGTCTGCTTGGT-3′;
β-actin, forward, 5′-GCCCTATAAAACCCAGCGGC-3′, reverse,
5′-TCGATGGGGTACTTCAGGGT-3′. The primers were synthesized by Sangon
Co. Ltd. (Shanghai, China). RT-qPCR was performed with the
following thermocycling conditions: 95.0°C for 3 min, and 39 cycles
of 95.0°C for 10 sec and 60°C for 30 sec.
GenElute Plasma/Serum RNA Purification Midi kit (cat
no. RNB600; Merck KGaA, Darmstadt, Germany) was used to isolate RNA
from 1.5 ml serum per subject, according to the manufacturer's
protocol. The quality and quantity of RNA was evaluated using
NanoDrop 2000 (Thermo Fisher Scientific, Inc.). The RNA yield from
these serum sample was ~50 ng/ml. The average absorbance ratio at
260/280 nm was ~1.93. A total of fifty ng RNA was reverse
transcribed using a cDNA ABScript II cDNA First-Strand Synthesis
kit (cat. no. RK20400, ABclonal Biotechnology Co., Ltd, Wuhan,
China) according to the manufacturer's protocol The RT-PCR data was
analysed by using 2−ΔΔCq method (10).
Cell Counting kit-8 proliferation
assay (CCK-8)
HCG27 and MKN45 cells proliferation rates were
measured using CCK-8 (Beyotime Institute of Biotechnology,
Hangzhou, China) following Ephrin B2 knockdown. Cells were seeded
at 0.5×104 cells per well in a 96-well plate for 24 h,
and further incubated for 24, 48 or 72 h. A total of 10 µl CCK-8
reagent was added to each well 1 h prior to the endpoint of
incubation. The optical density value per well was determined by a
microplate reader at a wavelength of 570 nm. The experiments were
repeated three times. Triplicate wells were used in each group.
Flow cytometry
An Annexin V-FITC/PI Apoptosis Detection kit (cat
no. 40302ES50, Yeasen, Shanghai, China; http://www.yeasen.com/) was used for analysis of
apoptosis. HGC27 and MKN-45 cells were harvested and washed twice
with PBS. A total of 2×105 cells were resuspended in 500
µl binding buffer from the kit. A total of 10 µl Annexin V-FITC and
10 µl propidium iodide (PI) were added and mixed. After 15 min
incubation, the cells were analyzed using a flow cytometer (BD
Biosciences, San Jose, CA, USA) according to the manufacturer's
protocol. The experiments were repeated three times. FlowJo
(version 10.4.2, FlowJo LLC, Ashland, OR, USA) was used to analyse
the apoptotic rate.
Statistical analysis
All data from three independent experiments were
expressed as the mean ± standard deviation and processed using SPSS
17.0 statistical software (SPSS Inc., Chicago, IL, USA). The
overall survival rate estimates were calculated using the
Kaplan-Meier method with the log-rank test. The clinical
association between ephrin-B2 expression and clinicopathological
variables in patients with gastric cancer was evaluated by the
χ2 test. The difference between two groups was
calculated by Student's t-test and the difference between multiple
groups were determined using a one-way analysis of variance with
Tukey's post-hoc test. P<0.05 was considered to indicate a
statistically significant difference. In addition, factors that
could predict the prognosis of patients with gastric cancer were
investigated by univariate and multivariate analyses.
Results
Ephrin-B2 expression is upregulated in
gastric cancer tissues and serum samples
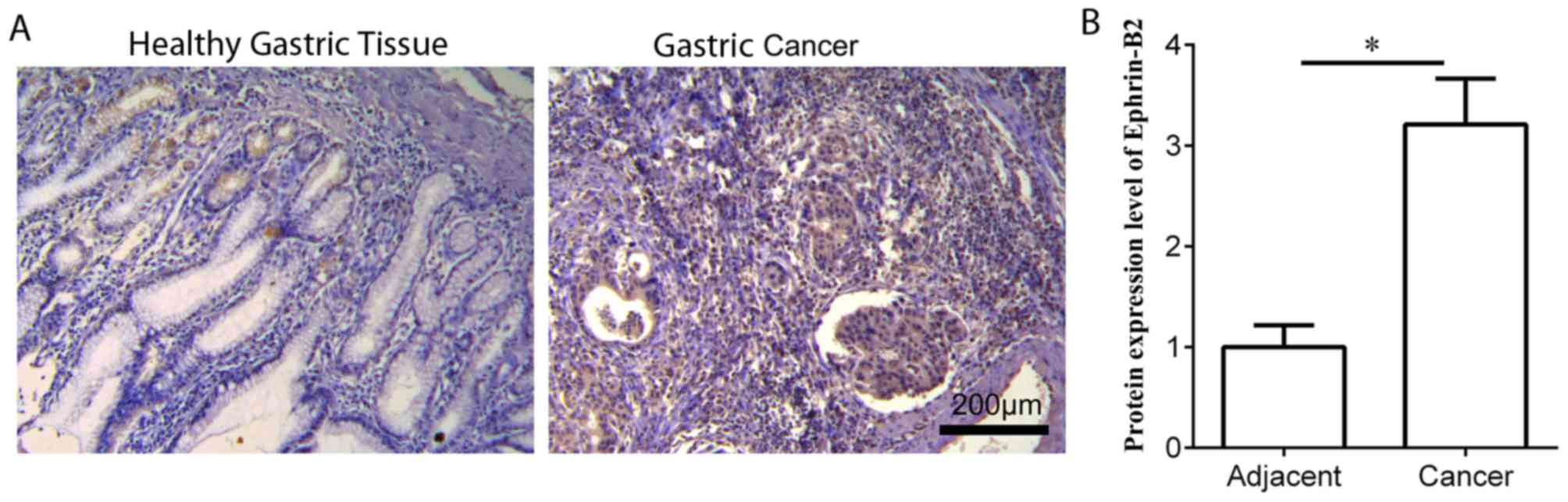
IHC staining was performed to measure the protein
expression level of ephrin-B2 in gastric cancer tissues (n=20) and
the matched healthy adjacent tissues. Ephrin-B2 was expressed in
the cytoplasm of gastric cancer cells (Fig. 1). Ephrin-B2 expression was
significantly increased in gastric cancer tissues compared with
adjacent healthy control tissues (Fig.
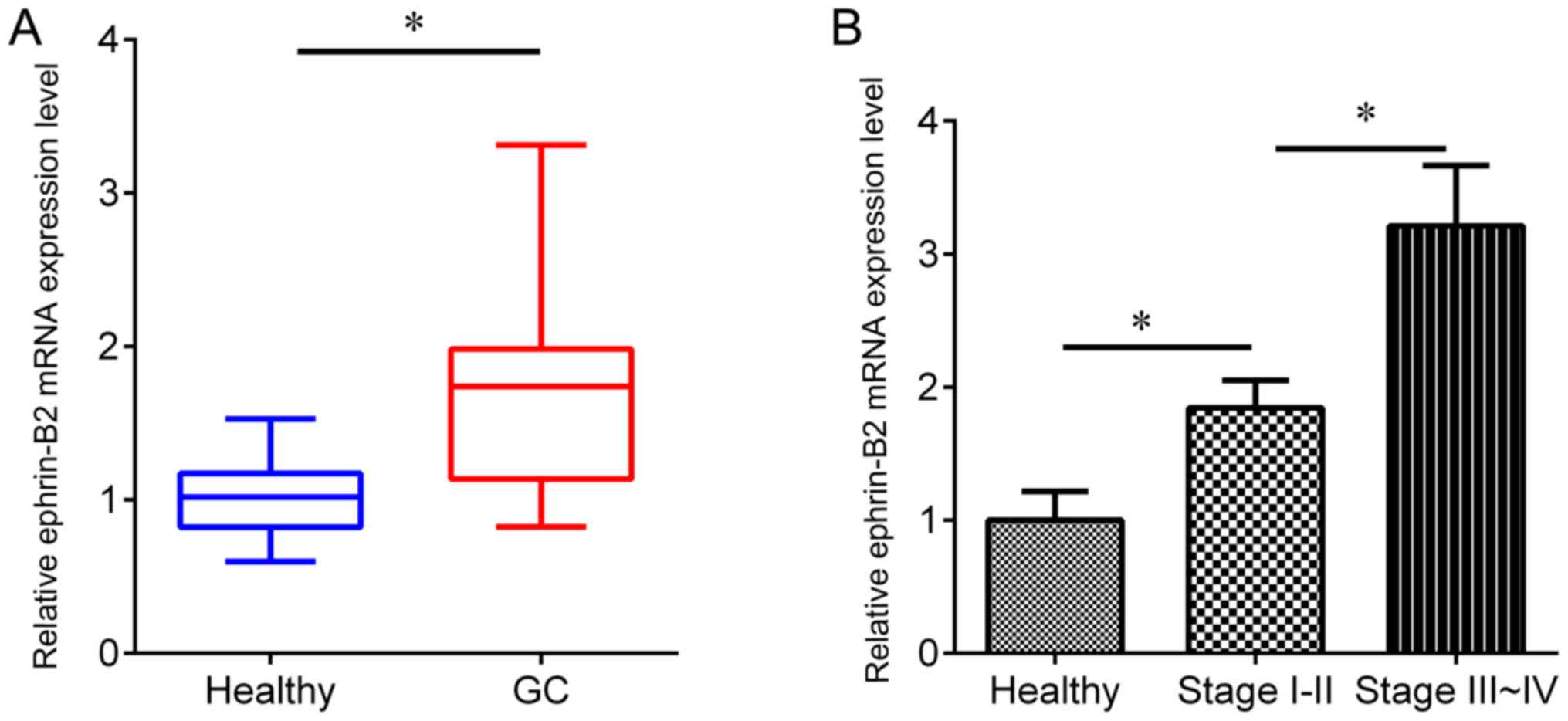
1). In addition, RT-qPCR was performed to detect the protein
expression level of ephrin-B2 in an independent cohort, including
gastric cancer serum samples (n=162) and healthy serum samples
(n=165). The expression of ephrin-B2 was revealed to be
significantly upregulated in gastric cancer serum samples compared
with the adjacent healthy samples (Fig.
2A), and the ephrin-B2 protein expression level was higher in
serum from patients with TNM stage III–IV than TNM stage I–II
disease (Fig. 2B), suggesting an
association of ephrin-B2 with gastric cancer development.
Ephrin-B2 is associated with
clinicopathoclinical features of patients with gastric cancer
The cases of gastric cancer were divided into high
and low ephrin-B2 protein expression groups, according to the mean
mRNA expression value. It was revealed that ephrin-B2 protein
expression was associated with tumor size (P<0.001), metastasis
(P=0.02) and TNM stage (P=0.03) (Table
II). Univariate analysis indicated that the serum ephrin-B2
protein expression level (P=0.02), as well as tumor size (P=0.01),
distant metastasis (P=0.02) and TNM stage (P=0.03) were
significantly associated with prognosis (Table III). Multivariate analysis indicated
that the serum ephrin-B2 protein expression level (P=0.01), tumor
size (P=0.01), distant metastasis (P=0.03) and TNM stage (P=0.02)
were independent factors for predicting the prognosis of patients
with gastric cancer (Table IV).
 | Table II.Clinical association between serum
ephrin-B2 levels and clinicopathological variables in gastric
cancer. |
Table II.
Clinical association between serum
ephrin-B2 levels and clinicopathological variables in gastric
cancer.
|
| Serum ephrin-B2 |
|
|---|
|
|
|
|
|---|
| Variable | Low expression
(n=69) | High expression
(n=93) | P-value
(χ2 test) |
|---|
| Age |
|
| 0.52 |
|
<60 | 33 | 50 |
|
| ≥60 | 36 | 43 |
|
| Sex |
|
| 0.63 |
| Male | 35 | 43 |
|
|
Female | 34 | 50 |
|
| Tumor size |
|
| <0.001 |
| <5
cm | 46 | 32 |
|
| ≥5
cm | 23 | 61 |
|
| Distant
metastasis |
|
| 0.02 |
| No | 39 | 35 |
|
| Yes | 30 | 58 |
|
| Tumor-Node-Metastasis
stage |
|
| 0.03 |
| I–II | 36 | 33 |
|
|
III–IV | 33 | 60 |
|
 | Table III.Univariate analysis of factors
associated with gastric cancer. |
Table III.
Univariate analysis of factors
associated with gastric cancer.
| Variable | Hazard ratio (95%
confidence intervals) | P-value |
|---|
| Age (≥60/<60) | 1.16 (0.66–1.66) | 0.68 |
| Sex
(male/female) | 1.07 (0.67–1.47) | 0.72 |
| Tumor size (≥5
cm/<5 cm) | 2.34 (2.24–2.44) | 0.01 |
| Distant metastasis
(yes/no) | 4.21 (3.81–4.61) | 0.02 |
| Tumor-Node-Metastasis
stage (III–IV/I–II) | 2.55 (2.35–2.75) | 0.03 |
| Serum ephrin-B2
levels (high/low) | 3.71 (3.51–3.91) | 0.02 |
 | Table IV.Multivariate analysis of potential
independent prognostic factors of gastric cancer. |
Table IV.
Multivariate analysis of potential
independent prognostic factors of gastric cancer.
| Variable | Hazard ratio (95%
confluence intervals) | P-value |
|---|
| Tumor size | 2.06
(1.96–2.16) | 0.01 |
| Distant
metastasis | 3.24
(2.94–3.54) | 0.03 |
|
Tumor-Node-Metastasis stage | 2.46
(2.16–2.76) | 0.02 |
| Serum ephrin-B2
levels | 3.23
(3.03–3.53) | 0.01 |
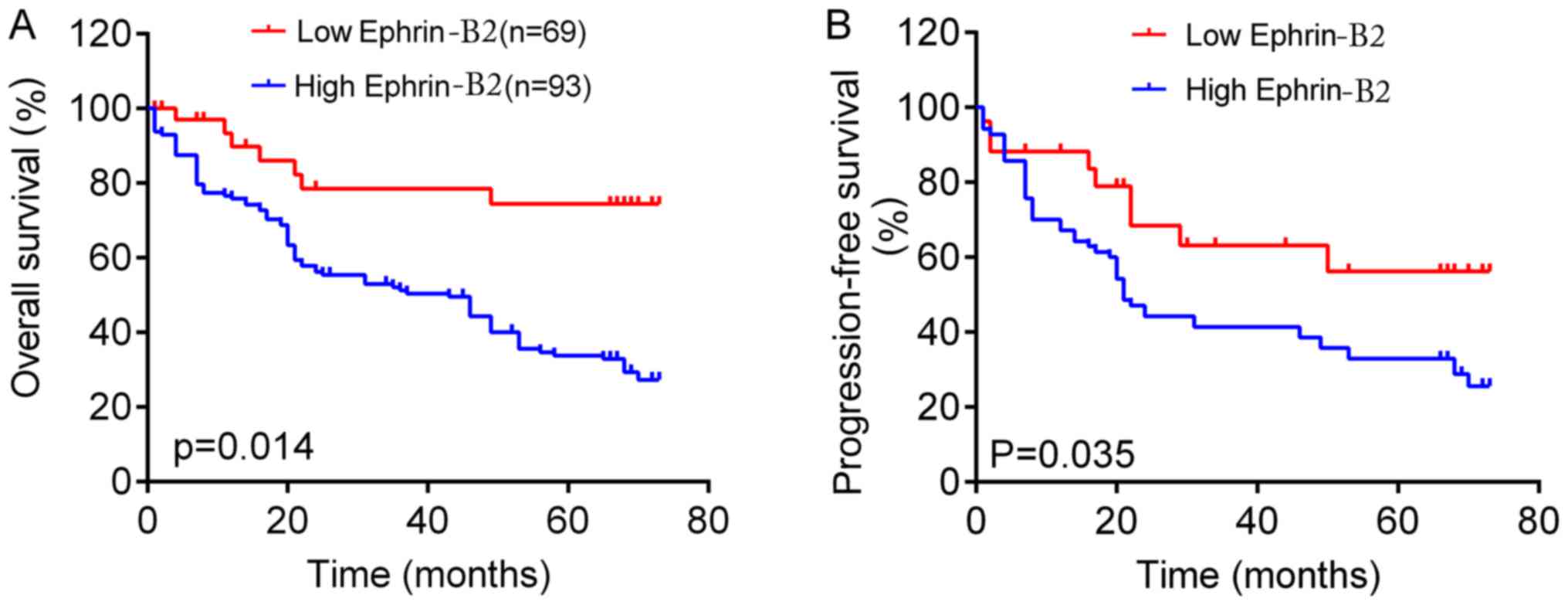
High serum ephrin-B2 levels predict
short overall and progression-free survival rates for patients with
gastric cancer
The association between serum ephrin-B2 levels and
survival time was analyzed in patients with gastric cancer. The
Kaplan-Meier survival curve revealed that patients with high
ephrin-B2 protein expression had shorter overall survival rates
than those with low ephrin-B2 protein expression (Fig. 3A). It was also revealed that patients
with low serum ephrin-B2 had longer progression-free survival times
than those with high ephrin-B2 levels (Fig. 3B). Thus, the results suggest that
ephrin-B2 served a role in the development of gastric cancer.
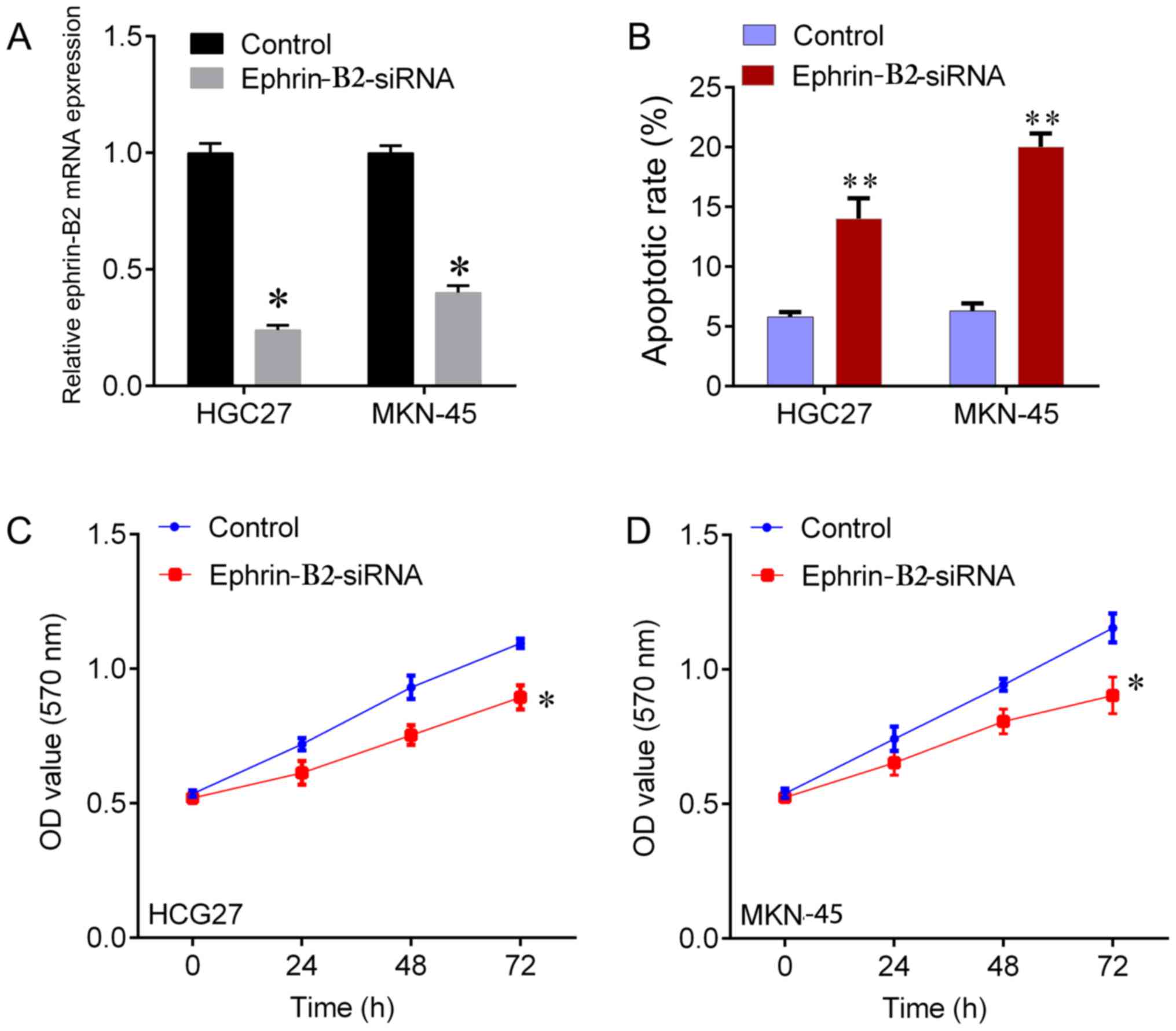
Knockdown of ephrin-B2 by siRNA
inhibits the proliferation and promotes apoptosis of gastric cancer
cells
To investigate whether ephrin-B2 affected the
proliferation of gastric cells, ephrin-B2 expression was knocked
down by siRNA in HGC27 and MKN-45 cells (Fig. 4A). The corresponding effect on
apoptosis was observed in a flow cytometry assay (the data was not
shown), which indicated a significant induction of apoptosis by
ephrin-B2-knockdown in HGC27 and MKN-45 cells compared with the NC
group (Fig. 4B). Ephrin-B2-knockdown
was indicated to significantly increase the viability of HGC27 and
MKN-45 cells (Fig. 4C and D).
Discussion
In the present study, ephrin-B2 was indicated to be
significantly upregulated in gastric cancer serum samples compared
with the adjacent healthy samples. Ephrin-B2 was revealed to be
associated with tumor size, metastasis and TNM stage. Furthermore,
patients with gastric cancer with high ephrin-B2 protein expression
were indicated to have shorter survival rates than those with low
ephrin-B2 protein expression. Ephrin-B2 has been demonstrated to be
upregulated in various types of cancer, including papillary thyroid
carcinoma, ovarian cancer and glioblastoma (11). Protein expression of ephrin-B2 in
arterial endothelium has been reported to be significantly
increased in tumor sections of the kidney, the bladder and the
prostate compared with non-tumor sections (12). An association has been identified
between the protein expression level of ephrin-B2 in glioma tissues
and the Karnofsky performance scale (KPS) score of patients with
glioma. Positive protein expression rates of ephrin-B2 in glioma
tissues were significantly higher in patients with low KPS scores
(4). A significant increase in the
mRNA expression levels of ephrin-B2 in ovarian cancer tissues with
clinical stage has been identified in ovarian cancers. The 24-month
survival rates of patients with high ephrin-B2 protein expression
were indicated to be poor (3). In
cervical cancer tissues, an increase of ephrin-B2 has been detected
with disease advancement, based on clinical stage, lymph node
metastasis, tumor size and poor patient prognoses (6). The aforementioned findings suggest that
ephrin-B2 may be a prognostic indicator in several types of cancer.
In the present study, it was reported that ephrin-B2 was
significantly upregulated in gastric cancer serum samples compared
with healthy samples. Taking into consideration the association of
serum ephrin-B2 with tumor size, metastasis and TNM stage of
gastric cancer, and the non-invasiveness of collecting serum
samples, we speculate that ephrin-B2 function is a promising
prognostic indicator for gastric cancer.
Ephrin-B2 regulates endothelial cell death (13), suggesting that ephrin-B2 is involved
in angiogenesis (14). A previous
study has reported that blocking ephrin-B2 with highly specific
antibodies inhibits angiogenesis, lymphangiogenesis and tumor
growth (8). Krusche et al
(7) revealed that upregulation of the
ephrin-B2 ligand in glioblastoma stem-like cells enabled
perivascular migration through homotypic forward signaling. In
human glioblastoma stem-like cell-derived orthotopic xenografts,
ephrin-B2-knockdown was indicated to block tumor initiation.
Furthermore, a combined treatment, involving an ephrin-B2-specific
antibody, was indicated to have an additive activity that inhibited
the migration and invasion of Kaposi sarcoma cells (15). An association between ephrin-B2 and
chemoresistance has also been reported (16). Ephrin-B2 has been identified as a
target gene of the gain-of-function mutant p53, which is
responsible for chemoresistance (17). The mutant p53 complex has been
reported to transcriptionally upregulate ephrin-B2 protein
expression in response to DNA damage. Ephrin-B2-silencing has been
demonstrated to restore chemosensitivity in mutant p53-harboring
tumors, involving the c-Jun N-terminal kinase signaling pathway and
the c-Src/extracellular signal-regulated kinase pathway (17). To evaluate the function of ephrin-B2
in gastric cancer, a loss-function assay was performed in two
gastric cancer cell lines. The results indicated that
ephrin-B2-knockdown significantly inhibited cell viability and
induced apoptosis. Thus, ephrin-B2 was demonstrated to function as
an oncogene in gastric cancer. However, the underlying mechanism by
which ephrin-B2 promotes gastric cancer cell proliferation requires
further examination.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
All data generated or analysed during this study are
included in this published article.
Authors' contributions
WJY and ZPC made substantial contributions to the
design of the study. YPL, ML and CXL analysed and interpreted the
patient data. SJM and QKL performed cell biological experiments.
WJY and SJM performed quantitative polymerase chain reaction and
immunohistochemical staining. All authors contributed to writing
the manuscript. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
The present study was approved by the Ethics
Committee of the Qianfoshan Hospital of Shandong University
(Shandong, China). Written informed consent was obtained from all
patients.
Patient consent for publication
All subjects participating in the present study
provided written informed consent for the publication of any
data.
Competing interests
The authors have no conflicts of interest to
declare.
References
|
1
|
Wang Y, Nakayama M, Pitulescu ME, Schmidt
TS, Bochenek ML, Sakakibara A, Adams S, Davy A, Deutsch U, Lüthi U,
et al: Ephrin-B2 controls VEGF-induced angiogenesis and
lymphangiogenesis. Nature. 465:483–486. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Yavrouian EJ, Sinha UK, Rice DH, Salam MT,
Gill PS and Masood R: The significance of EphB4 and EphrinB2
expression and survival in head and neck squamous cell carcinoma.
Arch Otolaryngol Head Neck Surg. 134:985–991. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Alam SM, Fujimoto J, Jahan I, Sato E and
Tamaya T: Coexpression of EphB4 and ephrinB2 in tumour advancement
of ovarian cancers. Br J Cancer. 98:845–851. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Tu Y, He S, Fu J, Li G, Xu R, Lu H and
Deng J: Expression of EphrinB2 and EphB4 in glioma tissues
correlated to the progression of glioma and the prognosis of
glioblastoma patients. Clin Transl Oncol. 14:214–220. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Zhu XL, Chen P, Guo H, Hou WJ, Shi Y,
Zhang T, Li Q and Zhang N: Relationships among differentiation
degree, contrast-enhanced ultrasound and the expression of
Ephb4/Ephrinb2 in primary hepatocarcinoma. Hepatogastroenterology.
59:1164–1167. 2012.PubMed/NCBI
|
|
6
|
Alam SM, Fujimoto J, Jahan I, Sato E and
Tamaya T: Coexpression of EphB4 and ephrinB2 in tumor advancement
of uterine cervical cancers. Gynecol Oncol. 114:84–88. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Krusche B, Ottone C, Clements MP,
Johnstone ER, Goetsch K, Lieven H, Mota SG, Singh P, Khadayate S,
Ashraf A, et al: EphrinB2 drives perivascular invasion and
proliferation of glioblastoma stem-like cells. Elife. 5:e148452016.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Abengozar MA, de Frutos S, Ferreiro S,
Soriano J, Perez-Martinez M, Olmeda D, Marenchino M, Canamero M,
Ortega S, Megias D, et al: Blocking ephrinB2 with highly specific
antibodies inhibits angiogenesis, lymphangiogenesis, and tumor
growth. Blood. 119:4565–4576. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Li P, Chen W, Wang Y, Fu X, Wen K, Qian J,
Huang C and Fu Z: Effects of ephrinB2 gene siRNA on the biological
behavior of human colorectal cancer cells. Oncol Rep. 33:758–766.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Sharma GK, Dhillon VK, Masood R and Maceri
DR: Overexpression of EphB4, EphrinB2, and epidermal growth factor
receptor in papillary thyroid carcinoma: A pilot study. Head Neck.
37:964–969. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Ozgur E, Heidenreich A, Dagtekin O,
Engelmann U and Bloch W: Distribution of EphB4 and EphrinB2 in
normal and malignant urogenital tissue. Urol Oncol. 29:78–84. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Salvucci O, Ohnuki H, Maric D, Hou X, Li
X, Yoon SO, Segarra M, Eberhart CG, Acker-Palmer A and Tosato G:
EphrinB2 controls vessel pruning through STAT1-JNK3 signalling. Nat
Commun. 6:65762015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Yamanda S, Ebihara S, Asada M, Okazaki T,
Niu K, Ebihara T, Koyanagi A, Yamaguchi N, Yagita H and Arai H:
Role of ephrinB2 in nonproductive angiogenesis induced by
Delta-like 4 blockade. Blood. 113:3631–3639. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Scehnet JS, Ley EJ, Krasnoperov V, Liu R,
Manchanda PK, Sjoberg E, Kostecke AP, Gupta S, Kumar SR and Gill
PS: The role of Ephs, Ephrins, and growth factors in Kaposi sarcoma
and implications of EphrinB2 blockade. Blood. 113:254–263. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Depner C, Zum BH, Bogurcu N, Cuesta AM,
Aburto MR, Seidel S, Finkelmeier F, Foss F, Hofmann J, Kaulich K,
et al: EphrinB2 repression through ZEB2 mediates tumour invasion
and anti-angiogenic resistance. Nat Commun. 7:123292016. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Alam SK, Yadav VK, Bajaj S, Datta A, Dutta
SK, Bhattacharyya M, Bhattacharya S, Debnath S, Roy S, Boardman LA,
et al: DNA damage-induced ephrin-B2 reverse signaling promotes
chemoresistance and drives EMT in colorectal carcinoma harboring
mutant p53. Cell Death Differ. 23:707–722. 2016. View Article : Google Scholar : PubMed/NCBI
|