Introduction
Prostate cancer was a common global malignancy among
men in 2012 (1,2). Ionizing radiation is used to treat
various types of cancer (3),
including localized prostate cancer (4). External beam radiotherapy (RT) has been
demonstrated to be a curative method for localized prostate cancer
(5) by the European Association of
Urology guidelines (6).
In addition to killing cancerous cells, ionizing
radiation damages healthy cells. The hematopoietic system is an
important system in radiosensitive patients who are affected by the
acute-phase effects of radiation (7).
Ionizing radiation results in vascular damage through exertion of a
cytotoxic effect on the vascular endothelium (8,9).
Furthermore, homeostatic responses are activated due to oxidative
damage that develops in the endothelium, including the activation
of platelets in irradiated tissues, which results in platelet
aggregation (9). The probability of
thrombosis differs based on the type of cancer, with this
probability varying between 6.9 and 11.4% in prostate cancer
(10). Platelets are known to serve
notable functions in the formation of thrombosis (11,12).
Since the mid-90s, developments in techniques used
in RT have allowed researchers to increase control over the tumor
while decreasing the side effects that result from the treatment.
These developments have additionally benefitted radiation therapy
used for prostate cancer (5). A
previous study performed with three-dimensional conformal RT
(3D-CRT) in prostate cancer revealed that toxicity may be decreased
if the target and the RT volume are compatible (6). Intensity-modulated RT (IMRT), a
technique used in 3D-CRT, is the most common method used to treat
prostate cancer. This method provides a better distribution of
conformal doses, thus increasing the targeted dose distribution and
decreasing the toxicity effect on normal tissues (5). A recently developed technique named
volumetric-modulated arc therapy (VMAT) delivers the radiation dose
more efficiently with a dynamic modulated arc (5,13);
however, there are a limited number of studies focusing on the
effect on platelet functions in acute and chronic phases of
radiation therapy applied using the VMAT technique. Studies
performed on the effects of RT on platelet functions usually focus
on specific markers, including platelet count and mean platelet
volume (MPV) (7,14–16). In
addition to platelet aggregation (17), the levels of P-selectin (18,19),
thrombospondin-1 (20) and platelet
factor 4 (PF 4) (21) are less
commonly used parameters as markers of platelet function.
Previous molecular studies have revealed an
association between serum/plasma levels and platelet levels and a
number of microRNAs (miRNA/miRs), which direct gene expression by
targeting mRNA at the post-transcriptional level (22,23). As
miRNA expression levels may be altered in various types of disease,
these tissue-specific molecules may be used as disease markers by
measuring their levels in circulation (24). It has been suggested that a number of
miRNAs may additionally be used as platelet activation markers
(25–27).
A number of miRNAs are associated with platelet
function, miR-223 and miR-126, which is expressed in platelets, are
among the most commonly studied molecules. One previous study
revealed that miR-223 and miR-126 serve a critical function in
regulating the expression of vascular cell adhesion protein 1 and
P2Y12 receptors in platelets (25).
It has been reported that the altered plasma levels of these two
miRNAs in cardiovascular diseases is associated with platelet
function (24,26,27). This
change may be plausible in post-treatment serum/plasma levels as a
result of miRNAs released from platelets due to the toxic effect of
RT. However, to the best of our knowledge, no studies have
investigated this type of change caused by treatment with RT,
particularly VMAT, on patients with prostate or other types of
cancer. In the light of the aforementioned information, the present
study was conducted to analyze the effect of VMAT on platelet
function in patients with prostate cancer using previously
determined markers, and to evaluate the association of plasma
levels of miR-223 and miR-126 with treatment.
Materials and methods
Case selection
A total of 25 male patients (mean age 66.57, range
55–76 years) diagnosed with histologically confirmed adenocarcinoma
of the prostate and treated with RT at the Istanbul University
Oncology Institute, Department of Radiation Oncology (Istanbul,
Turkey) between June 2013 and February 2014 were included in the
study. A total of 25 healthy male volunteers of similar age (mean
age 63.76, range 52–76 years), who did not receive any medication,
constituted the control group. Medical history, including
hematological disorders that affect platelet count or platelet
function, distant metastases or other malignant diseases, diabetes,
hypertension, chronic inflammatory diseases, infectious diseases
and autoimmune diseases, were designated as exclusion criteria for
the patients with prostate cancer. A known history of chronic,
inflammatory or malignant diseases and the use of antithrombotic
drugs were the exclusion criteria for the healthy control subjects.
Patients with prostate cancer received therapy with VMAT (Varian
Trilogy Rapid Arc Radiotherapy Device; Varian Medical Systems,
Inc., Palo Alto, CA, USA) with a daily dose of 180–200 cGy
fractions, 5 days a week for 30–37 days. The doses of the planned
target volumes for prostate and seminal vesicles were 66–74 and
50–54 Gy, respectively. The total postoperative dose administered
to the prostatic fossa was 66 Gy. Whole pelvis irradiation was not
given. All patients gave written informed consent, and the present
study was approved by the Istanbul University Cerrahpasa Medical
Faculty Ethics Committee (approval number: 28052/2012) and the
present study was performed in accordance with The Declaration of
Helsinki.
Sample collection
Venous blood was collected into tubes containing
3.8% sodium citrate and EDTA separately from each person the day
prior to RT (pre-RT group), the day RT was completed (post-RT day 0
group) and 40 days following the end of RT (post-RT day 40 group)
from patients with prostate cancer. The same volume of blood was
collected into the tubes from healthy control subjects.
Platelet count and MPV values
Platelet counts and MPV values were determined using
Cell-DYN C1600 (Abbott Pharmacuetical Co., Ltd., Lake Bluff, IL,
USA) blood count device using blood collected as
aforementioned.
Platelet aggregation
Platelet aggregation was determined according to the
method of Angiolillo et al (28) using a light transmittance aggregometer
(Chrono-log 500; Chrono Log Corp., Havertown, PA, USA). Venous
blood samples were collected in tubes containing 3.8% sodium
citrate. Platelet-rich plasma (PRP) was obtained as a supernatant
subsequent to centrifugation of citrated blood at 160 × g for 10
min at 22°C. The remaining blood was centrifuged at 2,500 × g for
10 min at 22°C to obtain platelet poor plasma (PPP). PPP and PRP
were incubated for 3 min at 37°C. Following incubation, 1 µM of
adenosine diphosphate (Chrono Log Corp.) was added into PRP to
induce the platelet aggregation. Platelet aggregation curves were
recorded for 3 min (29). Results
were expressed as the extent of maximal aggregation (% of maximal
amplitude).
Platelet activation
Venous blood samples containing 3.8% sodium citrate
were centrifuged for 15 min at 1,000 × g at 4°C, and the obtained
plasma samples were stored at −80°C until plasma thrombospondin-1,
P-selectin and PF 4 were measured using an ELISA. Subsequent to the
thawing of each plasma sample at 4°C, the measurements of plasma
thrombospondin-1 (EIAab Science Co. Ltd., Wuhan, Hubei, China; cat.
no. E0611 h), P-selectin (Wuhan EIAab Science Co. Ltd.; cat. no.
E0115 h) and PF 4 (Wuhan EIAab Science Co. Ltd.; cat. no. E0172 h)
were performed using commercial kits according to the
manufacturer's protocol.
miRNA detection with reverse
transcription-quantitative polymerase chain reaction (RT-qPCR)
EDTA-plasma samples stored at −80°C were thawed at
4°C prior to use and miRNA was immediately isolated by using the
Qiagen miRNeasy Serum/Plasma kit (Qiagen GmbH, Hilden, Germany)
following manufacturer's protocol. cDNA synthesis was synthesized
using ABM miRNA cDNA Synthesis kit with Poly(A) Polymerase Tailing
(ABM Inc., Vancouver, Canada) according to manufacturer's protocol.
Samples were stored at −80°C until further use. The expression
levels of miRNAs were determined using the Eco™ Real-Time PCR
system (Illumina, Inc., San Diego, CA, USA). The primers used were
as follows: Homo sapiens (hsa)-miR-223 forward,
5′-GTCCGCTGTCAGTTTGTCAAATAC-3′ and reverse,
5′-GTGCGTGTCGTGGAGTC-3′; hsa-miR-126 forward,
5′-GTCCGCTCGTACCGTGAGTAATA-3′ and reverse 5′-GTGCGTGTCGTGGAGTC-3′;
U6-2 forward 5′-GCCCCTGCGCAAGGATGAC-3′ and reverse,
5′-CCAGTGCAGGGTCCGAGGTA-3′. All miRNA primers were procured from
ABM Inc. RT-qPCR was conducted by using the EvaGreen miRNA qPCR
MasterMix (Applied Biological Materials, Inc., Richmond, BC,
Canada). The reaction conditions were as follows: 1 cycle for 10
min at 95°C, 40 cycles for (10 sec at 95°C, 15 sec at 60°C and 5
sec at 72°C). Melting curve analysis was performed with a
sensitivity of 0.1°C at temperatures between 55 and 90°C. PCR
analyses were performed by calculating the standard curve and the
number of duplicates. The Eco study software v5.0 (Illumina, Inc.)
was used to calculate quantification cycle (Cq) expression values
(30) for all genes. The U6-2 small
nuclear RNA was used as an internal control to detect
hsa-miR-223/miR-U6-2 and hsa-miR-126/miR-U6-2 ratios in the
plasma.
Statistical analysis
Data are presented as mean ± the standard deviation
(SD). Statistical analysis was performed using the Wilcoxon
signed-rank and Mann-Whitney U-tests. Correlation analysis using
Spearman's rank was used to study the association between markers.
P<0.05 was considered to indicate a statistically significant
difference. Variation coefficiency (% Cv) was used to calculate the
scattering due to individual variations. All calculations were
performed using GraphPad Prism version 5.00 for Windows (GraphPad
Software, Inc., La Jolla, CA, USA). Cv values were calculated as: %
Cv=(SD of measurement/measurement average) ×100.
Results
Patient data
Demographic data of patient and control groups are
presented in Table I. Platelet count,
MPV value and platelet activation markers are presented in Table II as the mean ± SD.
 | Table I.Demographic data of patient and
control groups. |
Table I.
Demographic data of patient and
control groups.
| Variables | Patients | Control | P-value |
|---|
| Age, years |
66.57±6.65a |
63.76±8.49a | 0.386 |
| Histology |
|
|
|
|
Adenocarcinoma, n (%) | 25 (100) | NA |
|
| Tumor stage |
|
|
|
| T2c, n
(%) | 4
(16%) | NA |
|
| T3b, n
(%) | 12
(48%) | NA |
|
| T3c, n
(%) | 9
(36%) | NA |
|
| Total radiation
dose, cGy | 6,600-7,400 | NA |
|
| Fractions, n | 30–37 | NA |
|
| Family history |
|
|
|
| Yes, n
(%) | 3
(12%) | NA |
|
| No, n
(%) | 22 (88%) | NA |
|
 | Table II.Differences in the level of
activation markers of platelets in patient and control groups. |
Table II.
Differences in the level of
activation markers of platelets in patient and control groups.
| Patient group | Platelet counts,
103/mm3 | Mean platelet
volume, µm3 | Platelet
aggregation, % | Plasma P-selectin,
ng/ml | Plasma
thrombospondin-1, ng/ml | Plasma platelet
factor 4, ng/ml |
|---|
| Pre-RT | 228.80±43.39 |
8.81±0.81a | 72.78±19.05 | 1.65±0.39 | 1.57±0.99 | 3.52±1.73 |
| Post-RT day 0 |
208.90±37.45b |
8.34±0.79c | 75.10±22.57 | 1.71±0.38 | 1.84±0.99 | 3.68±1.69 |
| Post-RT day 40 |
232.80±44.79d |
8.19±0.81e | 71.31±22.46 | 1.62±0.36 | 1.84±0.95 | 3.80±1.59 |
| Control | 231.60±40.89 | 7.97±1.17 | 77.25±16.27 | 1.62±0.26 | 1.68±0.98 | 3.26±1.77 |
Platelet count and MPV value
The platelet count of the post-RT day 0 group was
significantly reduced vs. the pre-RT and the post-RT day 40 groups
(208.90±37.45 vs. 228.80±43.39 and 232.80±44.79; P=0.002 and
P=0.001, respectively). There were no significant differences
identified (P>0.05) between the platelet count of the post-RT
day 40 group and the pre-RT group, and all RT groups compared with
the control group. Platelet count values are presented as the mean
± SD in Table II.
The MPV values of the pre-RT group were identified
to be higher than the post-RT day 0 and post-RT day 40 groups
(8.81±0.81 vs. 8.34±0.79 and 8.19±0.81; P<0.001 and P<0.001,
respectively). No significant difference in MPV values was
identified between the post-RT day 0 and post-RT day 40 groups
(P>0.05). Furthermore, there were no significant differences
identified (P>0.05) in the control group compared with the
post-RT day 0 and post-RT day 40 groups. However, a significant
difference was identified between the pre-RT and control groups
(P<0.05). MPV values are presented as the mean ± SD in Table II.
Platelet activation markers
Platelet aggregation, plasma P-selectin, plasma
thrombospondin-1 and plasma PF 4 were measured as platelet
activation markers. There was no significant difference (P>0.05)
between RT and control groups and among RT groups when platelet
aggregation, plasma levels of P-selectin, thrombospondin-1 and PF 4
were measured (Table II). However,
high values of plasma thrombospondin-1 and PF 4 were observed in
the coefficient of variation calculations used to determine the
scattering due to personal variations (Cv 51.6 to 63.4 and 41.8 to
54.5%, respectively; data not shown).
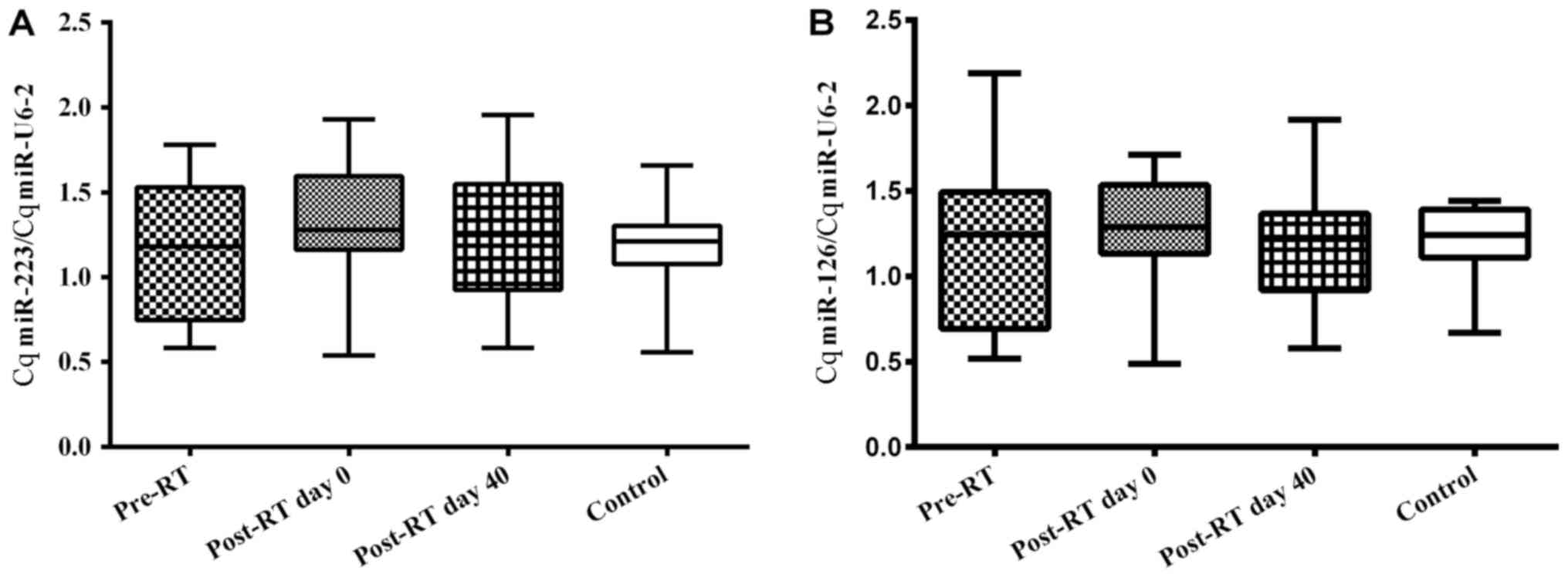
miR-223 and miR-126 expression
Plasma expression levels of miR-223 and miR-126 are
given as Cq miR-223/Cq miR-U6-2 and Cq miR-126/Cq miR-U6-2 ratios
(Fig. 1A and B, respectively). No
significant difference (P>0.05) was identified between the
plasma miR-223 expression levels of the RT-treated and control
groups and amongst the RT groups. Similarly, the miR-126 expression
levels did not vary significantly (P>0.05) between any of the
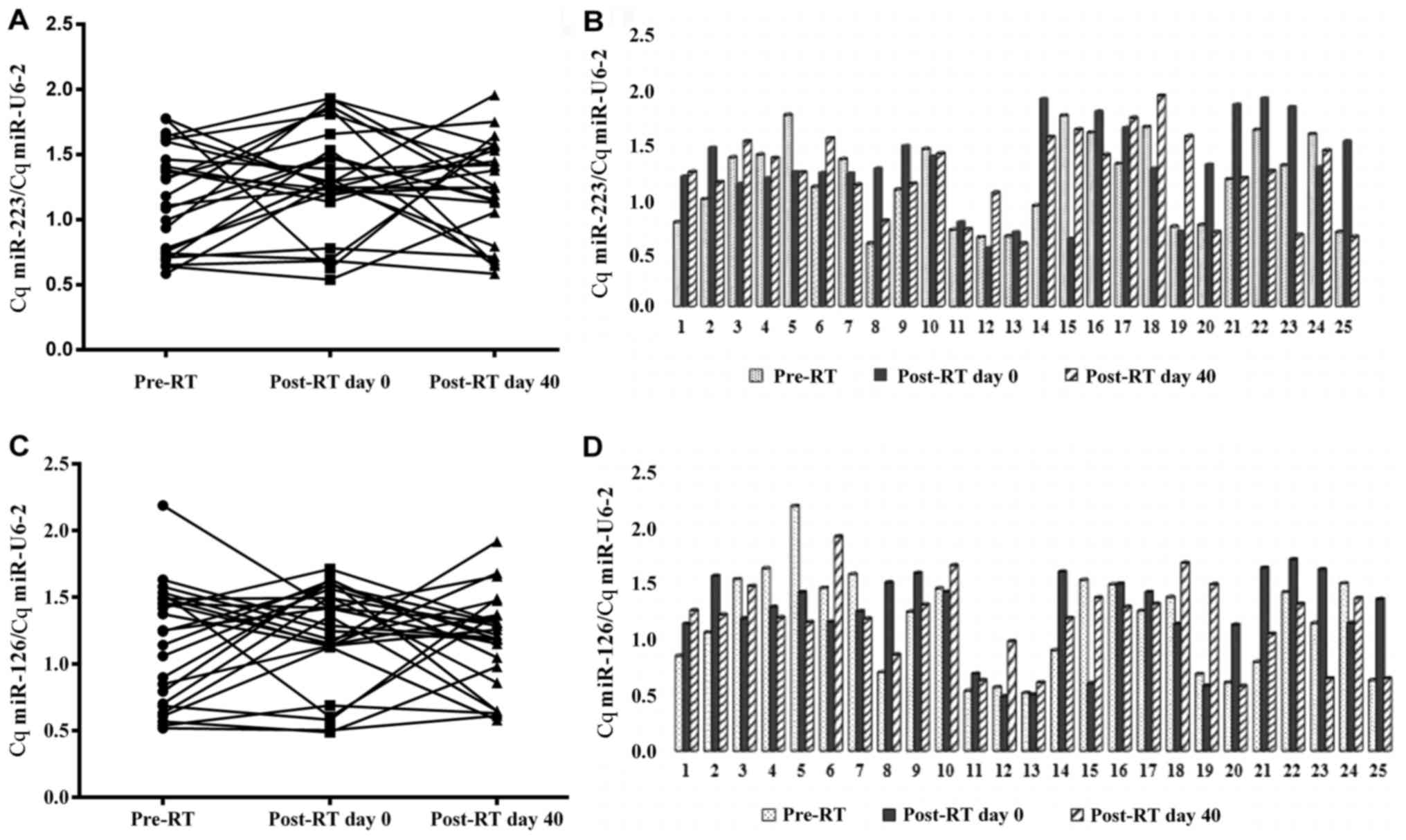
groups (Table III). Fig. 2A-D shows the individual changes in Cq
miR-223/Cq miR U6-2 and Cq miR-126/Cq miR-U6-2 ratios in RT-treated
groups. Ct miR-223/Ct miR U6-2 and Ct miR-126/Ct miR U6-2 ratios in
patient and control groups values are presented as the mean ± SD in
Table III.
 | Table III.Cq miR-223/Cq miR U6-2 ratios in
patient and control groups. |
Table III.
Cq miR-223/Cq miR U6-2 ratios in
patient and control groups.
| Patients (n=25 per
group) | Cq miR-223/Cq miR
U6-2 | Cq miR-126/Cq miR
U6-2 |
|---|
| Pre-RT | 1.17±0.40 | 1.15±0.44 |
| Post-RT day 0 | 1.31±0.41 | 1.22±0.38 |
| Post-RT day 40 | 1.23±0.38 | 1.17±0.36 |
| Control | 1.19±0.23 | 1.21±0.20 |
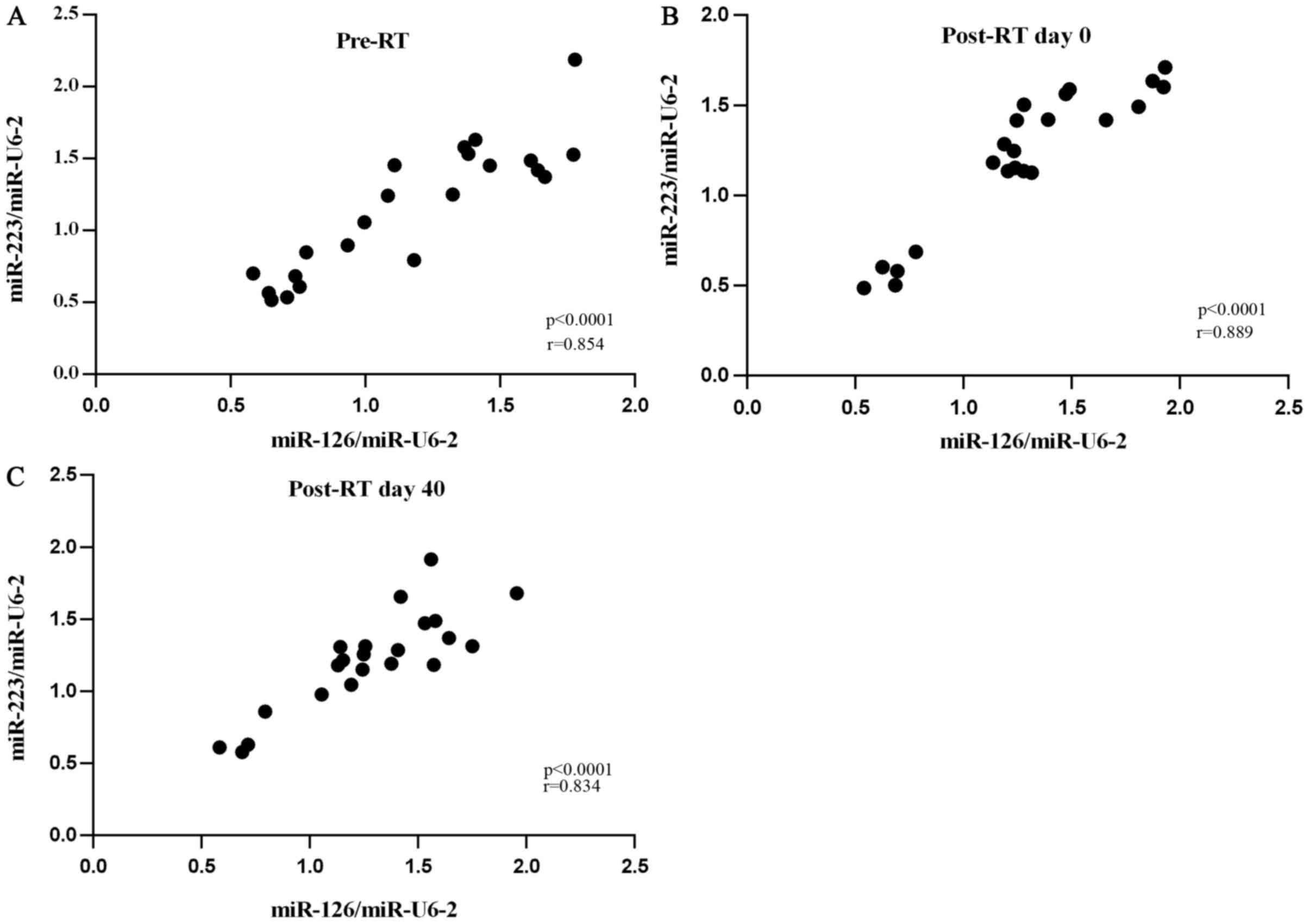
Correlation between miRNA expression
levels and platelet function markers
A positive correlation was identified between Cq
miR-223/Cq miR-U6-2 and Cq miR-126/Cq miR-U6-2 ratios in all
RT-treated groups. Correlation between Cq miR-223/Cq miR-U6-2 and
Cq miR-126/Cq miR-U6-2 ratios in all RT-treated groups are
presented in Fig. 3A-C.
Discussion
RT kills target cells by inducing DNA damage
(31). However, RT may also harm the
cells of healthy tissues and mitotically active bone marrow as
collateral damage (14). Various
studies have reported the existence of occlusions in the vascular
system in the chronic phase of irradiation and aggregation of
platelets in the vascular lumen during the 2 h following
irradiation (32–34). Platelet adhesion to vascular wall
structures due to radiation results in radiation-associated
thrombosis (32).
VMAT was developed as a novel form of arc therapy in
2007 to decrease the side effects of RT (35). There are a number of benefits of VMAT,
including increased target specificity and improved healthy tissue
avoidance, in addition to improving coverage of the target area by
adjusting the gantry rotation and speed, better dose distribution
and significantly reduced RT time (36).
Although previous studies have revealed that
targeted dose distributions are better managed by VMAT (36,37), to
the best of our knowledge there is no concrete data elucidating the
effect of VMAT on platelet markers. Therefore, the present study
aimed to measure the platelet parameters and activation markers
prior to and following RT to determine the effects of radiation
administered with VMAT on the platelet count, MPV value, platelet
aggregation, levels of P-selectin, thrombospondin-1 and PF 4
markers. Furthermore, their association with miR-226 and miR-123
expression levels in patients with prostate cancer was also
determined.
The results of the present study indicated the
presence of a significant difference in platelet count and MPV
value in pre- and post-RT with VMAT treatment groups of patients
with prostate cancer. According to the results, the platelet count
was decreased by 8.7% in the post-RT day 0 group in comparison with
the pre-RT group and increased by 10% in the post-RT day 40 group
compared with the post-RT day 0 group. Previous studies
investigating conventional RT and IMRT techniques have reported a
decrease in platelet count following treatment, thus corroborating
the results of the present study (7,15,38,39).
Furthermore, it has been demonstrated that sub-lethal radiation
doses may result in abnormal hemostasis characteristics and
coagulation biomarker values observed up to 21 days
post-irradiation (40). Therefore,
the post-RT decrease in platelet counts observed in the present
study may be due to the suppressive effect of radiation on the
hematopoietic system in the acute phase. The increase in the
platelet count in the post-RT day 40 group may be associated with
the removal of the suppressive effect of RT on bone marrow.
MPV is a marker of platelet function and activation.
An increase in platelet volume may result in increased platelet
activity. The changes in platelet volume may be used as a
diagnostic marker for the prevention and follow-up of the
platelet-associated diseases (41).
The formation and development of thrombi largely depends on
platelet activation, in which a change in platelet parameters may
affect the activation process (42).
The MPV is the average measurement of platelet size, and as the
platelet increases in size, the potential of thrombus formation
increases (41).
The present study revealed that MPV values were
increased by 10% in the pre-RT group in comparison with the control
group and decreased by 6% in the pre-RT day 0 group and 10% in the
pre-RT day 40 group comapred with the pre-RT group. These results
indicated that MPV values were increased owing to cancer and
decreased owing to RT. The MPV values were investigated in various
types of cancer, including prostate cancer (43,44);
however, to the best of our knowledge, there are no studies
investigating the change in MPV values associated with RT.
The platelet function markers investigated in the
present study include platelet aggregation, a marker of platelet
hyperactivity (45); P-selectin, an
adhesion molecule, which is present in the alpha granules of
platelets and released as a response to cellular activities
(46); thrombospondin-1, which is
released during hemostatic plaque formation and an active player in
thrombus formation (47), and PF 4,
which serves a function in thrombosis and is released by platelet
activation (48). When the data was
evaluated, no significant change was identified in the levels of
platelet aggregation, plasma P-selectin, thrombospondin-1 and PF 4
associated with RT between any of the RT groups.
Various studies reported a notable but unsignificant
increase in platelet aggregation levels in vitro and in
patients with metastatic prostate cancer compared with pre-RT
conditions (17,49). It has been suggested that
extracorporeal irradiation does not affect the platelet functions
that are already in systemic circulation, however, it may affect
bone marrow platelet stem cells (17).
Experimental molecular studies that determine the
effects of ionizing radiation on P-selectin levels revealed an
increase in the concentration of P-selectin in association with RT
(18,33,50).
However, Zekanowska et al (19) reported that the levels of soluble
P-selectin do not change following RT in patients with prostate
cancer. The results of the present study revealed that RT did not
affect the plasma P-selectin concentration in patients with
prostate cancer.
It has been revealed that the concentration of
thrombospondin-1 changes owing to an increasing dose of radiation
over time (51,52). Lewinski et al (20) reported that thrombospondin-1
concentration did not change in association with the radioactive
iodine treatment in patients with thyrotoxicosis.
Although contradictory results exist in clinical
studies investigating PF 4 levels in various types of cancer
(53–55), to the best of our knowledge no
clinical studies have focused on the changes in plasma PF 4 levels
associated with RT. A number of in vitro studies have
provided evidence of the protective effect of PF 4 against the
damaging effect of radiation (56,57).
At present, the basis for the lack of effect of VMAT
on platelet function remains unknown, although VMAT has a partial
effect on the platelet count and MPV value. A potential explanation
for this is that the highly developed dose distribution of VMAT may
result in platelet activation in the target area below detectable
levels in systemic circulation.
miRNAs are small molecules that target specific
genes and regulate their transcription (57). In previous years, it has been revealed
that miRNAs serve functions in various pathological and
physiological processes (57).
A previous study has reported alterations in miRNA
expression in different diseases, and that platelet miRNA levels
were biologically and clinically important markers of the
following: i) Platelet protein translation and expression; ii)
mature megakaryocyte miRNA level; iii) hematological disease and
platelet reactivity; and iv) basic mechanisms of
megakaryocyte/platelets (58).
Although the exact function of miRNAs in platelet function is not
clearly understood, it is thought that miRNAs have notable
functions in hemostasis and thrombosis (57).
Studies published in previous years provide evidence
that a number of miRNAs are specific to cells and tissues and are
differentially expressed (24,59,60).
It has been demonstrated that miRNAs can enter into systemic
circulation via certain mechanisms, including exosome secretion,
cell death and blebbing, and that these circulating miRNAs could be
used as potential markers for and in the follow-up of certain
diseases (24). So far, studies have
focused on the changes of miRNA expression levels associated with
cancer (1,61). However, to the best of our knowledge
there are no studies that have investigated the effects of
radiation on platelet miRNA expression. In experimental and total
body irradiation studies, it has been reported that the expression
rates of a number of miRNAs, including miR-126 and miR-223, change
in plasma and peripheral blood cells. miR-223 and miR-126 are
miRNAs identified in platelets. It has been revealed that miR-223
targets the P2Y12 receptor that is present on platelets and
inhibits it, thus suppressing platelet aggregation and activation
(58). miR-126 is downregulated
during megakaryocytopoiesis, indicating that it serves a function
in differentiation (62).
Various studies have reported that changes in the
plasma levels of miR-223 and miR-126 are associated with platelet
function in cardiovascular diseases (24,26,27).
Considering the effect of radiation on platelet functions from
these previous studies, a change in the expression levels of miRNAs
may be expected to exert an effect on platelet
activation-associated RT. In vitro and experimental studies
revealed that the expression levels of miR-223 and miR-126 changed
over time with increasing radiation dose (63,64).
Following RT, changes to RT-associated serum/plasma level changes
can be expected as platelets release miRNAs as RT damages the
cells. These changes may depend on the type and duration of
treatment and the dose distribution. Thus, the present study
additionally investigated the expression levels of miR-223 and
miR-126 in the plasma and the changes in the expression of these
miRNAs following VMAT application.
The results of the present study did not reveal a
significant change in miR-223 and miR-126 expression levels in
association with VMAT in patients with prostate cancer. The lack of
significant differences in miRNA expression between pre-RT and
control groups may indicate that these miRNAs are not
tumor-specific in serum/plasma. The VMAT technique achieved highly
conformal treatment plans and decreased the normal tissue toxicity
compared with other conventional RT techniques (65). Therefore, no significant changes in
the miRNA expression levels were observed. Dynamic changes to miRNA
expression levels in response to antitumor therapy may have
resulted in this non-significant difference (66). However, in the present study, there
existed a clear inter-individual variability to radiation response
in terms of the expression of miRNAs. Notably, this situation may
not be easily explained by previous observations. The cells of
patients with cancer respond differently to RT, even when they are
treated with the same curative dose. Furthermore, numerous
different factors, including genetic variations, environmental
stress, differences in nutritional state between patients and
controls, and exposure to genotoxic chemical agents may affect
individual responses to ionizing radiation (67–70).
Therefore, further studies are required to understand the molecular
events underlying the substantial inter-individual differences in
miRNA expression in response to radiation.
There are a number of limitations to the present
study, including its relatively small sample size, investigation of
a limited number of miRNAs rather than all miRNAs associated with
platelet activation and the focus on plasma samples. However, the
levels of miRNA and activation markers in platelets may provide
valuable data to evaluate the platelet function.
In summary, the present study demonstrated that
there were no significant changes in platelet aggregation, plasma
P-selectin, thrombospondin-1 and PF 4 levels and miR-226 and
miR-123 expression in plasma associated with RT. However, a
significant change in platelet count and MPV values were identified
to be associated with RT. The results of the present study
indicated that VMAT may not be a risk factor for platelet
activation in patients with prostate cancer, despite the fact that
it reduced the platelet count and MPV values, which may also be a
result of the suppressive effect of radiation on the hematopoietic
system.
Acknowledgements
Not applicable.
Funding
The present study was supported by the Research Fund
of the University of Istanbul (grant no. 376).
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
NB, İO and BA were responsible for study conception
and design. NB, İO, BA, OB, ST, FYA and MCA performed data analysis
and interpretation. FYA provided the patient specimens. All authors
approved the final version of this manuscript.
Ethics approval and consent to
participate
The present study was approved by the Ethics
Committee of the Cerrahpasa Medical Faculty of Istanbul University
(Grant no. 28052/2012), and informed consent to participate in the
study was obtained from all patients involved.
Patient consent for publication
No identifying patient information is included in
the published manuscript. Participants provided their consent for
the publication of the data.
Competing interests
All the authors declare that they have no competing
interests.
References
|
1
|
Sun X, Liu Z, Yang Z, Xiao L, Wang F, He
Y, Su P, Wang J and Jing B: Association of microRNA-126 expression
with clinicopathological features and the risk of biochemical
recurrence in prostate cancer patients undergoing radical
prostatectomy. Diagn Pathol. 8:2082013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Ferlay J, Soerjomataram I, Dikshit R, Eser
S, Mathers C, Rebelo M, Parkin DM, Forman D and Bray F: Cancer
incidence and mortality worldwide: Sources, methods and major
patterns in GLOBOCAN 2012. Int J Cancer. 136:E359–E386. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
He J, Hua J, Ding N, Xu S, Sun R, Zhou G,
Xie X and Wang J: Modulation of microRNAs by ionizing radiation in
human gastric cancer. Oncol Rep. 32:787–793. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Li M, Li GF, Hou XY, Gao H, Xu YG and Zhao
T: A dosimetric comparison between conventional fractionated and
hypofractionated image-guided radiation therapies for localized
prostate cancer. Chin Med J (Engl). 129:1447–1454. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Hayden AJ, Catton C and Pickles T:
Radiation therapy in prostate cancer: A risk-adapted strategy. Curr
Oncol. 17 Suppl 2:S18–S24. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Mottet N, Bellmunt J, Bolla M, Briers E,
Cumberbatch MG, De Santis M, Fossati N, Gross T, Henry AM, Joniau
S, et al: EAU-ESTRO-SIOG guidelines on prostate cancer Part 1:
Screening, diagnosis, and local treatment with curative intent. Eur
Urol. 71:618–629. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Yang FE, Vaida F, Ignacio L, Houghton A,
Nauityal J, Halpern H, Sutton H and Vijayakumar S: Analysis of
weekly complete blood counts in patients receiving standard
fractionated partial body radiation therapy. Int J Radiat Oncol
Biol Phys. 33:6171995. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Verheij M, Dewit LG, Boomgaard MN,
Brinkman HJ and van Mourik JA: Ionizing radiation enhances platelet
adhesion to the extracellular matrix of human endothelial cells by
an increase in the release of von Willebrand factor. Radiat Res.
137:202–207. 1994. View
Article : Google Scholar : PubMed/NCBI
|
|
9
|
Hallahan DE, Chen AY, Teng M and Cmelak
AJ: Drug-radiation interactions in tumor blood vessels. Oncology
(Williston Park). 13 10 Suppl 5:S71–S77. 1999.
|
|
10
|
Sørensen HT, Mellemkjaer L, Olsen JH and
Baron JA: Prognosis of cancers associated with venous
thromboembolism. N Engl J Med. 343:1846–1850. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kroll MH, Hellums JD, McIntire LV, Schafer
AI and Moake JL: Platelets and shear stress. Blood. 88:1525–1541.
1996.PubMed/NCBI
|
|
12
|
Ni H and Freedman J: Platelets in
hemostasis and thrombosis: Role of integrins and their ligands.
Transfus Apher Sci. 28:257–264. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Arcangeli S and Greco C: Hypofractionated
radiotherapy for organ-confined prostate cancer: Is less more? Nat
Rev Urol. 13:400–408. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zachariah B, Jacob SS, Gwede C, Cantor A,
Patil J, Casey L and Zachariah AB: Effect of fractionated regional
external beam radiotherapy on peripheral blood cell count. Int J
Radiat Oncol Biol Phys. 50:465–472. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Lundgren MSFS, Cavalcanti MSM and Sampaio
DA: Weekly monitoring of the effects of conventional external beam
radiation therapy on patients with head and neck, chest, and pelvis
cancer by means of blood cells count. Radiol Bras. 41:29–33. 2008.
View Article : Google Scholar
|
|
16
|
Ampil FL, Burton GV and Li BD: ‘Routine’
weekly blood counts during breast irradiation for early stage
cancer: Are they really necessary? Breast J. 7:450–452. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kalovidouris AE and Papayannis AG: Effect
of ionizing radiation on platelet function in vitro. Acta Radiol
Oncol. 20:333–336. 1981. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Mihaescu A, Thornberg C, Santén S,
Mattsson S, Jeppsson B and Thorlacius H: Radiation-induced
platelet-endothelial cell interactions are mediated by P-selectin
and P-selectin glycoprotein ligand-1 in the colonic
microcirculation. Surgery. 151:606–611. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zekanowska E, Rystok-Grabska D, Wisniewski
T, Ziolkowska E, Slomka A, Giemza-Kucharska P and Rosc D: C0141
Radiotherapy and platelets activation in patients with prostate
cancer. Thromb Res. 130:S163–S164. 2012. View Article : Google Scholar
|
|
20
|
Lewinski A, Brona A, Lewandowski KC,
Jedrzejuk D, Bohdanowicz-Pawlak A, Skowronska-Jozwiak E,
Bienkiewicz M and Milewicz A: Effects of radioiodine administration
on serum concentrations of matrix metalloproteinases, adiponectin
and thrombospondin-1. Thyroid Res. 6:92013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Lambert MP, Xiao L, Nguyen Y, Kowalska MA
and Poncz M: The role of platelet factor 4 in radiation-induced
thrombocytopenia. Int J Radiat Oncol Biol Phys. 80:1533–1540. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Willeit P, Zampetaki A, Dudek K, Kaudewitz
D, King A, Kirkby NS, Crosby-Nwaobi R, Prokopi M, Drozdov I,
Langley SR, et al: Circulating microRNAs as novel biomarkers for
trombosit activation. Circ Res. 112:595–600. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Nagalla S, Shaw C, Kong X, Kondkar AA,
Edelstein LC, Ma L, Chen J, McKnight GS, López JA, Yang L, et al:
Platelet microRNA-mRNA coexpression profiles correlate with
platelet reactivity. Blood. 117:5189–5197. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
de Boer HC, van Solingen C, Prins J, Duijs
JM, Huisman MV, Rabelink TJ and van Zonneveld AJ: Aspirin treatment
hampers the use of plasma microRNA-126 as a biomarker for the
progression of vascular disease. Eur Heart J. 34:3451–3457. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Tian HS, Zhou QG and Shao F: Relationship
between arterial atheromatous plaque morphology and
platelet-associated miR-126 and miR-223 expressions. Asian Pac J
Trop Med. 8:309–314. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Yu XY, Chen JY, Zheng ZW, Wu H, Li LW,
Zhang ZW, Chen ZH, Lin QX, Han YL and Zhong SL: Plasma miR-126 as a
potential marker predicting major adverse cardiac events in dual
antiplatelet-treated patients after percutaneous coronary
intervention. EuroIntervention. 9:546–554. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Zhang YY, Zhou X, Ji WJ, Shi R, Lu RY, Li
JL, Yang GH, Luo T, Zhang JQ, Zhao JH, et al: Decreased circulating
microRNA-223 level predicts high on-treatment platelet reactivity
in patients with troponin-negative non-ST elevation acute coronary
syndrome. J Thromb Thrombolysis. 38:65–72. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Angiolillo DJ, Fernandez-Ortiz A, Bernardo
E, Ramírez C, Cavallari U, Trabetti E, Sabaté M, Jimenez-Quevedo P,
Hernández R, Moreno R, et al: Lack of association between the P2Y12
receptor gene polymorphism and platelet response to clopidogrel in
patients with coronary artery disease. Thromb Res. 116:491–497.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Ruggeri ZM: New insights into the
mechanisms of platelet adhesion and aggregation. Semin Hematol.
31:229–239. 1994.PubMed/NCBI
|
|
30
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta DeltaC (T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Burdak-Rothkamm S and Prise KM: New
molecular targets in radiotherapy: DNA damage signalling and repair
in targeted and non-targeted cells. Eur J Pharmacol. 625:151–155.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Mouthon MA, Vereycken-Holler V, Van der
Meeren A and Gaugler MH: Irradiation increases the interactions of
platelets with the endothelium in vivo: Analysis by intravital
microscopy. Radiat Res. 160:593–599. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Hallahan DE, Staba-Hogan MJ, Virudachalam
S and Kolchinsky A: X-ray-induced P-selectin localization to the
lumen of tumor blood vessels. Cancer Res. 58:5216–5220.
1998.PubMed/NCBI
|
|
34
|
Hallahan DE and Virudachalam S:
Accumulation of P-selectin in the lumen of irradiated blood
vessels. Radiat Re. 152:6–13. 1999. View Article : Google Scholar
|
|
35
|
Otto K: Volumetric modulated arc therapy:
IMRT in a single gantry arc. Med Phys. 35:310–317. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Cozzi L, Dinshaw KA, Shrivastava SK,
Mahantshetty U, Engineer R, Deshpande DD, Jamema SV, Vanetti E,
Clivio A, Nicolini G and Fogliata A: A treatment planning study
comparing volumetric arc modulation with RapidArc and fixed field
IMRT for cervix uteri radiotherapy. Radiother Oncol. 89:180–191.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Nabavizadeh N, Simeonova AO, Waller JG,
Romer JL, Monaco DL, Elliott DA, Tanyi JA, Fuss M, Thomas CR Jr and
Holland JM: Volumetric-modulated arc radiotherapy for pancreatic
malignancies: Dosimetric comparison with sliding-window
intensity-modulated radiotherapy and 3-dimensional conformal
radiotherapy. Med Dosim. 39:256–260. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Blank KR, Cascardi MA and Kao GD: The
utility of serial complete blood count monitoring in patients
receiving radiation therapy for localized prostate cancer. Int J
Radiat Oncol Biol Phys. 44:317–321. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Pinkawa M, Djukic V, Klotz J, Petz D,
Piroth MD, Holy R and Eble MJ: Hematologic changes during prostate
cancer radiation therapy are dependent on the treatment volume.
Future Oncol. 10:835–843. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Kennedy AR, Maity A and Sanzari JK: A
review of radiation-induced coagulopathy and new findings to
support potential prevention strategies and treatments. Radiat Res.
186:121–140. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Mutlu H, Berk V, Karaca H, Erden A, Aslan
T and Akca Z: Treatment regimen with bevacizumab decreases mean
platelet volume in patients with metastatic colon cancer. Clin Appl
Thromb Hemost. 18:546–548. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Khode V, Sindhur J, Kanbur K, Ruikar K and
Nallulwar S: Mean platelet volume and other platelet volume indices
in patients with stable coronary artery disease and acute
myocardial infarction: A case control study. J Cardiovasc Dis Res.
3:272–275. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Riedl J, Kaider A, Reitter EM, Marosi C,
Jäger U, Schwarzinger I, Zielinski C, Pabinger I and Ay C:
Association of mean platelet volume with risk of venous
thromboembolism and mortality in patients with cancer. Results from
the vienna cancer and thrombosis study (CATS). Thromb Haemost.
111:670–678. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Inagaki N, Kibata K, Tamaki T, Shimizu T
and Nomura S: Prognostic impact of the mean platelet
volume/platelet count ratio in terms of survival in advanced
non-small cell lung cancer. Lung Cancer. 83:97–101. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Cooke NM, Egan K, McFadden S, Grogan L,
Breathnach OS, O'Leary J, Hennessy BT and Kenny D: Increased
platelet reactivity in patients with late-stage metastatic cancer.
Cancer Med. 2:564–570. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Johnson RC, Mayadas TN, Frenette PS,
Mebius RE, Subramaniam M, Lacasce A, Hynes RO and Wagner DD: Blood
cell dynamics in P-selectin-deficient mice. Blood. 86:1106–1114.
1995.PubMed/NCBI
|
|
47
|
Esemuede N, Lee T, Pierre-Paul D, Sumpio
BE and Gahtan V: The role of thrombospondin-1 in human disease. J
Surg Res. 122:135–142. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Sachais BS, Higazi AA, Cines DB, Poncz M
and Kowalska MA: Interactions of platelet factor 4 with the vessel
wall. Semin Thromb Hemost. 30:351–358. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Weiss K, Palumbo B, Palumbo I, Palumbo R,
Granegger S, Hiltunen J and Sinzinger H: Platelet function after
single [153Sm]EDTMP therapy in prostate cancer. Q J Nucl Med Mol
Imaging. 50:330–333. 2006.PubMed/NCBI
|
|
50
|
El Kaffas A, Smith K, Pradhan P, Machtaler
S, Wang H, von Eyben R, Willmann JK and Hristov D: Molecular
contrast-enhanced ultrasound imaging of radiation-induced
P-Selectin expression in healthy mice colon. Int J Radiat Oncol
Biol Phys. 97:581–585. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Rofstad EK, Henriksen K, Galappathi K and
Mathiesen B: Antiangiogenic treatment with thrombospondin-1
enhances primary tumor radiation response and prevents growth of
dormant pulmonary micrometastases after curative radiation therapy
in human melanoma xenografts. Cancer Res. 63:4055–4061.
2003.PubMed/NCBI
|
|
52
|
Rofstad EK, Galappathi K and Mathiesen B:
Thrombospondin-1 treatment prevents growth of dormant lung
micrometastases after surgical resection and curative radiation
therapy of the primary tumor in human melanoma xenografts. Int J
Radiat Oncol Biol Phys. 58:493–499. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Wiesner T, Bugl S, Mayer F, Hartmann JT
and Kopp HG: Differential changes in platelet VEGF, Tsp, CXCL12,
and CXCL4 in patients with metastatic cancer. Clin Exp Metastasis.
27:141–149. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Poruk KE, Firpo MA, Huerter LM, Scaife CL,
Emerson LL, Boucher KM, Jones KA and Mulvihill SJ: Serum platelet
factor 4 is an independent predictor of survival and venous
thromboembolism in patients with pancreatic adenocarcinoma. Cancer
Epidemiol Biomarkers Prev. 19:2605–2610. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Lam YW, Mobley JA, Evans JE, Carmody JF
and Ho SM: Mass profiling-directed isolation and identification of
a stage-specific serologic protein biomarker of advanced prostate
cancer. Proteomics. 5:2927–2938. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Chen JJ, Gao Y, Tian Q, Liang YM and Yang
L: Platelet factor 4 protects bone marrow mesenchymal stem cells
from acute radiation injury. Br J Radiol. 87:201401842014.
View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Gatsiou A, Boeckel JN, Randriamboavonjy V
and Stellos K: MicroRNAs in platelet biogenesis and function:
Implications in vascular homeostasis and inflammation. Curr Vasc
Pharmacol. 10:524–531. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Edelstein LC, McKenzie SE, Shaw C,
Holinstat MA, Kunapuli SP and Bray PF: MicroRNAs in platelet
production and activation. J Thromb Haemost. 11 Suppl 1:S340–S350.
2013. View Article : Google Scholar
|
|
59
|
Corsten MF, Dennert R, Jochems S,
Kuznetsova T, Devaux Y, Hofstra L, Wagner DR, Staessen JA, Heymans
S and Schroen B: Circulating MicroRNA-208b and MicroRNA-499 reflect
myocardial damage in cardiovascular disease. Circ Cardiovasc Genet.
3:499–506. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Wang GK, Zhu JQ, Zhang JT, Li Q, Li Y, He
J, Qin YW and Jing Q: Circulating microRNA: A novel potential
biomarker for early diagnosis of acute myocardial infarction in
humans. Eur Heart J. 31:659–666. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Li X, Zhang Y, Zhang H, Liu X, Gong T, Li
M, Sun L, Ji G, Shi Y, Han Z, et al: miRNA-223 promotes gastric
cancer invasion and metastasis by targeting tumor suppressor
EPB41L3. Mol Cancer Res. 9:824–833. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Garzon R, Pichiorri F, Palumbo T, Iuliano
R, Cimmino A, Aqeilan R, Volinia S, Bhatt D, Alder H, Marcucci G,
et al: MicroRNA fingerprints during human megakaryocytopoiesis.
Proc Natl Acad Sci USA. 103:pp. 5078–5083. 2006; View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Templin T, Paul S, Amundson SA, Young EF,
Barker CA, Wolden SL and Smilenov LB: Radiation-induced micro-RNA
expression changes in peripheral blood cells of radiotherapy
patients. Int J Radiat Oncol Biol Phys. 80:549–557. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Cui W, Ma J, Wang Y and Biswal S: Plasma
miRNA as biomarkers for assessment of total-body radiation exposure
dosimetry. PLoS One. 6:e229882011. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Jin L, Wang R, Jiang S, Yue J, Liu T, Dou
X, Zhu K, Feng R, Xu X, Chen D and Yin Y: Dosimetric and clinical
toxicity comparison of critical organ preservation with
three-dimensional conformal radiotherapy, intensity-modulated
radiotherapy, and RapidArc for the treatment of locally advanced
cancer of the pancreatic head. Curr Oncol. 23:e41–e48. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Ponomaryova AA, Morozkin ES, Rykova EY,
Zaporozhchenko IA, Skvortsova TE, Dobrodeev АY, Zavyalov AA,
Tuzikov SA, Vlassov VV, Cherdyntseva NV, et al: Dynamic changes in
circulating miRNA levels in response to antitumor therapy of lung
cancer. Exp Lung Res. 42:95–102. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Smirnov DA, Brady L, Halasa K, Morley M,
Solomon S and Cheung VG: Genetic variation in radiation-induced
cell death. Genome Res. 22:332–339. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Foray N, Bourguignon M and Hamada N:
Individual response to ionizing radiation. Mutat Res. 770:369–386.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Mei N, Imada H, Nomoto S, Kunugita N and
Norimura T: Individual variation and age dependency in the
radiosensitivity of peripheral blood T-lymphocytes from normal
donors. J Radiat Res. 37:235–245. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Barber JB, West CM, Kiltie AE, Roberts SA
and Scott D: Detection of individual differences in
radiation-induced apoptosis of peripheral blood lymphocytes in
normal individuals, ataxia telangiectasia homozygotes and
heterozygotes, and breast cancer patients after radiotherapy.
Radiat Res. 153:570–578. 2000. View Article : Google Scholar : PubMed/NCBI
|