Introduction
Lung cancer, one of the most aggressive tumors with
a high incidence rate, is the leading cause of cancer-associated
mortality both worldwide and in China (1). Nearly 85% of all lung cancer cases are
classified as non-small cell lung cancer (NSCLC) and its 5-year
survival rate does not often reach 15% (2). In clinic, according to the histological
characteristics, NSCLC is divided into 3 types, including
adenocarcinoma, squamous cell carcinoma, and large cell lung cancer
(3). At present, >70% of NSCLC
patients exhibiting metastases to the regional lymph nodes or to
distant sites, are at advanced stages at diagnosis (4). Therefore, it is urgent to investigate
the targets and explore the mechanisms of NSCLC for early diagnosis
and comprehensive treatment.
The metastatic process of cancer can be categorized
into 3 stages, namely, tumor cell invasion into surrounding tissue,
intravasation into blood or lymphatic vessels, and extravasation
into a new host environment (5–7).
Epithelial-to-mesenchymal transition (EMT), the ability of
epithelial cells to convert from a polarized morphology to a loose
mesenchymal phenotype, plays an important role in the process of
cancer metastases (8). Accumulating
evidence shows that EMT occurs through numerous cellular and
molecular alterations, including a gain of Vimentin and Fibronectin
expression, and loss of E-cadherin and Keratin at the cell membrane
(9,10). Moreover, EMT allows the transient
cells to have increased cell mobility, tumor invasion and
metastatic dissemination and to be more resistant to cytotoxic
drugs (11). Secreted growth factors,
notably transforming growth factor-β (TGF-β) and epidermal growth
factor (EGF), can lead to the activation of EMT of cancer cells
through the activation of downstream pathways, such as PI3K/AKT,
ERK/MAPK and Smad pathways.
Leptin, encoded by the ob gene on chromosome
7, is a 17 kDa protein composed of 167 amino acids, which primarily
regulates appetite and weight. Furthermore, leptin is also
considered to play a role in the pathogenesis of several cancer
types, including breast and thyroid cancer, hepatocellular
carcinoma, colorectal and pancreatic cancer (12–20). With
regards to lung cancer, studies have mainly focused on the role of
leptin in the carcinogenesis and proliferation of lung cancer
cells. However, few studies have investigated the association
between leptin and the metastasis of lung cancer. A previous study
demonstrated that leptin is differentially expressed in lung cancer
tissues that do not occur or metastasize, and the expression of
leptin is increased in lung cancers with bone metastasis (21), indicating that the leptin pathway may
be involved in the metastasis of lung cancer. However, the effect
and mechanisms of leptin on metastasis of lung cancer have not yet
been fully elucidated.
The present study, therefore, examined the effect of
leptin on EMT, a crucial stage in the metastatic process, and
explored the underlying molecular mechanisms in A549 lung cancer
cells. Our results demonstrated that leptin promoted EMT and
regulated the expressions of EMT-related markers and transcription
factors through the activation of the ERK signaling pathway.
Furthermore, leptin promoted EMT-induced migration and invasion in
A549 lung cancer cells.
Materials and methods
Reagents and antibodies
Leptin was obtained from the leptin protein
(Sigma-Aldrich, St. Louis, MO, USA). Antibodies against human
E-cadherin, Vimentin, Keratin, Fibronectin, ZEB-1 and Twist were
purchased from the Cell Signaling Technology, Inc. (Danvers, MA,
USA). Antibodies for p-ERK, total-ERK, p-AKT, total-AKT and β-actin
were obtained from EMD Millipore (Billerica, MA, USA). Matrigel (BD
Biosciences, Franklin Lakes, NJ, USA), 24-well Transwell inserts,
6-well and 96-well plates (both from Corning Corp, Corning, NY,
USA) were used.
Cell culture and grouping
A549 cell line [obtained from the American Type
Culture Collection (ATCC) Rockville, MD, USA] was maintained in
RPMI-1640 medium. The medium was supplemented with 10% fetal calf
serum (FCS), 100 U/ml of penicillin and 100 U/ml of streptomycin
and all the cells were kept at 37°C in a humidified atmosphere
containing 5% CO2 and 95% air. Cells were divided into
the following groups: i) Control group (n=6), cells were treated
under normal conditions; ii) leptin group (n=6), cells were treated
with leptin (100 ng/ml) for the indicated time period; and iii)
TGF-β1 group (n=6), cells were treated with TGF-β1 (5 ng/ml) for
the indicated time period.
Western blotting
After protein quantitation using a Coomassie
brilliant blue assay, 50 µg protein was boiled in loading buffer,
resolved on 10% SDS-polyacrylamide gels, electrotransferred to
nitrocellulose membranes, and probed with antibodies against
E-cadherin (1:2,500), Keratin (1:1,000), Fibronectin (1:1,000),
Vimentin (1:1,000), ZEB-1 (1:200), Twist (1:200), p-ERK (Thr
202/Tyr 204, 1:500), p-AKT (Ser 473, 1:200), total-ERK (1:500),
total-AKT (1:500) and β-actin (1:1,000) overnight. The secondary
antibody (anti-mouse or anti-rabbit IgG peroxidase conjugated;
1:1,000) was incubated with the membranes and the relative content
of target proteins was detected by chemiluminescence.
Wound healing assay
For the wound-healing assay, cells were plated into
6-well plates and grown under normal conditions. When cells grew
into a monolayer, a plastic pipette tip was drawn across the center
of the plate to produce a clean 1-mm-wide wound area after the
cells reached confluency. Then, cells were cultured in medium with
1% FCS for 24 h. The cell movement into the wound area was examined
by a phase-contrast microscope.
Matrigel invasion assay
The invasion assay was carried out using a Transwell
plate (Corning Costar Corp.) precoated with Matrigel (BD
Biosciences). Briefly, the Transwell plate was placed on a 24-well
plate, and 400 µl culture medium (10% FCS) was added to the lower
chamber as a chemoattractant. Then, 200 µl cells (1×105)
suspended in culture medium with 1% FCS were added to the upper
chamber. Cells in the invasion chambers were incubated in a
humidified incubator for 24 h. The cells that traversed the
membrane pore and spread to the lower surface of the filters were
stained with 5% Giemsa solution for visualization. Cell invasion
viability was expressed as a percentage of the value of the control
group.
Colony formation assay
A soft agar colony formation assay was performed to
assess the anchorage-independent growth ability of cells as a
characteristic of in vitro tumorigenicity. Briefly, A549
cells were detached and plated on 0.3% agarose with a 0.5% agarose
underlay in 6-wells (1.0×104 cells/well). The number of
foci (>100 µm) were counted after 17 days. Each experiment was
performed in triplicate.
Statistical analysis
Each experiment was repeated at least 3 times. Bands
from western blotting were quantified by Quantity One software
(Bio-Rad Laboratories, Inc., Hercules, CA, USA). Relative protein
levels were calculated by referring them to the amount of actin.
Data are expressed as the mean ± standard error of the mean. The
difference between means was analyzed by one-way analysis of
variance, followed by a Dunnett's test for multiple comparisons.
All statistical analyses were performed using SPSS 11.0 software
(SPSS, Inc., Chicago, IL, USA). A statistical difference was
accepted as significant if P<0.05.
Results
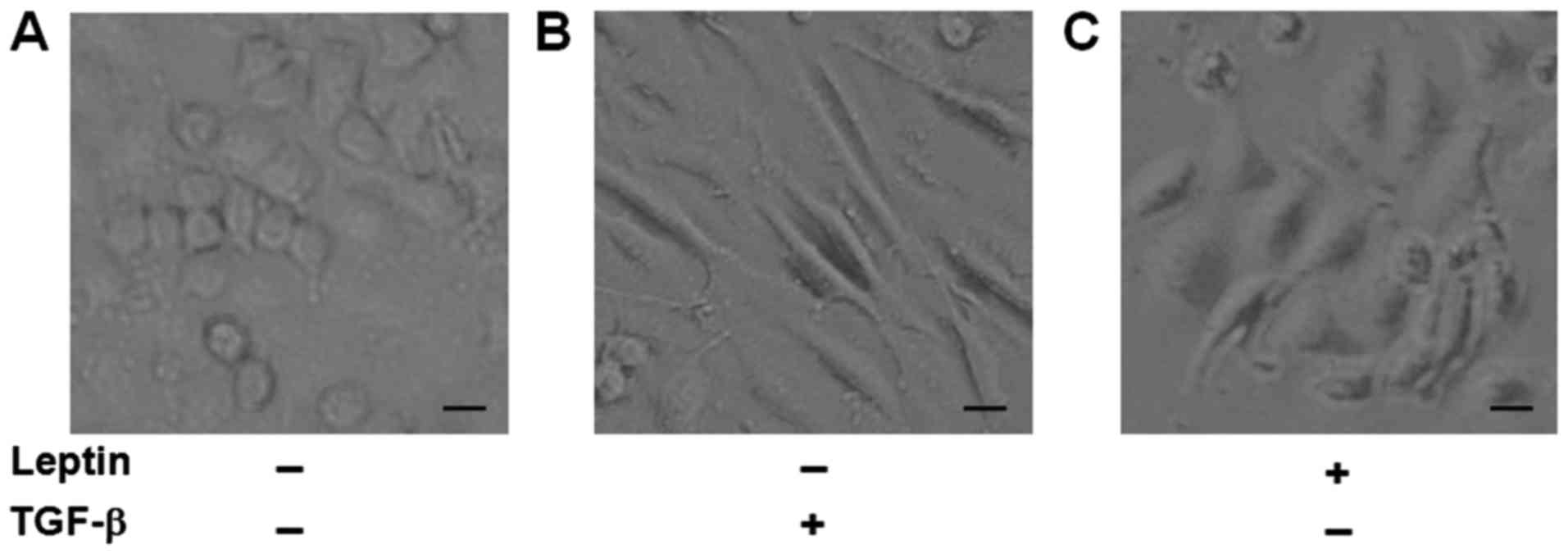
Leptin induces morphological changes
of EMT in A549 cells
It has been reported that A549 cells undergo EMT
phenotypic changes when cells are exposed to TGF-β1 (22,23). As a
result, we used TGF-β1 as a positive control and to see if leptin
also induced EMT in A549 cells. After exposure to leptin or TGF-β1
for 48 h, we found that A549 cells in the two groups all changed to
a mesenchymal phenotype, as revealed by an elongated and
disseminated appearance (Fig. 1).
These findings indicated that leptin induced EMT in A549 cells.
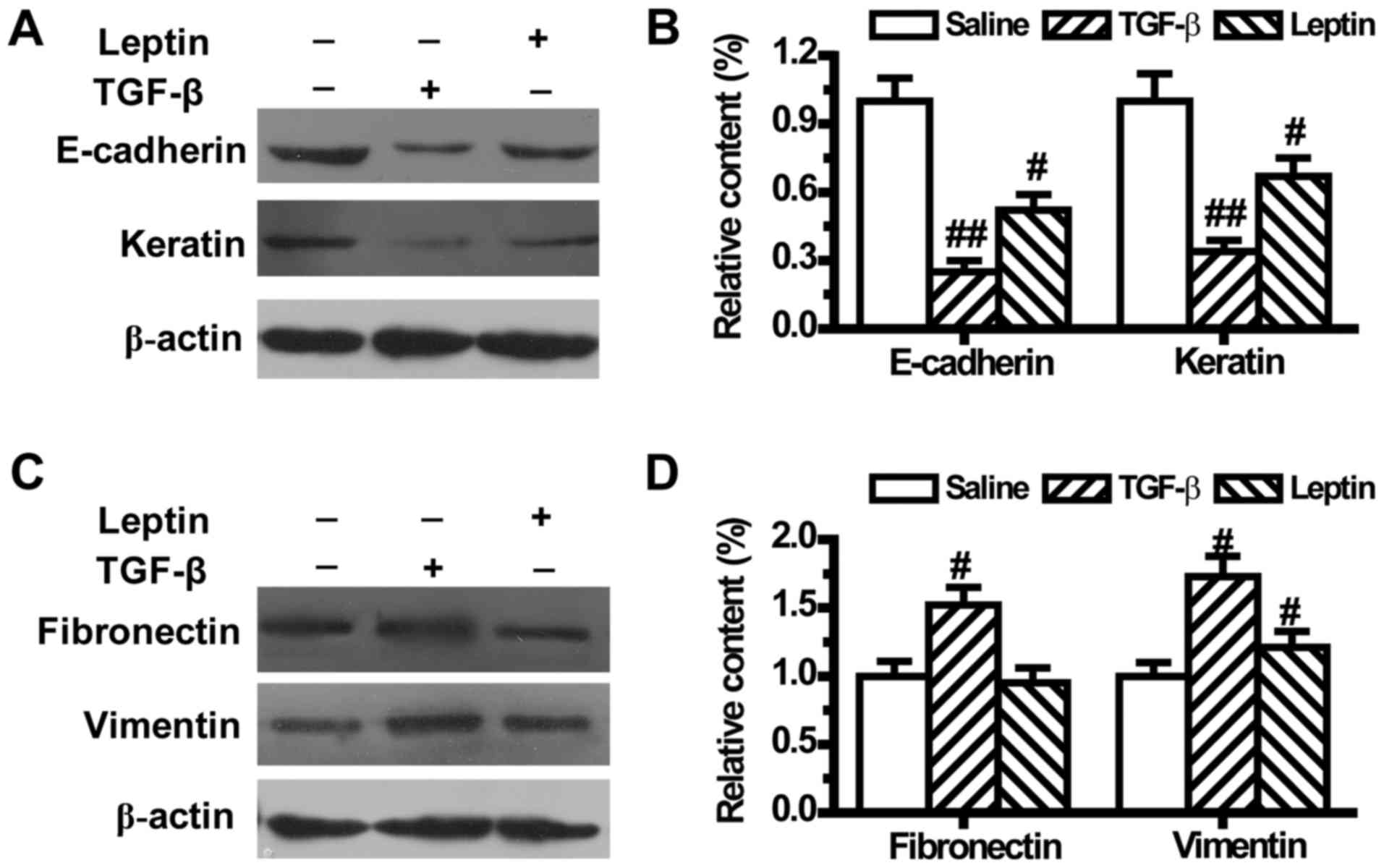
Leptin regulates the expression levels
of EMT markers in A549 cells
To further confirm that leptin could lead to EMT,
the expression levels of EMT-related markers were measured by
western blotting. As shown in Fig. 2,
compared with the control group, both leptin and TGF-β1
downregulated the expression levels of epithelial phenotype markers
E-cadherin and Keratin (Fig. 2A and
B). TGF-β1 also upregulated the expression levels of
mesenchymal phenotype markers Fibronectin and Vimentin (Fig. 2C and D). Meanwhile, leptin did not
change the expression of Fibronectin, but significantly increased
Vimentin expression in A549 cells (Fig.
2C and D).
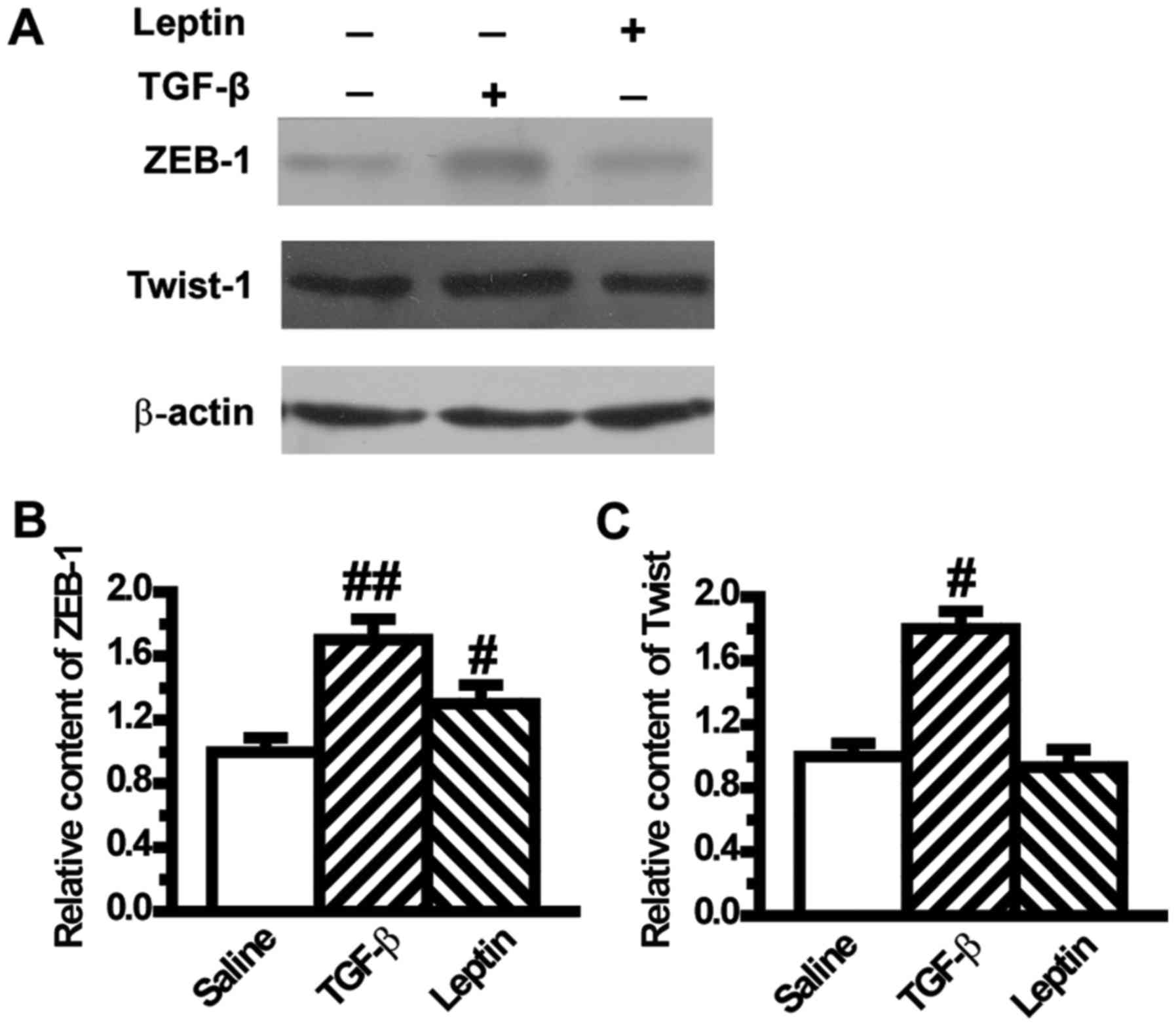
Leptin increases the expression levels
of EMT-induced transcription factors in A549 cells
To confirm the mesenchymal phenotype, we assessed
the expression levels of transcription factors of EMT such as ZEB-1
and Twist. Results from western blotting showed that ZEB-1 was
significantly increased in the leptin and TGF-β1 groups compared
with the control group (Fig. 3A and
B). The level of Twist was also upregulated by TGF-β1 (Fig. 3A and C), but its level was not
influenced by leptin (Fig. 3A and
C).
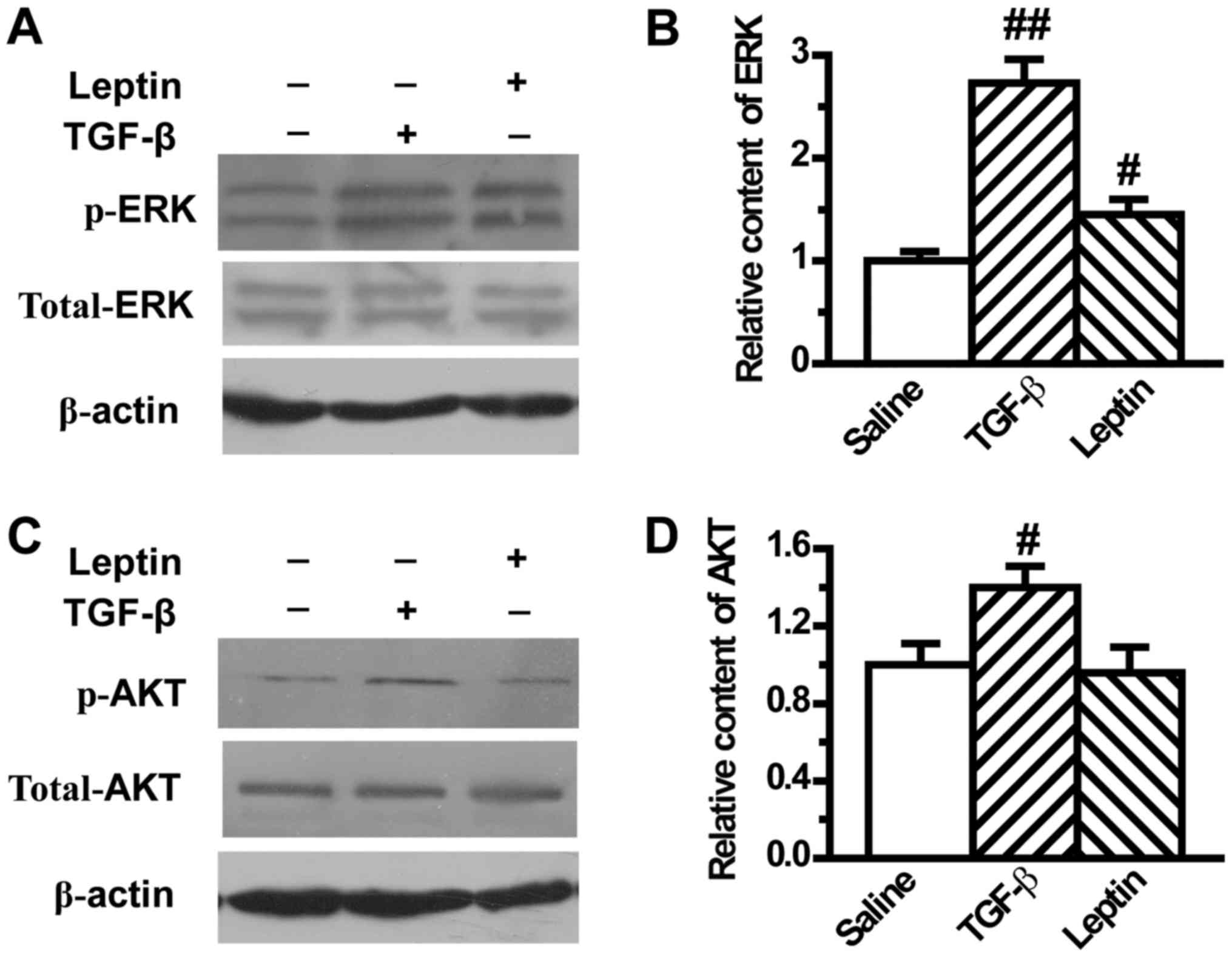
Leptin activates EMT-related ERK
signaling pathway in A549 cells
It has been reported that ERK and AKT pathways play
an important role in modulating EMT. Therefore, the present study
next investigated the effect of leptin on ERK and AKT activation in
A549 cells. Our results showed that TGF-β1 induced the activation
of ERK and AKT, in accordance with results from previous reports,
which was demonstrated by the upregulation of phosphorylated ERK
and AKT (Fig. 4). In addition, leptin
significantly increased the expression of phosphorylated ERK, which
indicated activation of the ERK pathway (Fig. 4A and B). However, leptin did not
change the expression of phosphorylated AKT (Fig. 4C and D).
Leptin enhances EMT-induced tumor
phenotypes in A549 cells
Previous studies have shown that tumor cells with
EMT phenotype are more motile resulting in increased migration,
invasion and metastatic abilities (24). Although leptin induced EMT in A549
cells, whether leptin could regulate EMT-induced aggressive
behaviors in A549 cells remained unclear. To investigate if leptin
influences cell migration, the present study compared the migratory
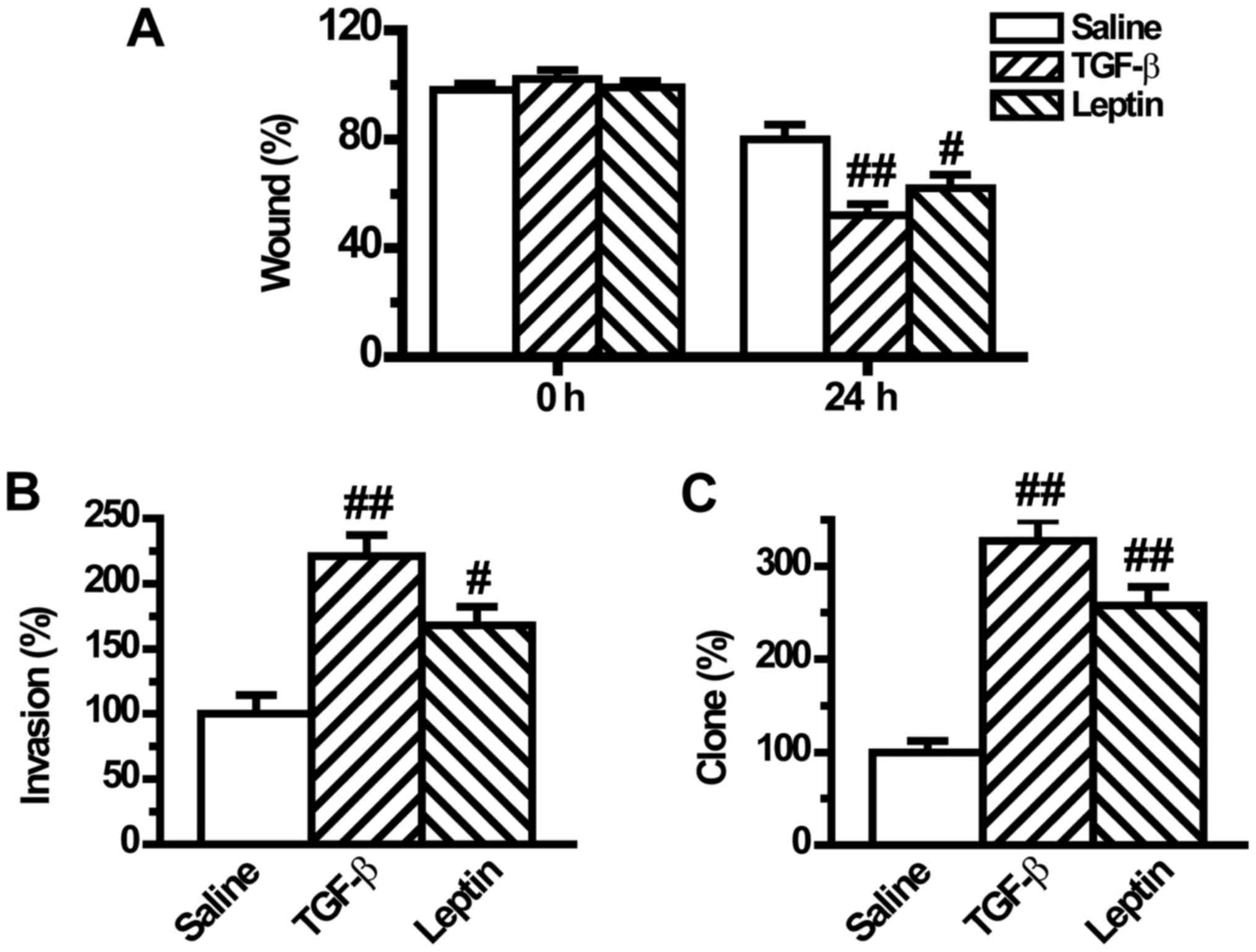
rate of the tumor cells in a wound-healing assay. We found that
leptin significantly increased cell migration after wound induction
for 24 h (Fig. 5A). Similarly, the
cell invasion potential, which was measured in a Matrigel-coated
Transwell assay, was significantly raised by leptin pretreatment
(Fig. 5B). Moreover, the tumorigenic
phenotype was also augmented by leptin as documented by the
clonogenic growth assay (Fig. 5C).
The regulatory mode of leptin was the same as that of TGF-β1
(Fig. 5).
Discussion
In the present study, we showed the important role
of leptin in the development of EMT in A549 cells. First, we found
that leptin could induce A549 cells to change from an epithelial
phenotype to a mesenchymal phenotype. Second, leptin downregulated
the expression levels of epithelial phenotype markers E-cadherin
and Keratin and upregulated the expression of mesenchymal phenotype
marker Vimentin in A549 cells. Third, leptin increased the
expression of EMT-induced transcription factor ZEB-1 and the
activation of ERK signaling pathway in A549 cells. Fourth, leptin
enhanced EMT-induced cell migration, invasion and tumorigenic
phenotypes in A549 cells. Together, these findings suggested that
leptin is important in inducing EMT in A549 lung cells.
Leptin is mainly synthesized and secreted in white
fat (25), but many other tissues
also secrete a small amount of leptin. Leptin mainly functions in
combination with its functional receptor Ob-R. The main role of
leptin is to regulate metabolism by inducing anorexigenic factors
and suppressing orexigenic neuropeptides (26). Moreover, recent reports suggest that
leptin is involved in the regulation of immune function,
reproduction, hematopoiesis, and blood pressure.
Studies have shown that leptin and its receptors are
highly expressed in lung cancer tissues (27), and the leptin receptor gene
polymorphism determines the susceptibility of NSCLC (28), indicating that the leptin pathway is
related to the occurrence of lung cancer. In A549 cells cultured
in vitro, leptin promotes the growth of lung cancer cells by
inhibiting apoptosis (29), and
promotes the immune escape of tumor cells by inducing
proinflammatory cytokines and inhibiting apoptosis in cells
(30). Leptin can promote the
proliferation of A549 cells through blocking endoplasmic reticulum
stress-mediated apoptosis and this blocking is mediated by the
p-Perk and ATF6 pathway through blocking the activation of CHOP
(29,31). Moreover, downregulation of leptin
inhibits growth and induces apoptosis of lung cancer cells via the
Notch and JAK/STAT3 signaling pathways, which suggests that leptin
knockdown could become a new approach for the prevention of lung
cancer progression (32). However,
few studies have been reported to investigate the effect of leptin
on invasion and migration and the underlying mechanisms in lung
cancer cells. In the present study, we attempted to observe the
involvement of a previously unknown mechanism, EMT, in the
leptin-induced invasion and migration in A549 cells.
EMT is characterized by a switch from an epithelial
phenotype of polarized cells expressing epithelial markers to a
mesenchymal phenotype of cells with downregulation of epithelial
markers and upregulation of mesenchymal markers that lack polarity
and are motile. As EMT is a critical step in the development of
metastases, it is an attractive target for anti-cancer therapeutic
strategies (33). In the present
study, we first investigated the effect of leptin on EMT in A549
cells. Results showed that leptin induced EMT in a similar way to
TGF-β1 in A549 cells, as proven by the morphological change
(Fig. 1). The effect of leptin was
further confirmed by the decrease in the expression levels of the
epithelial phenotype markers E-cadherin and Keratin and the
increase of the mesenchymal phenotype marker Vimentin (Fig. 2). Transcriptional factors such as
ZEB-1 and Twist play a central role in EMT, which have been
reported to serve as mesenchymal markers (34,35). Then,
we next examined whether leptin affected EMT-induced transcription
factors. Our results demonstrated that expression of ZEB-1 was
increased by leptin (Fig. 3).
To mechanistically understand how leptin affected
EMT, we investigated two main molecules, namely ERK and AKT, which
are involved in the MAPK and PI3K/AKT pathways, respectively, and
are key for EMT initiation and maintenance (36,37). TGF-β
can activate Akt and ERK signaling pathways that are activated by
tyrosine kinase receptors or other receptor types in response to
their respective ligands (37).
TGF-β-induced activation of Akt and ERK pathways has been linked to
the characteristics of EMT, such as cytoskeletal organization, cell
growth, survival, migration, and invasion (38). Our results showed that TGF-β1 greatly
increased the activation of ERK and AKT pathways (Fig. 4). Leptin activated the ERK signaling
pathway and did not influence the AKT pathway in A549 cells. Theses
results suggested that the activation of ERK was probably
responsible for the induction of leptin-induced EMT.
EMT is able to increase cell adhesion, migration,
invasion, tumorigenesis and drug resistance in cancer cells
(24,39,40).
Therefore, we next investigated whether leptin could increase
EMT-induced malignant phenotypes in A549 cells. Results from the
wound-healing assay and Transwell assay indicated that leptin
increased the movement and the migratory and invasive abilities of
A549 cells (Fig. 5A and B).
Furthermore, the clonogenic growth assay revealed that leptin also
promoted tumorigenesis in lung cancer cells (Fig. 6C). These data
suggested that leptin increased lung cancer cell invasion and
metastasis by inducing EMT.
Consistent with the results of the present study, a
previous report regarding the mechanism of leptin in the promotion
of EMT leading to metastasis in A549 lung cancer cells was studied
(21). In the previous study, the
incidence of EMT in A549 cells was examined by real-time PCR and
immunofluorescence staining. Furthermore, it was found that in
patient samples leptin was present at higher levels in samples
associated with diagnosis of lung cancer bone metastases tissue
than lung cancer tissue. The results also indicated that leptin
promoted the metastasis of A549 cell lines by inducing EMT in a
TGF-β-dependent manner, which is another mechanism accounting for
the effect of leptin on EMT.
Numerous studies in vivo have found that
leptin is involved in tumorigenesis and the progression of lung
cancer. The serum leptin level has been shown to increase in
patients with NSCLC. When stratifying the groups according to the
lung cancer histological subtypes, mean serum leptin level is
significantly higher in patients with adenocarcinoma compared with
squamous cell subtype (41). A
meta-analysis indicated that a subgroup analysis in high-study
quality group found a weak association between serum leptin
concentration and lung cancer in the Chinese population (42). Another study showed that the
expression levels of leptin and leptin receptor in primary
pulmonary adenocarcinoma tissues were associated with their
expression levels in bone metastatic tissue (43). Further results indicated that the
serum leptin level had prognostic indications in patients with
advanced lung adenocarcinoma during cisplatin/pemetrexed
chemotherapy (44). However, these
studies were inconsistent. Results found that patients present
significantly lower serum leptin levels compared with control group
(45,46) and that the serum leptin level has no
prognostic indications in advanced lung cancer patients (46). The reasons for the divergence may be
interpreted that the selected population and the inclusion criteria
were different. Based on the results obtained, the role of leptin
in vivo has not been fully elucidated and further studies
are required in order to clarify this.
In summary, the present study provides evidence that
leptin induces EMT via activating the ERK pathway. Furthermore,
leptin also increases EMT-induced invasion, metastasis and
tumorigenic characteristics in lung cancer cells. At present,
although further investigations are need to study the effects of
leptin in vivo, our findings suggest that leptin may be a
promising target for lung cancer treatment.
Acknowledgements
Not applicable.
Funding
This study was supported by the Natural Science
Foundation of Shandong Province (grant nos. ZR2015HQ028 and
ZR2015CQ015).
Availability of data and materials
The datasets analyzed during the current study are
available from the corresponding author on reasonable request.
Authors' contributions
MX and FLC conceived and designed the study, and
performed the cell culture and western blot analysis. NYL and XG
collected the samples and performed the statistical analysis. XJS
and XLJ performed the wound healing, Matrigel invasion and colony
formation assays.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
EMT
|
epithelial-to-mesenchymal
transition
|
|
NSCLC
|
non-small cell lung cancer
|
|
FCS
|
fetal calf serum
|
|
TGF-β
|
transforming growth factor-β
|
|
EGF
|
epidermal growth factor
|
References
|
1
|
Jemal A, Siegel R, Xu J and Ward E: Cancer
statistics, 2010. CA Cancer J Clin. 60:277–300. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Liu D, Yang Y and Zhao S: Autophagy
facilitates the EGFR-TKI acquired resistance of non-small-cell lung
cancer cells. J Formos Med Assoc. 113:141–142. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Petersen I and Petersen S: Towards a
genetic-based classification of human lung cancer. Anal Cell
Pathol. 22:111–121. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ma J, Ward EM, Smith R and Jemal A: Annual
number of lung cancer deaths potentially avertable by screening in
the united states. Cancer. 119:1381–1385. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Jones SE: Metastatic breast cancer: The
treatment challenge. Clin Breast Cancer. 8:224–233. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Bacac M and Stamenkovic I: Metastatic
cancer cell. Annu Rev Pathol. 3:221–247. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Chaffer CL and Weinberg RA: A perspective
on cancer cell metastasis. Science. 331:1559–1564. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Thiery JP: Epithelial-mesenchymal
transitions in tumour progression. Nat Rev Cancer. 2:442–454. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kalluri R and Weinberg RA: The basics of
epithelial-mesenchymal transition. J Clin Invest. 119:1420–1428.
2009. View
Article : Google Scholar : PubMed/NCBI
|
|
10
|
Tomaskovic-Crook E, Thompson EW and Thiery
JP: Epithelial to mesenchymal transition and breast cancer. Breast
Cancer Res. 11:2132009. View
Article : Google Scholar : PubMed/NCBI
|
|
11
|
Yilmaz M, Christofori G and Lehembre F:
Distinct mechanisms of tumor invasion and metastasis. Trends Mol
Med. 13:535–541. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Jarde T, Caldefie-Chezet F, Damez M,
Mishellany F, Penault-Llorca F, Guillot J and Vasson MP: Leptin and
leptin receptor involvement in cancer development: A study on human
primary breast carcinoma. Oncol Rep. 19:905–911. 2008.PubMed/NCBI
|
|
13
|
Liu CL, Chang YC, Cheng SP, Chern SR, Yang
TL, Lee JJ, Guo IC and Chen CP: The roles of serum leptin
concentration and polymorphism in leptin receptor gene at codon 109
in breast cancer. Oncology. 72:75–81. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Otvos L Jr and Surmacz E: Targeting the
leptin receptor: A potential new mode of treatment for breast
cancer. Expert Rev Anticancer Ther. 11:1147–1150. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Akinci M, Kosova F, Cetin B, Aslan S, Ari
Z and Cetin A: Leptin levels in thyroid cancer. Asian J Surg.
32:216–223. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Uddin S, Bu R, Ahmed M, Hussain AR, Ajarim
D, Al-Dayel F, Bavi P and Al-kuraya KS: Leptin receptor expression
and its association with pi3k/akt signaling pathway in diffuse
large b-cell lymphoma. Leuk Lymphoma. 51:1305–1314. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kitade M, Yoshiji H, Kojima H, Ikenaka Y,
Noguchi R, Kaji K, Yoshii J, Yanase K, Namisaki T, Asada K, et al:
Leptin-mediated neovascularization is a prerequisite for
progression of nonalcoholic steatohepatitis in rats. Hepatology.
44:983–991. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Ribatti D, Belloni AS, Nico B, Di Comite
M, Crivellato E and Vacca A: Leptin-leptin receptor are involved in
angiogenesis in human hepatocellular carcinoma. Peptides.
29:1596–1602. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Chia VM, Newcomb PA, Lampe JW, White E,
Mandelson MT, McTiernan A and Potter JD: Leptin concentrations,
leptin receptor polymorphisms and colorectal adenoma risk. Cancer
Epidemiol Biomark Prev. 16:2697–2703. 2007. View Article : Google Scholar
|
|
20
|
Stolzenberg-Solomon RZ, Newton CC,
Silverman DT, Pollak M, Nogueira LM, Weinstein SJ, Albanes D,
Mannisto S and Jacobs EJ: Circulating leptin and risk of pancreatic
cancer: A pooled analysis from 3 cohorts. Am J Epidemiol.
182:187–197. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Feng H, Liu Q, Zhang N, Zheng L, Sang M,
Feng J, Zhang J, Wu X and Shan B: Leptin promotes metastasis by
inducing an epithelial-mesenchymal transition in a549 lung cancer
cells. Oncol Res. 21:165–171. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Kim JH, Jang YS, Eom KS, Hwang YI, Kang
HR, Jang SH, Kim CH, Park YB, Lee MG, Hyun IG, et al: Transforming
growth factor beta1 induces epithelial-to-mesenchymal transition of
a549 cells. J Korean Med Sci. 22:898–904. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Kasai H, Allen JT, Mason RM, Kamimura T
and Zhang Z: TGF-beta1 induces human alveolar epithelial to
mesenchymal cell transition (EMT). Respir Res. 6:562005. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Zhang HJ, Wang HY, Zhang HT, Su JM, Zhu J,
Wang HB, Zhou WY, Zhang H, Zhao MC, Zhang L, et al: Transforming
growth factor-β1 promotes lung adenocarcinoma invasion and
metastasis by epithelial-to-mesenchymal transition. Mol Cell
Biochem. 355:309–314. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Malli F, Papaioannou AI, Gourgoulianis KI
and Daniil Z and Daniil Z: The role of leptin in the respiratory
system: An overview. Respir Res. 11:1522010. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Lago F, Dieguez C, Gomez-Reino J and
Gualillo O: Adipokines as emerging mediators of immune response and
inflammation. Nat Clin Pract Rheumatol. 3:716–724. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Xu YJ, Shao YF, Zhao X, Geng YT, Wang K
and Yin YM: Expression and clinical significance of leptin, the
functional receptor of leptin (OB-Rb) and her-2 in non-small-cell
lung cancer: A retrospective analysis. J Cancer Res Clin Oncol.
137:1841–1848. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Li Y, Geng J, Wang Y, Lu Q, Du Y, Wang W
and Li Z: The role of leptin receptor gene polymorphisms in
determining the susceptibility and prognosis of nsclc in chinese
patients. J Cancer Res Clin Oncol. 138:311–316. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Wang W, Yan H, Dou C and Su Y: Human
leptin triggers proliferation of a549 cells via blocking
endoplasmic reticulum stress-related apoptosis. Biochemistry.
78:1333–1341. 2013.PubMed/NCBI
|
|
30
|
Shen Y, Wang Q, Zhao Q and Zhou J: Leptin
promotes the immune escape of lung cancer by inducing
proinflammatory cytokines and resistance to apoptosis. Mol Med Rep.
2:295–299. 2009.PubMed/NCBI
|
|
31
|
Lai Q and Sun Y: Human leptin protein
induces proliferation of A549 cells via inhibition of PKR-like ER
kinase and activating transcription factor-6 mediated apoptosis.
Yonsei Med J. 54:1407–1415. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Zheng XJ, Yang ZX, Dong YJ, Zhang GY, Sun
MF, An XK, Pan LH and Zhang SL: Downregulation of leptin inhibits
growth and induces apoptosis of lung cancer cells via the notch and
jak/stat3 signaling pathways. Biol Open. 5:794–800. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Qi HW, Xin LY, Xu X, Ji XX and Fan LH:
Epithelial-to-mesenchymal transition markers to predict response of
berberine in suppressing lung cancer invasion and metastasis. J
Transl Med. 12:222014. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Sanchez-Tillo E, Lazaro A, Torrent R,
Cuatrecasas M, Vaquero EC, Castells A, Engel P and Postigo A: Zeb1
represses e-cadherin and induces an emt by recruiting the swi/snf
chromatin-remodeling protein brg1. Oncogene. 29:3490–3500. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Yang MH, Hsu DS, Wang HW, Wang HJ, Lan HY,
Yang WH, Huang CH, Kao SY, Tzeng CH, Tai SK, et al: Bmi1 is
essential in Twist1-induced epithelial-mesenchymal transition. Nat
Cell Biol. 12:982–992. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Chen XF, Zhang HJ, Wang HB, Zhu J, Zhou
WY, Zhang H, Zhao MC, Su JM, Gao W, Zhang L, et al: Transforming
growth factor-β1 induces epithelial-to-mesenchymal transition in
human lung cancer cells via PI3K/Akt and MEK/Erk1/2 signaling
pathways. Mol Biol Rep. 39:3549–3556. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Bakin AV, Tomlinson AK, Bhowmick NA, Moses
HL and Arteaga CL: Phosphatidylinositol 3-kinase function is
required for transforming growth factor beta-mediated epithelial to
mesenchymal transition and cell migration. J Biol Chem.
275:36803–36810. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Derynck R and Zhang YE: Smad-dependent and
smad-independent pathways in tgf-beta family signalling. Nature.
425:577–584. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Ahmad A, Maitah MY, Ginnebaugh KR, Li Y,
Bao B, Gadgeel SM and Sarkar FH: Inhibition of hedgehog signaling
sensitizes nsclc cells to standard therapies through modulation of
emt-regulating mirnas. J Hematol Oncol. 6:772013. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Gomes LR, Terra LF, Sogayar MC and
Labriola L: Epithelial-mesenchymal transition: Implications in
cancer progression and metastasis. Curr Pharm Biotechnol.
12:1881–1890. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Karatas F, Yalcin B, Sahin S, Akbulut H,
Utkan G, Demirkazik A and Icli F: The significance of serum leptin
level in patients with early stage nonsmall cell lung cancer. J
Cancer Res Ther. 13:204–207. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Tong X, Ma Y, Zhou Q, He J, Peng B, Liu S,
Yan Z, Yang X and Fan H: Serum and tissue leptin in lung cancer: A
meta-analysis. Oncotarget. 8:19699–19711. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Feng HL, Guo P, Wang J, Liu QY, Xu JF,
Yang HC and Zhang JM: Association of the expression of leptin and
leptin receptor with bone metastasis in pulmonary adenocarcinoma.
Zhonghua Zhong Liu Za Zhi. 38:840–844. 2016.(In Chinese).
PubMed/NCBI
|
|
44
|
Mou W, Xue H, Tong H, Sun S, Zhang Z,
Zhang C, Sun Q, Dong J, Wen X, Yan G, et al: Prognostic value of
serum leptin in advanced lung adenocarcinoma patients with
cisplatin/pemetrexed chemotherapy. Oncol Lett. 7:2073–2078. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Demiray G, Degirmencioglu S, Ugurlu E and
Yaren A: Effects of serum leptin and resistin levels on cancer
cachexia in patients with advanced-stage non-small cell lung
cancer. Clin Med Insights Oncol. 11:11795549176901442017.
View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Anar C, Deniz D, Erol S, Batum O, Bicmen C
and Yilmaz U: Are serum leptin levels a prognostic factor in
advanced lung cancer? Bratisl Lek Listy. 118:13–16. 2017.PubMed/NCBI
|