Introduction
Osteosarcoma, which frequently occurs in teenagers,
is the most common form of primary malignant bone tumor, with a
high incidence of fatality (1). The
specific molecular and pathological mechanisms underlying the
development of osteosarcoma have not been fully elucidated.
Therefore, there remains a lack of specific therapies for the
clinical treatment of this disease (2). Thus, there is an urgent requirement to
study the pathological mechanisms and to develop targeted drugs so
as to improve the prognosis of patients with osteosarcoma. Myeloid
differentiation factor 88 (MyD88) has been demonstrated to
contribute to the occurrence and development of multiple types of
tumors, including breast (3), ovarian
(4), liver (5), pancreatic and colon (6,7) cancer.
However, there has been a distinct lack of previous research
focusing on the expression and function of MyD88 in osteosarcoma
tissue.
Upon activation of toll-like or interleukin (IL)-1β
receptors, MyD88 activates a signaling cascade that culminates in
the activation of nuclear factor-κB (NF-κB) and the expression of
proinflammatory genes. NF-κB is an important modulator in tumor
progression (8). Activation of NF-κB
contributes to cancer metastasis by inducing expression of
metastasis-associated proteins. A previous study found that
patients whose osteosarcoma had active NF-κB experienced shorter
median overall survival time compared with those patients whose
osteosarcoma had inactive NF-κB (9).
Inhibitors of NF-κB signaling suppressed expression of
NF-κB-modulated metastasis-associated proteins and led to blockage
of the metastatic mechanism in osteosarcoma cells, in vitro
and in vivo (10,11). Based on these observations, we
hypothesized that MyD88 may serve a key role in osteosarcoma
pathogenesis and development. A specific inhibitor of MyD88,
ST2825, is a type of halogenated heptapeptide, which inhibits the
dimerization of MyD88 via competitive binding to the Toll
interleukin-1 receptor (TIR) domain of MyD88, thereby blocking the
Toll-like receptors/IL signaling pathway and inhibiting the
activation of NF-κB (11).
The aim of the present study was to elucidate the
expression and function of MyD88 in patients with osteosarcoma in
association with their clinicopathological aspects. In addition, in
the osteosarcoma U2OS cell line, the potential use of a MyD88
inhibitor, ST2825, was assessed as a novel drug targeting molecular
pathways involved in the progression of osteosarcoma.
Materials and methods
Patient information
A total of 98 patients with postoperatively
pathologically confirmed osteosarcoma following resection were
selected and enrolled into the present study at the Affiliated
Yixing Hospital of Jiangsu University (Yixing, China) between June
2001 and February 2010. During surgery, osteosarcoma tissues and
matched normal peritumoral bone tissues (control) were sampled.
Prior to the study, the research was approved by the by the Ethics
Committee of the Affiliated Yixing Hospital of Jiangsu University,
and written informed consent was provided by all patients or by an
appropriate guardian. Patient information was collected throughout
the study, including patient's name, age, anatomical site,
histological subtype, metastasis and Enneking grade (12). Exclusion criteria included patients
who had received radiotherapy or chemotherapy prior to surgery. All
patients were followed up for a 5-year period, either by telephone
or by regular hospital visits, every 3 months.
Immunohistochemistry (IHC) and
hematoxylin and eosin (H&E) staining
All fresh specimens incised during the surgery were
fixed in 10% formalin for 24 h at 4°C. Following conventional
paraffin embedding, tissue samples were sectioned at a thickness of
3 µm, and dried in the oven at 60°C for 4 h. Sections were dewaxed
in xylene and rehydrated in a series of anhydrous ethanol. Tissue
sections were washed in PBS, 3 times for 5 min each time. Antigen
retrieval was performed by placing tissue sections in sodium
citrate buffer and heating in a pressure cooker, until steam was
produced. The tissues were heated for a further 2 min from when the
pressure cooker let off steam, and were then cooled naturally to
room temperature. The sections were washed in distilled water twice
and in PBS twice, for 3 min each time. Endogenous peroxidase
activity was blocked by incubation of slides in peroxidase blocking
solution (3% hydrogen peroxide) at room temperature for 10 min.
Slides were washed in PBS 3 times, for 3 min each time.
Non-specific binding was blocked by incubation of slides in 5%
normal rabbit serum (Zhongshan Golden Bridge Biotechnology, Co.,
Ltd., Beijing, China) at room temperature for 10 min. Slides were
then incubated with the primary antibody against MyD88 (1:50
dilution; catalog no. sc-11356; Santa Cruz Biotechnology, Inc.,
Dallas, TX, USA) at room temperature for 60 min, and PBS replaced
the primary antibody as the negative control. Slides were washed in
PBS, 3 times, for 3 min each time. An appropriate biotin-labeled
goat-anti-rabbit IgG secondary antibody (1:50 dilution; catalog no.
DS-0002; Zhongshan Golden Bridge Biotechnology, Co., Ltd.) was
titrated and the sections were incubated at room temperature for 10
min. Slides were washed in PBS, 3 times, for 3 min each time.
Streptavidin-peroxidase solution (Zhongshan Golden Bridge
Biotechnology, Co., Ltd; goat-anti-rabbit IgG) was titrated and
sections were incubated at room temperature for 10 min. Slides were
washed in PBS, 3 times, for 3 min each time, and stained with
3,3′-diaminobenzidine for 3 min for antigen detection. Sections
were counterstained with hematoxylin, prior to dehydration with a
series of alcohols (70% ethanol, 95% ethanol and absolute ethanol),
and mounted with neutral balsam. H&E staining was performed
according to the standard protocols (13).
IHC analysis
IHC results were interpreted by three experienced
pathologists in the Affiliated Yixing Hospital of Jiangsu
University, and the specific methods of analysis used were
performed a previously described (14). The double-blind method was adopted to
count 15 fields of view for each section at ×400 magnification with
an optical microscope (Olympus, Tokyo, Japan). The positive cell
rate referred to the average number of cells with positive MyD88
staining/per 100 cells. Interpretation of positive IHC results was
performed following methods as previously described (5), according to the certain aspects. The
staining intensity of positive cells was graded as follows:
Negative staining, 0; pale yellow staining, 1; yellow staining, 2;
and tan staining, 3. The intensity of staining results were defined
as follows: 0–2, negative staining; or 3, positive staining. The
percentage of positive cells was as follows: 0–5%, negative
expression; 6–25%, weakly positive expression; 26–75%, moderately
positive expression; and >76%, strongly positive expression.
Weakly, moderately and strongly positive expression was defined as
positive staining.
Cell culture and grouping
The human osteosarcoma U2OS cell line was provided
by the Cell Bank of the Chinese Academy of Sciences (Shanghai,
China) and cultured in Dulbecco's modified Eagle's medium (DMEM;
Gibco; Thermo Fisher Scientific, Inc., Waltham, MA, USA) containing
10% fetal bovine serum (FBS; Sigma-Aldrich; Merck KGaA, Darmstadt,
Germany) in an incubator with 5% CO2 at 37°C.
Groups
U2OS cells at the logarithmic stage were selected
for the subsequent experiments. The MyD88 inhibitor, ST2825, was
purchased from MedChemexpress LLC (Princeton, NJ, USA). ST2825 was
firstly dissolved in dimethyl sulfoxide (DMSO), which was further
diluted to 15 and 30 µM with culture medium prior to each
experiment. The cells were divided into four groups: The blank
control group, the solvent (DMSO) treatment group, the low-dose
ST2825 treatment group (15 µM) and the high-dose ST2825 treatment
group (30 µM).
MTT assay for analysis of cell
proliferation
MTT and DMSO were obtained from Solarbio Science and
Technology Co., Ltd. (Beijing, China). Cells cultured with ST2825
(15 or 30 µM) were placed in a humidified incubator and maintained
at 37°C with 5% CO2 for 24, 48 and 72 h. A total of 20
µl MTT solution was added to each well and maintained for a further
4 h, prior to the termination of culture. DMSO (100 µl;
Sigma-Aldrich; Merck KGaA) was added to dissolve the formazan
crystals. An enzyme immunoassay analyzer (Bio-Rad Laboratories,
Inc. Hercules, CA, USA) was used to measure the optical density
(OD) values at a wavelength of 490 nm.
Cell apoptosis analysis
An Annexin V-fluorescein isothiocyanate (FITC)
staining kit (BioVision, Inc., Milpitas, CA, USA) was used to
detect apoptosis in human U2OS cells, according to the
manufacturers protocol. At 24, 48 and 72 h, cells were digested by
0.25% pancreatin (Gibco; Thermo Fisher Scientific, Inc.) and washed
by PBS twice, followed by centrifugation at 2,000 × g for 5 min.
Cells were washed in PBS and 1X binding buffer was added. A dual
staining method was used and U2OS cells were labeled with 5 µl
Annexin V-FITC and 10 µl propidium iodide, added to each well.
Transwell chamber migration assay
A cell migration assay was performed using 24-well
Transwell chambers (8-µm pore size; Merck KGaA), according to the
manufacturer's instructions.
Briefly, the cells were seeded at a density of
5×104/400 µl, and treated with ST2825 or vehicle (DMSO)
in DMEM, place into the upper chambers of the Transwell assay.
Fresh DMEM (600 µl/well) containing 20% FBS, was added to the
bottom chambers. Following 24 h of culture, cells that migrated to
the underside of the filter were fixed with methanol and stained
with crystal violet for 1 min at room temperature, and counted
using bright field microscopy. Each experiment was repeated on
three independent occasions.
Western blot analysis
Subsequent to washing twice with PBS, the
osteosarcoma cells treated for 48 h were centrifuged at 12,000 × g
for 15 min at 4°C. A total cellular protein extraction kit (Bi
Yuntian Biological Technology Institution, Nantong, China) and
nucleoprotein extraction reagents (Bi Yuntian Biological Technology
Institution, Nantong, China) were used to extract nucleoprotein
according to the manufacturer's protocols. Total protein
concentrations were quantified using the BCA method. Protein
samples (30 µg) were loaded and separated by 10% SDS-PAGE and
transferred to polyvinylidene difluoride membranes. The membranes
were blocked with 5% non-fat milk (Beyotime Institute of
Biotechnology) in TBST [20 mM Tris, HCl (pH 7.2), 150 mM NaCl and
0.05% Tween-20] for 1 h at room temperature. The membranes were
incubated with primary antibodies Histone 3 (H3) rabbit monoclonal
antibody (mAb) (catalog no. 4499; 1:1,000 dilution), cyclin D1
rabbit mAb (catalog no. 2978; 1:1,000 dilution), p65 rabbit mAb
(catalog no. 8242; 1:1,000 dilution), cleaved-caspase-3 rabbit mAb
(catalog no. 9664; 1:1,000 dilution) and β-actin rabbit mAb
(catalog no. 4970; 1:1,000 dilution) at 4°C overnight. All the
primary antibodies were purchased from Cell Signaling Technology,
Inc. (Danvers, MA, USA). The membranes were then washed and
incubated with 5% milk-diluted horseradish peroxidase-conjugated
goat-anti-rabbit secondary antibody (catalog no. 7074; 1:5,000
dilution; Cell Signaling Technology, Inc.) for 1 h at room
temperature. Membranes were washed again and proteins were detected
using enhanced chemiluminescence reagent (Pierce; Thermo Fisher
Scientific, Inc.). Images were captured on a gel imager (Tanon-3500
digital gel imaging system; Tanon Science and Technology Co., Ltd.,
Shanghai, China) and ImageJ (National Institutes of Health,
Bethesda, MD, USA) was used for band densitometry analysis.
Statistical analysis
Statistical analysis was conducted using SPSS,
v.19.0 software (SPSS, IBM Corp, Armonk, NY, USA). Data are
expressed as the mean ± standard deviation. A χ2 test
was used to analyze enumeration data, Kaplan-Meier method was used
for survival analysis and the one-way ANOVA test was used to
compare quantitative data. P<0.05 was considered to indicate a
statistically significant difference.
Results
H&E and IHC staining results of
osteosarcoma and normal peritumoral bone tissues
Osteosarcoma specimens used in the present study
were confirmed by postoperative pathological diagnosis. Typical
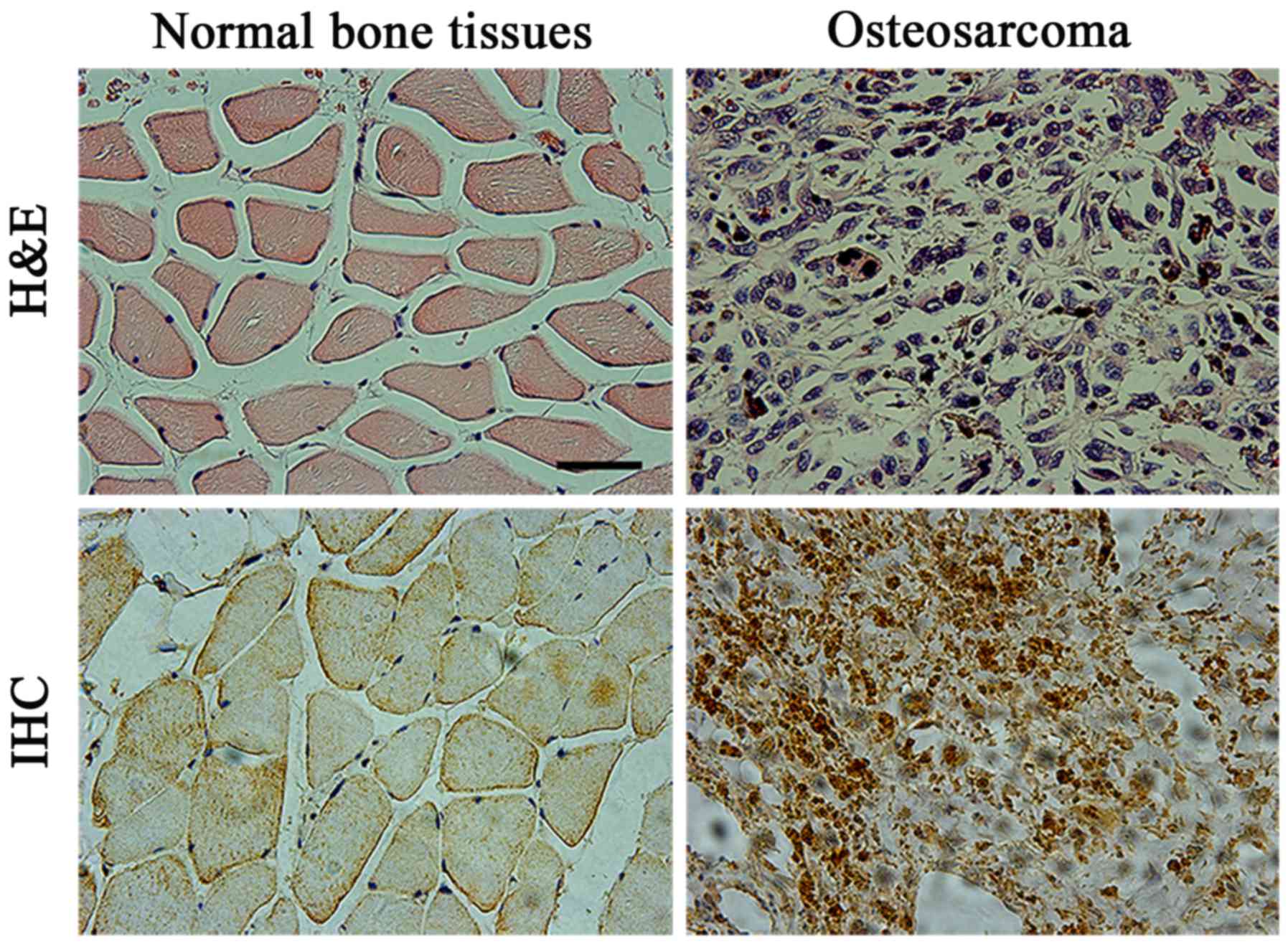
H&E staining results of osteosarcoma are demonstrated in
Fig. 1. Osteosarcoma cells were mixed
with trabecular or patchy osteoid tissues, and osteosarcoma cells
were in clear fusiform or polygonal form. The various nuclear forms
were large and strongly stained with marked nucleoli (Fig. 1A and B). MyD88 staining in
osteosarcoma tissues was mainly located in the cytoplasm. Positive
staining was evident as a pale yellow, tan or brown color,
representative of weak, moderate or strong staining, respectively.
There were relatively fewer MyD88-positive cells in normal
peritumoral bone tissues compared with osteosarcoma tissues
(Fig. 1C and D). Statistical analysis
demonstrated that the positive rate of MyD88 staining in
osteosarcoma tissues was significantly increased (71.4%) compared
with that in matched normal peritumoral bone tissues (6.1%;
P<0.05; Table I).
 | Table I.Analysis of MyD88 expression in
osteosarcoma and matched normal peritumoral bone tissues. |
Table I.
Analysis of MyD88 expression in
osteosarcoma and matched normal peritumoral bone tissues.
|
|
| MyD88 expression |
|
|---|
|
|
|
|
|
|---|
| Tissue type | Total, n | Positive, n (%) | Negative, n (%) | P-value |
|---|
| Osteosarcoma
tissues | 98 | 70 (71.4) | 28 (28.6) |
<0.001a |
| Adjacent non-tumor
tissues | 98 | 6 (6.1) | 91 (92.9) |
|
Association between MyD88 protein and
pathological characteristics and grading of patients with
osteosarcoma
Statistical analysis of the clinicopathological
status of patients with osteosarcoma in association with MyD88
protein revealed that MyD88 protein was not associated with gender,
age, tumor site or subtype of patients with osteosarcoma
(P>0.05). MyD88 positive expression is associated with
metastasis and Enneking stage (both P<0.05) (Table II).
 | Table II.Association between MyD88 and
clinicopathological factors of patients with osteosarcoma
(n=98). |
Table II.
Association between MyD88 and
clinicopathological factors of patients with osteosarcoma
(n=98).
|
|
| MyD88 expression |
|
|---|
|
|
|
|
|
|---|
| Factor | Total, n | Positive, n | Negative, n | P-value |
|---|
| Sex |
|
|
| 0.08 |
| Male | 64 | 42 | 22 |
|
|
Female | 34 | 28 | 6 |
|
| Age, years |
|
|
| 0.22 |
|
<18 | 72 | 49 | 23 |
|
| ≥18 | 26 | 21 | 5 |
|
| Anatomical site |
|
|
| 0.74 |
|
Femur | 49 | 36 | 13 |
|
|
Tibia | 42 | 30 | 12 |
|
|
Other | 7 | 4 | 3 |
|
| Histological
subtype |
|
|
| 0.37 |
|
Osteoblastic | 63 | 48 | 15 |
|
|
Chondroblastic | 24 | 15 | 9 |
|
|
Others | 11 | 7 | 4 |
|
| Metastasis |
|
|
|
<0.001a |
|
Yes | 43 | 40 | 3 |
|
| No | 55 | 30 | 25 |
|
| Enneking grade |
|
|
|
<0.001a |
| I | 10 | 2 | 8 |
|
|
IIa | 24 | 12 | 12 |
|
|
IIb | 53 | 46 | 7 |
|
|
III | 11 | 10 | 1 |
|
Association between MyD88 protein
expression and clinical prognosis of patients with
osteosarcoma
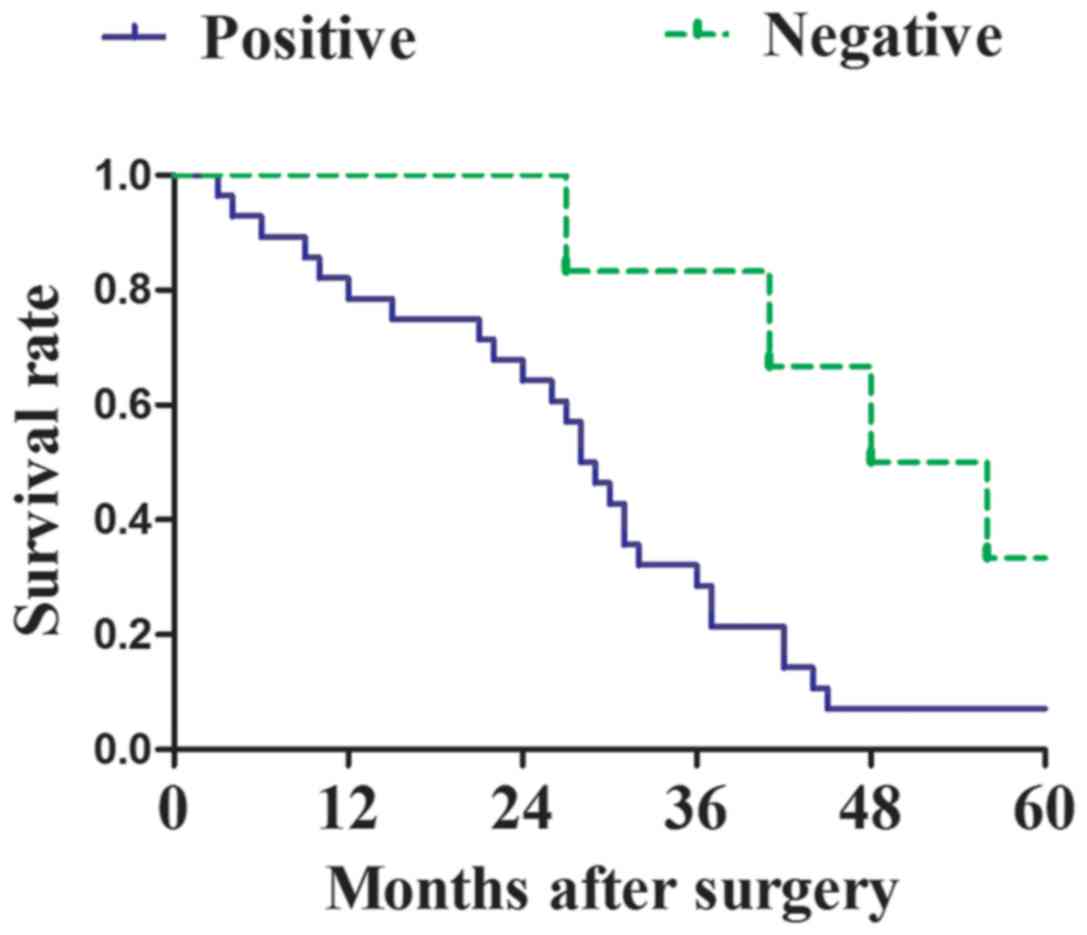
The Kaplan-Meier method was used to analyze the
survival rate of post-surgery patients with osteosarcoma, with
positive or negative MyD88 expression (Fig. 2). For patients with osteosarcoma with
positive MyD88 expression, 26 cases succumbed within 5 years, the
median survival time was 28.5 months and the overall 5-year
survival rate was 62.9%. For osteosarcoma patients with negative
MyD88 expression, 4 cases succumbed within 5 years, the median
survival time was 52 months and the overall 5-year survival rate
was 85.7%. The 5-year survival rate was significantly reduced in
patients with osteosarcoma displaying positive MyD88 expression
compared with that in patients with negative MyD88 expression
(P=0.023) (Fig. 2).
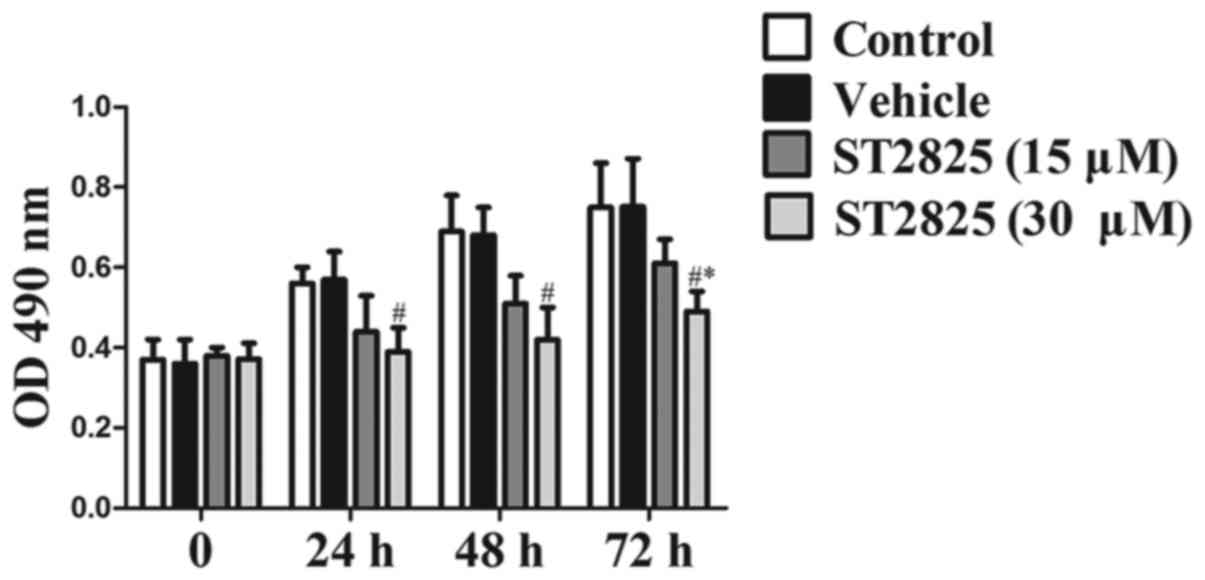
Effects of ST2825 on the proliferation
of osteosarcoma cells
The proliferation rate of osteosarcoma U2OS cells
was analyzed in the presence of the MyD88 selective inhibitor,
ST2825 (15 or 30 µM). A significant inhibition of the proliferation
of osteosarcoma cells was demonstrated at 72 h of culture compared
with that in the blank control and vehicle groups, in a
dose-dependent manner (P<0.05; Fig.
3). The inhibition rate was significantly increased in the
high-dose ST2825 treatment group compared with that in the low-dose
treatment group at each time (P<0.05).
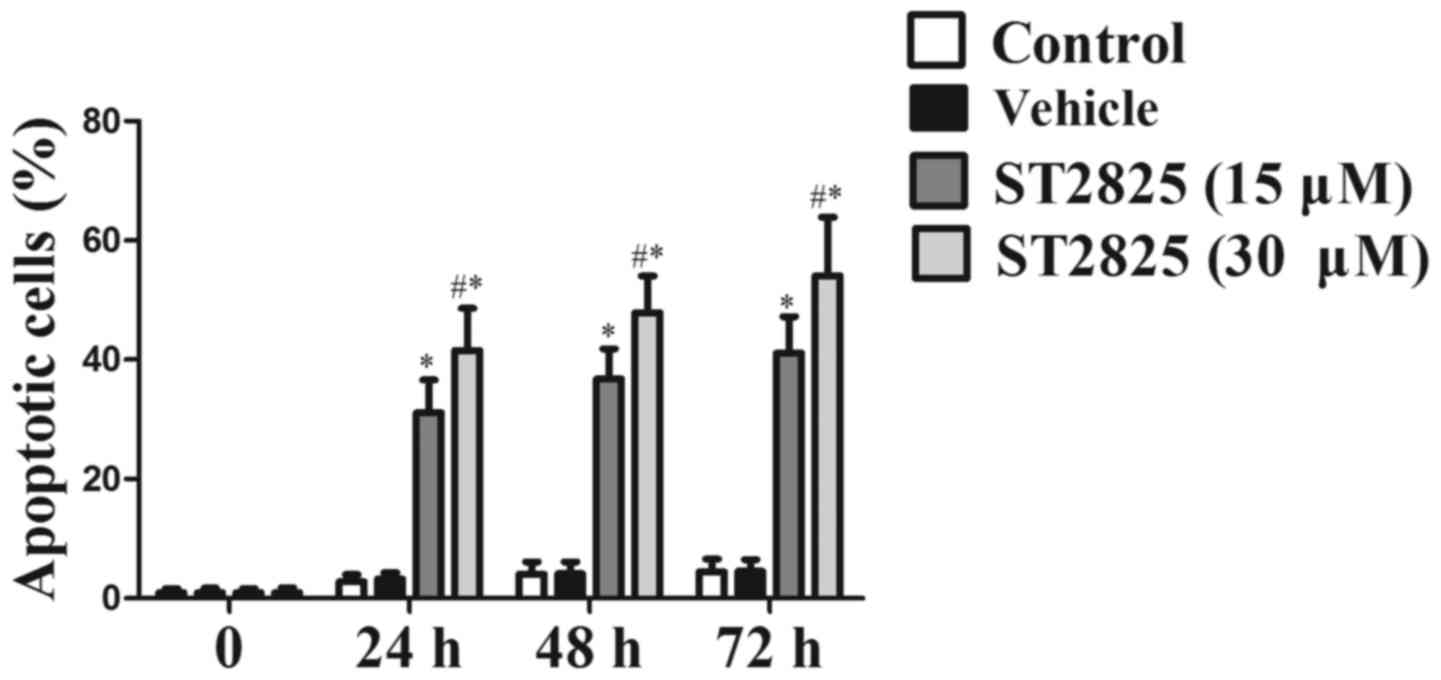
Effects of ST2825 on the apoptosis of
osteosarcoma cells
As shown in Fig. 4,
the apoptosis rate of the osteosarcoma cells was unchanged between
the solvent treatment group and the blank control group
(P>0.05). In response to ST2825 treatment (15 or 30 µM), there
was a significant dose-dependent increase in the apoptotic rate of
osteosarcoma cells compared with that of the control groups
(P<0.05). In addition, the apoptosis rate was significantly
increased in the high-dose treatment group compared with that in
the low-dose treatment group (P<0.05).
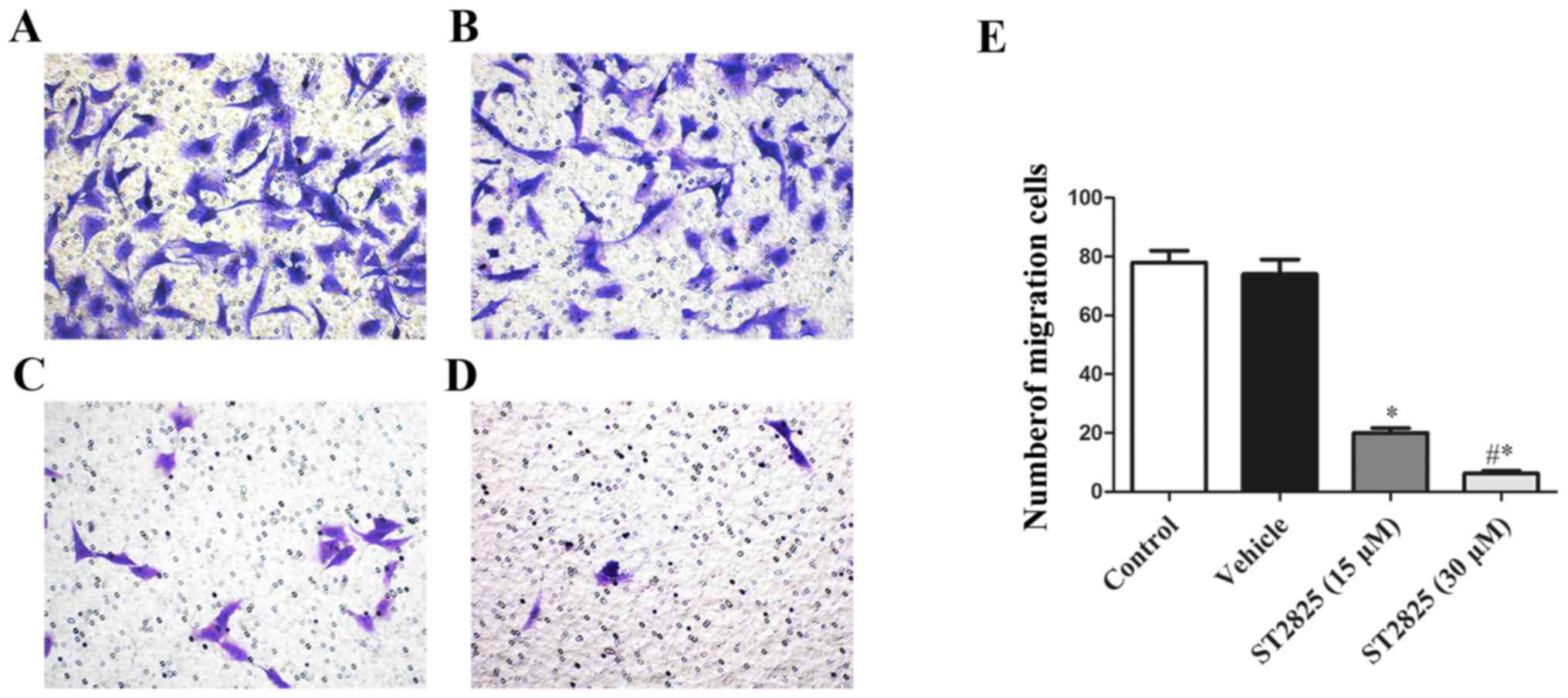
ST2825 inhibits osteosarcoma cell
migration in vitro
A Transwell migration assay was performed to detect
the effects of ST2825 treatment on osteosarcoma cell migration. The
effects of ST2825 treatment (15 or 30 µM) significantly decreased
the number of osteosarcoma cells that migrated in the Transwell
assay in a dose-dependent manner compared with that of the control
or vehicle-treated group (P<0.05; Fig.
5). In addition, the inhibitory effect of ST2825 treatment on
osteosarcoma cell migration was significantly increased in the
high-dose treatment group compared with that in the low-dose
treatment group (P<0.05).
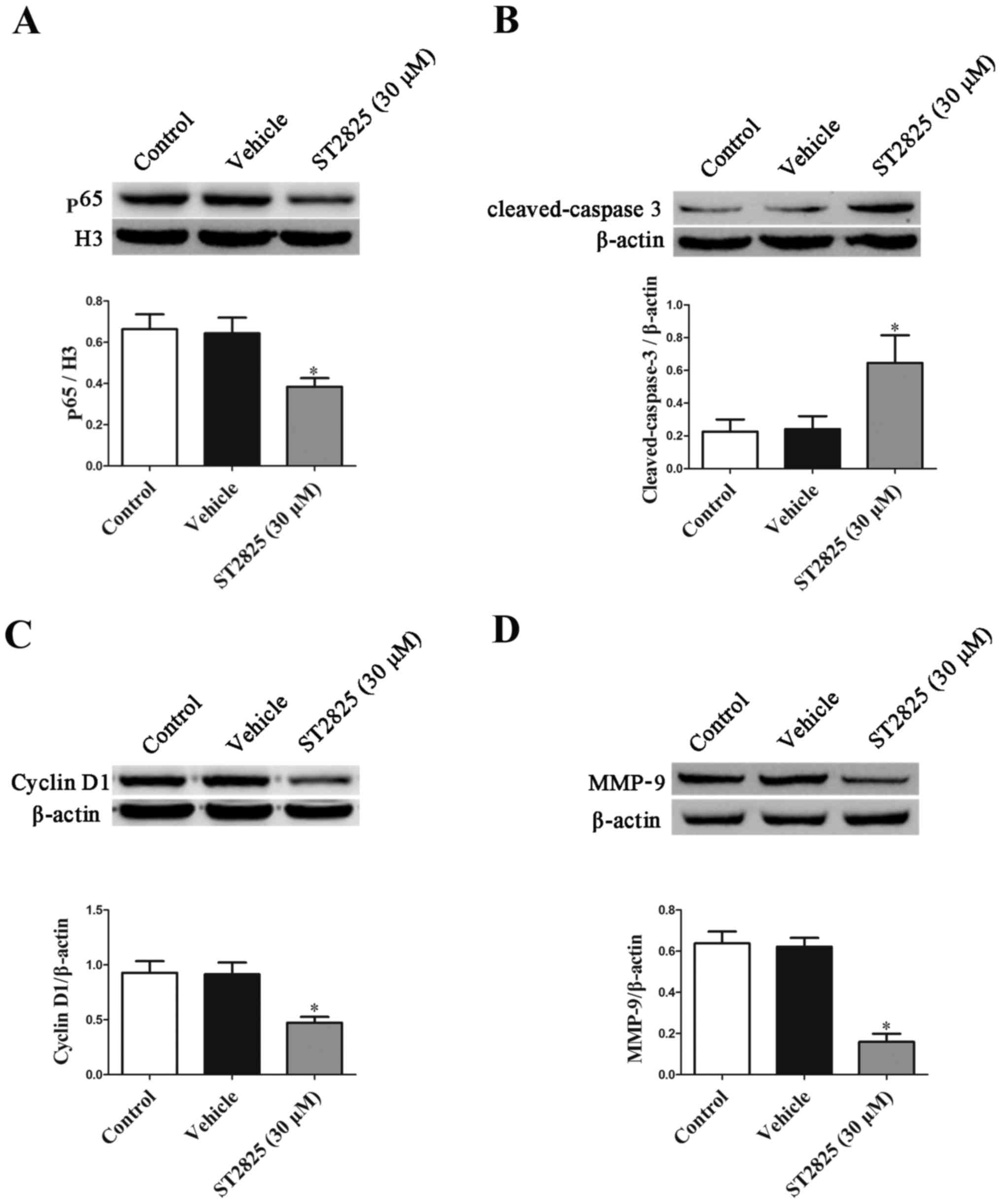
Effects of ST2825 on p65, cyclin D1,
matrix-metallopeptidase-9 (MMP-9) and cleaved-caspase-3 protein
expression in osteosarcoma cells
Given that high-dose ST2825 treatment was confirmed
in the present study to produce an enhanced anti-proliferative and
pro-apoptotic effect, this dose was used in subsequent experiments
to study the potential mechanisms that may be involved. The results
presented in Fig. 6 demonstrated that
MMP-9, cyclin D1 and nuclear p65 expression were significantly
decreased in osteosarcoma cells in the high-dose ST2825 treatment
group compared with the control group, whilst cleaved-caspase 3
protein expression was significantly increased (P<0.05).
Discussion
The results from the present study demonstrated that
an abnormally high level of MyD88 protein was apparent in human
osteosarcoma cells. However, MyD88 expression was not associated
with the gender, age, tumor site or subtype of patients with
osteosarcoma (P>0.05), yet the presence of osteosarcoma
metastasis was associated with Enneking stage. In addition, the
5-year survival rate of patients with osteosarcoma with positive
MyD88 expression was significantly lower compared with that of
patients with negative MyD88 expression. Furthermore, the effects
of ST2825, a selective inhibitor of MyD88, significantly inhibited
the proliferation and promoted the apoptosis of osteosarcoma cells
in vitro. The underlying mechanisms may involve inhibition
of the nuclear transfer of NF-κB and p65, inhibition of Cyclin D1
and MMP9, and enhanced cleaved-caspase 3 protein expression. The
results from the present study indicated that aberrant MyD88
expression may contribute to the development of osteosarcoma, and
may thus be a potential novel therapeutic target for the treatment
of this disease.
Previous studies have revealed abnormally high MyD88
expression in multiple types of tumor tissue, and the inhibition of
MyD88 expression has been confirmed to be associated with the
occurrence and development of various tumors (15). In the present study, inhibition of
MyD88 significantly inhibited the proliferation of tumor cells and
promoted apoptosis, indicating that MyD88 has potential as a novel
target for tumor treatment (16,17).
Previous studies have confirmed that MyD88 is located in activated
key nodes of multiple signal transduction pathways, and that
activated MyD88 can regulate the activity of mitogen-activated
protein kinases (MAPK) signaling pathways (18,19). In
addition, MyD88 can activate IκB kinase polymerase chain reaction,
and further degrade IκBα. Next NF-κBp65, which has been freed from
IκBα, transfers from the cytoplasm to the nucleus (20). In turn, NF-κBp65 binds to the specific
downstream target gene sequence, thus regulating the transcription
of associated-target genes. Previous studies have confirmed that
MAPK expression is elevated in osteosarcoma tissues, and that
inhibition of MAPK expression significantly inhibited the
proliferation of osteosarcoma cells and promoted their apoptosis
(21,22). NF-κB was also revealed to be
abnormally activated in osteosarcoma tissues (23), and inhibition of NF-κB activity was
shown to significantly inhibit the proliferation and promote the
apoptosis of osteosarcoma cells (23–25). This
suggests that MyD88 likely contributes an important function in the
occurrence and development of osteosarcoma. Immunohistochemical
analysis was adopted in the present study to investigate the MyD88
protein expression in the tumor tissues of patients with
osteosarcoma. The results from the present study demonstrated that
MyD88 expression was significantly higher in osteosarcoma tissues
compared with that in normal peritumoral bone tissues. Further
investigation revealed that MyD88 expression was not associated
with gender, age or tumor sites of patients with osteosarcoma, but
that it was associated with the presence or absence of osteosarcoma
metastasis and Enneking stage. This is indicative of an important
association of aberrant MyD88 expression in the development of
osteosarcoma. The Kaplan-Meier method was used for statistical
analysis of the survival rate of patients with osteosarcoma. The
results from the present study revealed that the 5-year survival
rate was significantly lower in patients with osteosarcoma with
positive MyD88 expression compared with that in osteosarcoma
patients with negative MyD88 expression, thus the promotion of
MyD88 expression may be an important factor affecting the prognosis
of osteosarcoma. Postoperative detection of the MyD88 expression in
osteosarcoma tissues may be of particular importance in the
prognosis of patients with osteosarcoma.
Following confirmation of the association between
high expression of MyD88 in osteosarcoma tissues and patient
prognosis, a specific inhibitor of MyD88, ST2825 was used to treat
U2OS osteosarcoma cells during in vitro cell culture in the
present study, to further investigate the function of MyD88 on the
proliferation and apoptosis of osteosarcoma cells. The doses of
ST2825 adopted in the present study were used as described in a
previous study (11). The results of
the present study demonstrated that ST2825 significantly inhibited
the proliferation and promoted the apoptosis of osteosarcoma cells
in a dose-dependent manner. The study revealed that p65 and cyclin
D1 expression in the nuclei of osteosarcoma cells was significantly
decreased in the ST2825 treatment group compared with that in the
control group, indicating that this may be a mechanism of ST2825
inhibition of tumor cell proliferation. Cleaved-caspase 3 is the
primary protein of apoptosis (25).
In the present study, cleaved-caspase 3 protein was significantly
increased in the ST2825 intervention group compared with the
solvent group, suggesting that this may be a mechanism by which
ST2825 inhibits the proliferation of osteosarcoma cells and
promotes apoptosis. As, this effect of the selective inhibitor of
MyD88 was only confirmed in the in vitro experiments,
further in vivo studies are required in order to lay a solid
theoretical basis for the treatment of osteosarcoma by targeting
MyD88.
In conclusion, MyD88 expression is significantly
increased in osteosarcoma tissues, and is significantly associated
with clinical stage and prognosis. ST2825, a specific inhibitor of
MyD88, inhibited the proliferation and promoted the apoptosis of
osteosarcoma cells in vitro, suggesting that MyD88 may be a
novel target for the treatment of osteosarcoma.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Bishop MW, Janeway KA and Gorlick R:
Future directions in the treatment of osteosarcoma. Curr Opin
Pediatr. 28:26–33. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Ferrari S and Serra M: An update on
chemotherapy for osteosarcoma. Expert Opin Pharmacother.
16:2727–2736. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Xiang F, Ni Z, Zhan Y, Kong Q, Xu J, Jiang
J, Wu R and Kang X: Increased expression of MyD88 and association
with paclitaxel resistance in breast cancer. Tumour Biol.
37:6017–6025. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Zhu Y, Huang JM, Zhang GN, Zha X and Deng
BF: Prognostic significance of MyD88 expression by human epithelial
ovarian carcinoma cells. J Transl Med. 10:772012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Liang B, Chen R, Wang T, Cao L, Liu Y, Yin
F, Zhu M, Fan X, Liang Y, Zhang L, et al: Myeloid differentiation
factor 88 promotes growth and metastasis of human hepatocellular
carcinoma. Clin Cancer Res. 19:2905–2916. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Salcedo R, Worschech A, Cardone M, Jones
Y, Gyulai Z, Dai RM, Wang E, Ma W, Haines D, O'Huigin C, et al:
MyD88-mediated signaling prevents development of adenocarcinomas of
the colon: Role of interleukin 18. J Exp Med. 207:1625–1636. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kuo AH and Scheeren FA: Cell-intrinsic
TLR2/MyD88 pathway in breast and colon cancer. Cell Cycle.
13:3785–3786. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Di Padova F, Quesniaux VFJ and Ryffel B:
MyD88 as a therapeutic target for inflammatory lung diseases.
Expert Opin Ther Targets. 22:401–408. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Tang QL, Xie XB, Wang J, Chen Q, Han AJ,
Zou CY, Yin JQ, Liu DW, Liang Y, Zhao ZQ, et al: Glycogen synthase
kinase-3β, NF-κB signaling, and tumorigenesis of human
osteosarcoma. J Natl Cancer Inst. 104:749–763. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Xu HY, Fang W, Huang ZW, Lu JC, Wang YQ,
Tang QL, Song GH, Kang Y, Zhu XJ, Zou CY, et al: Metformin reduces
SATB2-mediated osteosarcoma stem cell-like phenotype and tumor
growth via inhibition of N-cadherin/NF-kB signaling. Eur Rev Med
Pharmacol Sci. 21:4516–4528. 2017.PubMed/NCBI
|
|
11
|
Lu Y, Li F, Xu T and Sun J: Tetrandrine
prevents multidrug resistance in the osteosarcoma cell line, U-2OS,
by preventing Pgp overexpression through the inhibition of NF-κB
signaling. Int J Mol Med. 39:993–1000. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Loiarro M, Capolunghi F, Fantò N, Gallo G,
Campo S, Arseni B, Carsetti R, Carminati P, De Santis R, Ruggiero V
and Sette C: Pivotal Advance: Inhibition of MyD88 dimerization and
recruitment of IRAK1 and IRAK4 by a novel peptidomimetic compound.
J Leukoc Biol. 82:801–810. 2017. View Article : Google Scholar
|
|
13
|
Zhang Y, Meng W and Cui H: LncRNA CBR3-AS1
predicts unfavorable prognosis and promotes tumorigenesis in
osteosarcoma. Biomed Pharmacother. 102:169–174. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zhou Y, Zhang W, Tang F, Luo Y, Min L,
Zhang W, Shi R, Duan H and Tu C: A case report of apatinib in
treating osteosarcoma with pulmonary metastases. Medicine
(Baltimore). 96:e65782017. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Chen X, Zhao F, Zhang H, Zhu Y, Wu K and
Tan G: Significance of TLR4/MyD88 expression in breast cancer. Int
J Clin Exp Pathol. 8:7034–7039. 2015.PubMed/NCBI
|
|
16
|
Kfoury A, Virard F, Renno T and Coste I:
Dual function of MyD88 in inflammation and oncogenesis:
Implications for therapeutic intervention. Curr Opin Oncol.
26:86–91. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Deng Y, Sun J and Zhang LD: Effect of
ST2825 on the proliferation and apoptosis of human hepatocellular
carcinoma cells. Genet Mol Res. 15:150168262016. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Shiratori E, Itoh M and Tohda S: MYD88
inhibitor ST2825 suppresses the growth of lymphoma and leukaemia
cells. Anticancer Res. 37:6203–6209. 2017.PubMed/NCBI
|
|
19
|
Warner N and Núñez G: MyD88: A critical
adaptor protein in innate immunity signal transduction. J Immunol.
190:3–4. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Cervantes JL: MyD88 in Mycobacterium
tuberculosis infection. Med Microbiol Immunol. 206:187–193. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Deguine J and Barton GM: MyD88: A central
player in innate immune signaling. F1000Prime Rep. 6:972014.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Chandhanayingyong C, Kim Y, Staples JR,
Hahn C and Lee FY: MAPK/ERK signaling in osteosarcomas, ewing
sarcomas and chondrosarcomas: Therapeutic implications and future
directions. Sarcoma. 2012:4048102012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Meng Q, Zheng M, Liu H, Song C, Zhang W,
Yan J, Qin L and Liu X: TRAF6 regulates proliferation, apoptosis,
and invasion of osteosarcoma cell. Mol Cell Biochem. 371:177–186.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Miwa S, Sugimoto N, Yamamoto N, Shirai T,
Nishida H, Hayashi K, Kimura H, Takeuchi A, Igarashi K, Yachie A
and Tsuchiya H: Caffeine induces apoptosis of osteosarcoma cells by
inhibiting AKT/mTOR/S6K, NF-κB and MAPK pathways. Anticancer Res.
32:3643–3649. 2012.PubMed/NCBI
|
|
25
|
Zhao Z, Wu MS, Zou C, Tang Q, Lu J, Liu D,
Wu Y, Yin J, Xie X, Shen J, et al: Downregulation of MCT1 inhibits
tumor growth, metastasis and enhances chemotherapeutic efficacy in
osteosarcoma through regulation of the NF-κB pathway. Cancer Lett.
342:150–158. 2014. View Article : Google Scholar : PubMed/NCBI
|