Introduction
Sepsis is a systemic inflammatory response syndrome
(SIRS) caused by a variety of pathogenic microorganisms and
endotoxin release; it is also a serious complication for severe
patients with acute and severe trauma and shock (1). HMGB1 serves a key role in the occurrence
and progression of sepsis, and its production is induced by
secretions of immune cells, including mononuclear cells, dendritic
cells, macrophages stimulated by endotoxins, and inflammatory
cytokines (2). It is one of the most
important inflammatory mediators for the lethal effect of sepsis
(3). The interaction of HMGB1 with
other inflammatory mediators serves an important role in mediating
the signaling pathways of the inflammatory response (4). A previous study demonstrated that HMGB1
could promote the migration of macrophages and the release of
various inflammatory cytokines, causing aggregation of a variety of
immune cells and inducing the inflammatory responses of sepsis
(3). Another study also demonstrated
that the elevated expression of HMGB1 is closely associated with
the occurrence of sepsis (5). miRs
are highly conserved, endogenous, non-coding small RNAs, which can
regulate the expression of target genes by complete or incomplete
complementary pairing with the 3′-untranslated region (3′-UTR) of
the mRNA, serving an important role in immune cell activation,
inflammatory cytokine release and the immune response (6,7). Previous
research has revealed that miR-25 was involved in the occurrence
and progression of sepsis and has potential as a diagnostic marker
of sepsis (8,9). miR-25 expression in peripheral blood in
patients with sepsis is abnormal, suggesting that miR-25
abnormalities may be associated with the pathogenesis of sepsis
(1,2).
Bioinformatics analysis revealed that a target binding site existed
between miR-25 and the HMGB1 3′-UTR. However, the specific role of
miR-25 in the regulation of HMGB1 and sepsis remains unclear. In
the present study, by analyzing the expression of miR-25 and HMGB1
in patients with sepsis, the association between miR-25, HMGB1 and
sepsis was investigated.
Materials and methods
Reagents and materials
The Ficoll-Paque Plus was purchased from GE
Healthcare (Chicago, IL, USA; cat. no. 17-1440-02). α-MEM was from
Hyclone (GE Healthcare) and fetal bovine serum was from Gibco
(Thermo Fisher Scientific, Inc., Waltham, MA, USA; cat. no.
10099141). Streptomycin was from Lonza Group, Ltd. (Basel,
Switzerland; cat. no. 17-602E). Lipopolysaccharide (LPS) was from
Sigma-Aldrich (Merck KGaA, Darmstadt, Germany; cat. no. L6529).
TRIzol (cat. no. 15596018), and Lipofectamine® 2000
(cat. no. 11668019) were from Thermo Fisher Scientific, Inc. The
PrimeScript™ RT reagent kit was from Takara Biotechnology Co., Ltd.
(Dalian, China; cat. no. RR037A) and the SYBR Green Real-Time PCR
Master mix was from Thermo Fisher Scientific, Inc., (cat. no.
4309155). miR-25 nucleotide fragments and primers were synthesized
by Guangzhou RiboBio Co., Ltd., Guangzhou, China. The mouse
anti-human HMGB-1 antibody was purchased from Cell Signaling
Technology, Europe, B.V. (Leiden, The Netherlands; cat. no. 6893).
Mouse anti-p-NF-κB p65 was purchased from Santa Cruz Biotechnology,
Inc. (Dallas, TX, USA; cat. no. sc-166748). Rabbit anti-GAPDH (cat.
no. ab181602) and Lamin B1 (cat. no. ab133741) were purchased from
Abcam (Cambridge, UK). Horseradish peroxidase-labeled anti-mouse
(cat. no. 115-035-206) and anti-rabbit (cat. no. 323-005-021)
secondary antibodies were purchased from Jackson ImmunoResearch
Laboratories, Inc. (West Grove, PA, USA). Transwell chambers were
purchased from EMD Millipore (Billerica, MA, USA; cat. no.
PSET010R1). The pLUC Luciferase vector was purchased from Ambion;
Thermo Fisher Scientific, Inc. The Dual-Luciferase®
Reporter Assay system was purchased from Promega Corporation
(Madison, WI, USA; cat. no. E1910). The tumor necrosis factor α
(TNF-α; cat. no. ELH-TNFa-1), interleukin 6 (IL-6; cat. no.
ELH-IL6-1) and HMGB-1 (cat. no. ELH-HMGB1-1) ELISA detection kits
were purchased from RayBiotech (Norcross, GA USA). Human
recombinant M-CSF was purchased from R&D Systems, Inc.
(Minneapolis, MN, USA; cat. no. 216-MC-025). Mouse anti-human CD68
was purchased from BD Pharmingen (BD Biosciences, Biosciences,
Franklin Lakes, NJ, USA; cat. no. 562117). The BCA protein
quantification kit was purchased from Beyotime Institute of
Biotechnology (Haimen, China; cat. no. P0010).
Clinical data
A total of 39 patients with sepsis were admitted to
the ICU of our hospital between July 2015 and April 2016. All
patients met the American College of Chest Physicians/Society of
Critical Care Medicine diagnostic criteria for sepsis (1992)
(10). These criteria include
definite or suspected infection, and (i) Body temperature >38°C
or <36°C; (ii) Heart rate >90 beats/min; (iii) Breathing
>20 times/min or pCO2 <32 mmHg; (iii) White blood
cell count >12×109/l or <4×109/l, or a
proportion of immature rod-shaped cells >10%. Among the 39 cases
of sepsis, 22 were male and 17 were female, aged 33–65 years (mean
age 43.9±12.6 years). A total of 32 healthy subjects without signs
of infection included 19 males and 13 females, aged 35–64 years
(mean age, 44.6±11.8 years). There was no significant difference in
age or sex ratio between the healthy and septic patients. All
specimens were collected with the informed consent of all
participants. The present study was reviewed and approved by Hefei
Second People's Hospital Biomedical Ethics Committee (Hefei,
China).
A total of 4 ml peripheral venous blood was
collected from patients with sepsis after admission, or from
healthy subjects in a fasting state, of which 2 ml was used to
isolate peripheral blood mononuclear cells (PBMCs), and 2 ml was
used to isolate serum. For serum isolation, blood was kept at room
temperature for 1 h, then centrifuged for 10 min at 800 × g (21°C).
The separated upper layer was then collected and stored at −80°C
until further use.
Isolation of PBMCs
A total of 2 ml blood was diluted in 2 ml PBS, and
slowly dripped onto the surface of 5 ml Ficoll-Paque Plus. The
mixture was centrifuged for 30 min at 300 × g at room temperature,
then the cells in the upper white layer were transferred into
another 15 ml centrifuge tube. PBS was added at a volume of 3:1
(PBS: cell solution) and mixed gently. The mixture was centrifuged
for 5 min at 200 × g, the supernatant was discarded, and the cell
pellet was resuspended in 1 ml ice-cold 1X PBS to obtain a PMBC
cell precipitate.
Induced differentiation of
macrophages
PBMCs were placed in α-MEM medium containing 10% FBS
and 1% streptomycin and cultured at 37°C in 5% CO2.
After 24 h, the culture supernatant was collected and non-adherent
cells were removed. Medium containing 30 ng/ml M-CSF, 10% FBS and
1% streptomycin was replenished every 72 h for 1 week prior to
detection of the macrophage marker, cluster of differentiation 68
(CD68).
Flow cytometry analysis
Samples were prepared using a PrimeScript™ RT
reagent kit according to the manufactures' instructions, (Takara
Biotechnology Co., Ltd). The phenotype of macrophages was analyzed
using an intracellular CD68 assay (cat. no. Y1/82A; BD
Biosciences). Cells were fixed with 4% paraformaldehyde (room
temperature, 20 min), permeabilized by FACS permeabilizing solution
(BD PharMingen, San Diego, CA) and then stained with the
aforementioned reagents for 30 min at room temperature. Flow
cytometry analysis was performed using a FACS Aria II flow
cytometer (BD Biosciences). Data were analyzed with FlowJo software
(version 7.6.1; FlowJo LLC, Ashland, OR, USA).
Construction of luciferase reporter
gene vector and dual-luciferase reporter gene assay
The 293 cell genome was used as a template to
amplify the HMGB1 3′-UTR full length fragment, or
mutation-containing fragments, then cloned into the pLUC vector to
transform DH5α competent cells (Thermo Fisher Scientific, Inc.).
The efficient plasmids were sequenced, screened and named
pLUC-HMGB1-3′-UTR-wt and pLUC-HMGB1-3′-UTR-mut. The
pLUC-HMGB1-3′-UTR-wt or pLUC-HMGB1-3′-UTR-mut and miR-25 mimics
were co-transfected into 293 cells (Thermo Fisher Scientific, Inc.)
using Lipofectamine® 2000, according to the
manufacturer's instructions. Luciferase activity was detected after
48 h. Firefly and Renilla luciferase activities were measured for
each sample using the Dual-Luciferase® Reporter Assay
System (cat. no. E1960; Promega Corporation).
Macrophage transfection and LPS
treatment
The microRNA.org
online target gene prediction tool was used to predict the relation
between miR-25 and the 3′-UTR of HMGB1 mRNA (11). Therefore, the successfully
differentiated macrophages were divided into 5 groups: miR-negative
control (NC) transfection group, miR-25 mimic transfection group,
short interfering RNA (si)-NC transfection group, si-HMGB1 group,
and miR-25 mimic+si-HMGB1 group. The siRNA sequences are as
follows: si-HMGB1 sense strand, 5′-CGGCCUCUGUUUGAUUUCUTT-3′,
si-HMGB1 antisense strand, 5′-AGAAAUCAAACAGAGGCCGTT-3′; si-NC sense
strand, 5′-UUCUCCGAACGUGUCACGUTT-3′, si-NC antisense strand,
5′-ACGUGACACGUUCGGAGAATT-3′; miR-25 mimic sense strand,
5-CAUUGCACUUGUCUCGGUCUGA-3; miR-25 mimic antisense strand,
5-UUCAAGUAAUCCAGGAUAGGCU-3; control sense strand,
5-UCACAAGUCAGGCUCUUGGGAC-3, control antisense strand,
5-ACGGGUUAGGCUCUUGGGAGCU-3. Lipofectamine® 2000 was used
to transfect the cells (5×104 cell/well, 50 nM si-HMGB1
or si-NC in each well) according to the manufacturer's protocol. A
total of 72 h after the transfection, cells were sequenced to
confirm transfection and used for subsequent experiments. Cells in
all transfection groups were treated with 100 ng/ml LPS for 48 h
prior to collection of cells and culture supernatants.
Detection of gene expression by
reverse transcription- quantitative polymerase chain reaction
(RT-qPCR)
Total RNA was isolated using TRIzol, according to
the manufacturer's instructions, following extraction with 99%
chloroform for 2 min at room temperature, precipitation with 95%
isopropanol for 10 min at room temperature, and washing with 75%
ethanol followed by centrifuging at 7,500 × g for 5 min at 4°C,
then hydrolysis with RNAse free H2O. cDNA was obtained
using the PrimeScript™ RT reagent kit, according to the
manufacturer's instructions, and the resulting cDNAs were stored in
a −20°C refrigerator. PCR was performed using Taq DNA polymerase
from the SYBR Green Real-Time PCR Master mix and the following
primers: miR-25, forward, 5′-CGGCGGCATTGCACTTGGTCTC-3′, reverse,
5′-GTGCAGGGTCCGAGGT-3′; U6, forward, 5′-ATTGGAACGATACAGAGAAGATT-3′,
reverse, 5′-GGAACGCTTCACGAATTTG-3′; HMGB1, forward,
5′-TATGGCAAAAGCGGACAAGG-3′, reverse, 5′-CTTCGCAACATCACCAATGGA-3′;
GAPDH, forward, 5′-ACAACTTTGGTATCGTGGAAGG-3′, reverse,
5′-GCCATCACGCCACAGTTTC-3′. The PCR reaction contained 5.0 µl 2X
SYBR Green Mixture, 0.5 µl 2.5 µm/l forward primer, 0.5 µ of 2.5
µm/l reverse primer, 1 µl cDNA and ddH2O to make the
volume up to 10 µl. The thermocycling conditions were as follows:
40 cycles of 95°C for 15 sec, 60°C for 30 sec and 74°C for 30 sec
in an ABI 7500-type fluorescence quantitative PCR system (Thermo
Fisher Scientific, Inc.). Results were expressed by using the
2−ΔΔCq method (12), where
the number of cycles (Ct) at which the fluorescence signal exceeded
a defined threshold for GAPDH was subtracted from Ct values for
target genes (Cttargets-CtGAPDH=ΔCP), and
values were calculated as 2−ΔCP and normalized to each
other.
Western blotting
Protein was extracted using radioimmunoprecipitation
assay buffer (Thermo Fisher Scientific, Inc.). The protein
concentration was determined using a BCA protein quantification
kit, according to the manufacturer's protocol. A total of 50 µg of
protein was loaded for separation by 8% SDS-PAGE, then transferred
into polyvinylidene difluoride membranes by electroporation for 60
min. The membranes were blocked with 5% skimmed milk for 60 min at
room temperature, followed by incubation with the primary
antibodies (HMGB1, phosphorylated (p-)p65, GAPDH and Lamin B1
antibodies were used at dilutions of were 1:100, 1:100, 1:800 and
1:300, respectively) overnight at 4°C, then washed 3 times
PBS-Tween (PBST) prior to incubation with the HRP-labeled
anti-mouse or anti-rabbit secondary antibodies (dilution, 1:8,000
dilution) for 60 min at room temperature. The membranes were washed
3 times in PBST, and visualized using an enhanced chemiluminescent
system (Thermo Fisher Scientific, Inc.).
Detection of inflammatory cytokine
content by ELISA assay
All ELISA procedures strictly followed the protocols
provided by the ELISA kit manufacturer.
Detection of macrophage migration
ability by Transwell assay
Collagen IV added to the upper chamber of Transwell
inserts with 8-µm pore size (Corning Incorporated, Corning, NY,
USA). Macrophages were plated into the upper chamber
(5×105) and medium (α-MEM without FBS) containing 100
ng/ml LPS was added to the lower chamber. After 48 h of culture,
the culture solution was discarded, and the insert was washed twice
with PBS, and fixed with methanol for 30 min at room temperature,
stained by 0.1% crystal violet for 20 min at room temperature. The
number of migratory cells was counted under a light microscope at
×400 magnification.
Statistical analysis
All statistical analysis was performed using SPSS
ver. 18.0 (SPSS, Inc., Chicago, IL, USA). Data are expressed as
mean ± standard deviation. Differences between 2 groups were
compared using Student's t-test. One-way analysis of variance was
used to compare differences between multiple groups, followed by
Bonferroni correction. Pearson correlation coefficient analysis was
used to observe the relation between miR-25 level in serum and
sepsis. P<0.05 was considered to indicate a statistically
significant difference.
Results
The expression of HMGB1 is elevated
and the expression of miR-25 is decreased significantly in patients
with sepsis
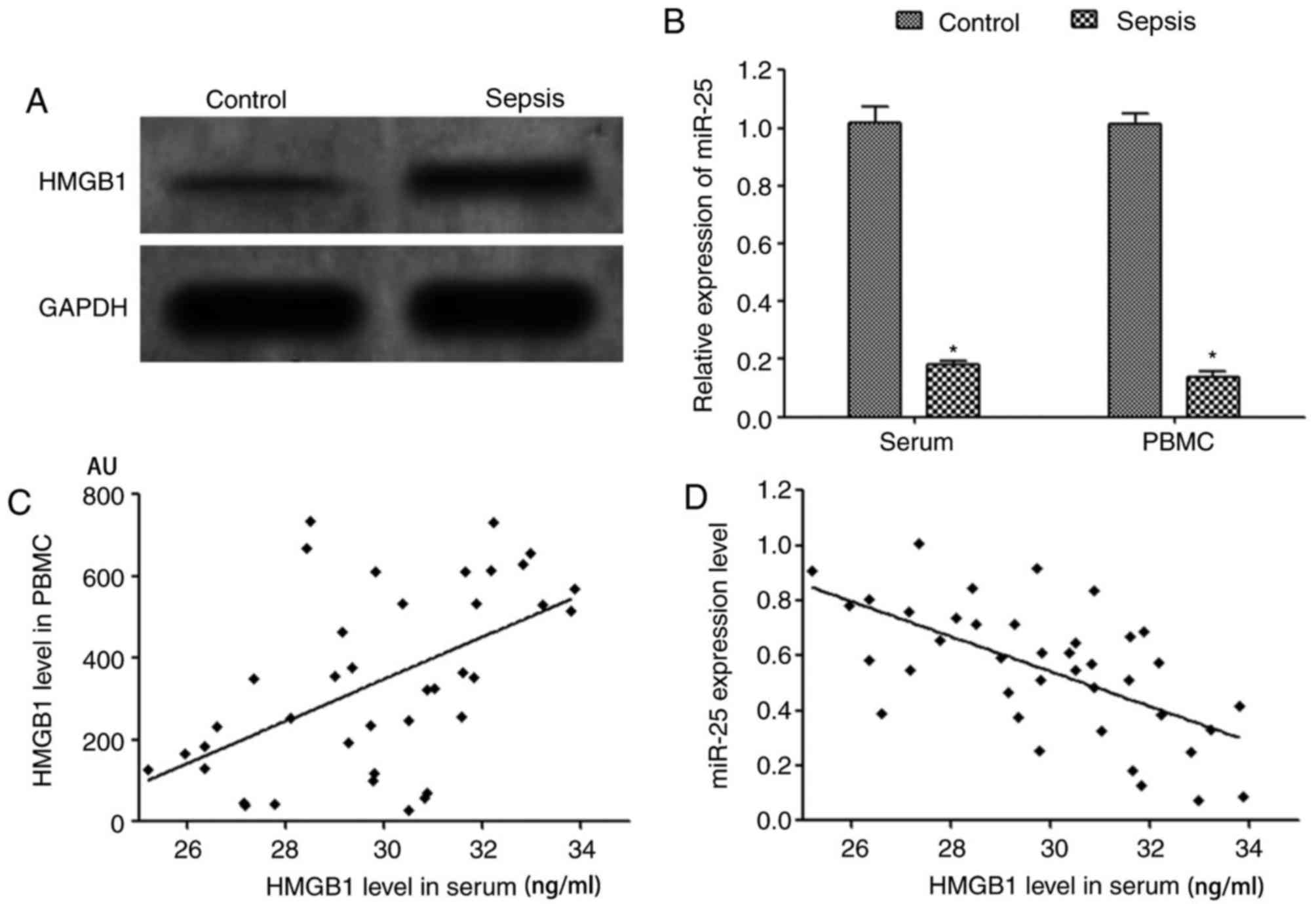
ELISA assay results demonstrated that the level of
HMGB1 in the serum of peripheral blood in patients with sepsis was
significantly increased compared with the healthy control group
(Table I). Western blotting results
revealed that the expression level of HMGB1 protein the PBMCs of
patients with sepsis was significantly increased compared with that
of healthy controls (Fig. 1A).
RT-qPCR demonstrated that the expression of miR-25 in the serum and
PBMCs of patients with sepsis was significantly decreased compared
with that of healthy controls (Fig.
1B). Pearson correlation coefficient analysis demonstrated that
the expression of miR-25 in serum of patients with sepsis was
negatively correlated that of HMGB1 (r=−0.591, P=0.041). A
significant positive correlation was identified between serum HMGB1
levels and HMGB1 protein expression in PBMCs (r=0.537, P<0.001),
and a significant negative correlation was identified between serum
miR-25 expression and HMGB1 protein content (r=−0.622, P<0.001),
as presented in Fig. 1C and D.
 | Table I.HMGB1 content of serum in the two
groups. |
Table I.
HMGB1 content of serum in the two
groups.
| Group | Number | HMGB1 (ng/ml) |
|---|
| Healthy controls | 32 |
2.17±0.23 |
| Patients with
sepsis | 39 |
29.61±4.52a |
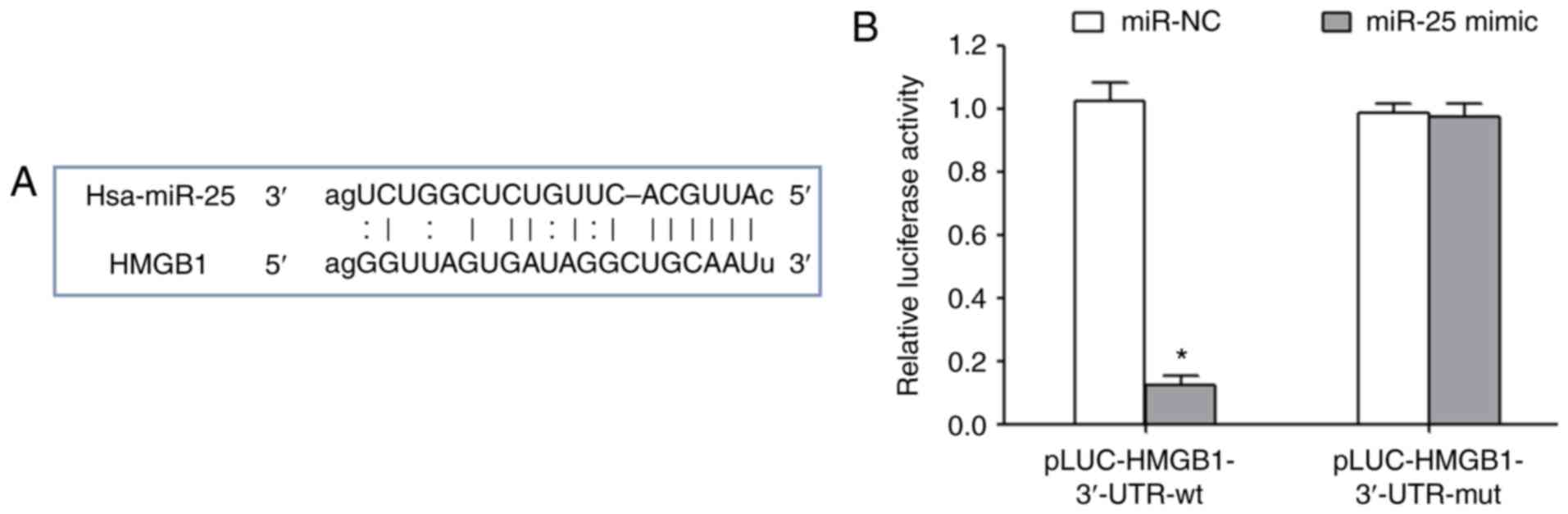
miR-25 inhibits HMGB1 expression
MicroRNA.org online target gene
prediction results indicated that there was a targeted binding site
between miR-25 and the 3′-UTR of HMGB1 mRNA (Fig. 2A). Transfection of miR-25 mimics
significantly reduced the relative luciferase activity, suggesting
that miR-25 could target the 3′-UTR region of HMGB1 and inhibit its
expression (Fig. 2B).
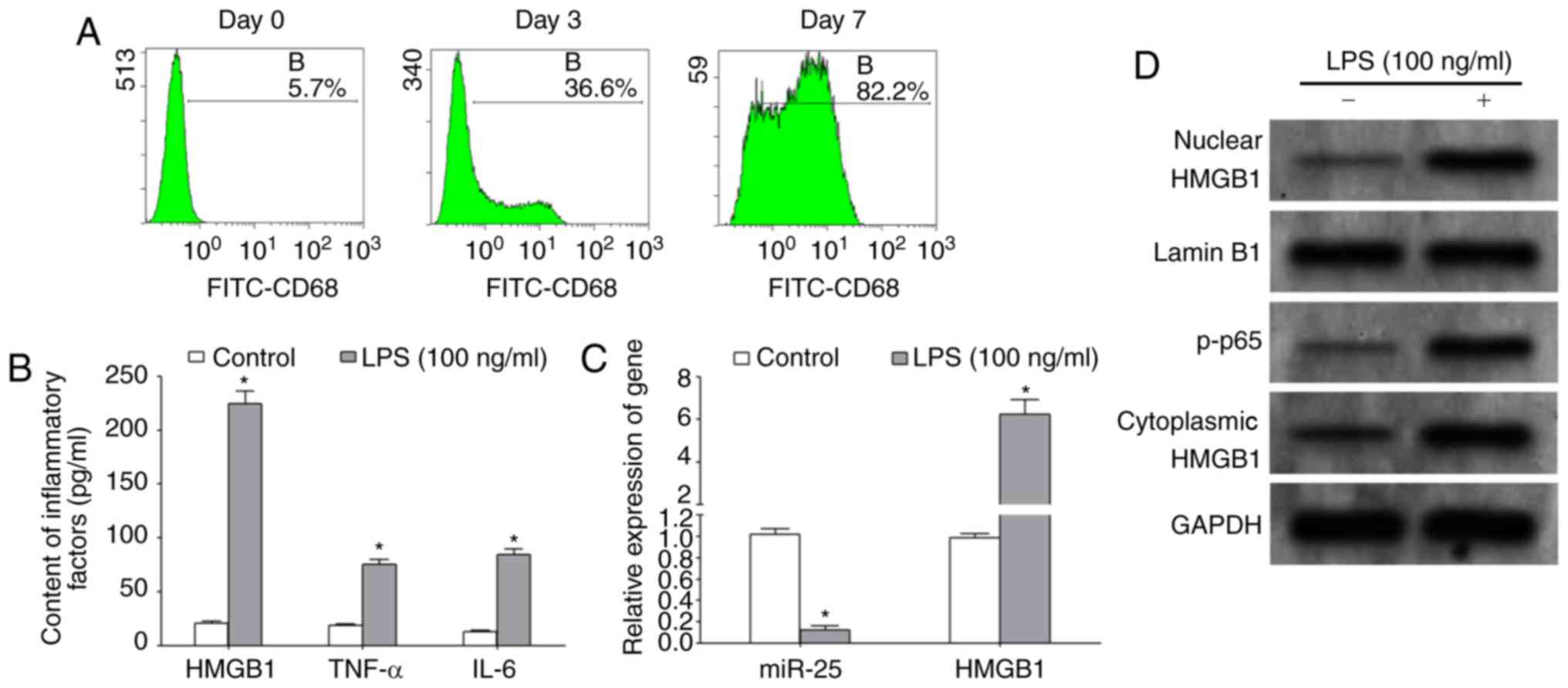
LPS induces macrophages to secrete
HMGB1 and inhibit miR-25 expression
The results of flow cytometry demonstrated that the
macrophage-specific marker, CD68, was increased by >80%
following a 7-day induction with M-CSF, indicating that the
differentiation of macrophages was induced successfully, allowing
subsequent experiments to be performed (Fig. 3A). ELISA assays demonstrated that,
following treatment with 100 ng/ml LPS for 48 h, the protein levels
of HMGB-1, TNF-α and IL-6 in the culture supernatant were
significantly increased (Fig. 3B).
The results of RT-qPCR demonstrated that LPS treatment
significantly upregulated the expression of HMGB1 mRNA and
significantly decreased the expression of miR-25 in macrophages
(Fig. 3C). Western blotting results
revealed that LPS treatment significantly enhanced the
transcriptional activity of NF-κB and upregulated the expression of
HMGB1 protein in the nucleus and cytoplasm of macrophages (Fig. 3D).
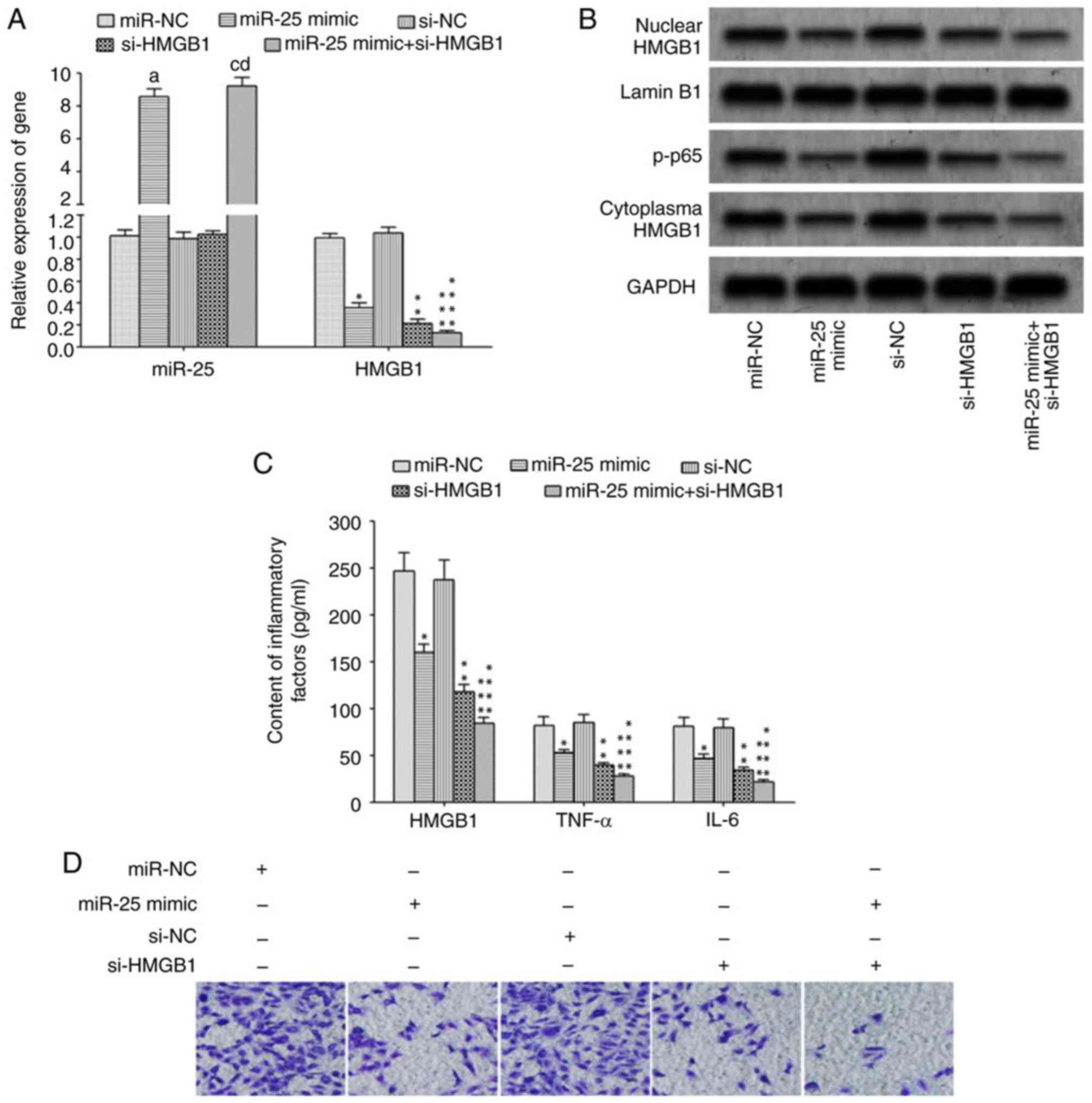
Elevated miR-25 could inhibit HMGB1
expression and migration in macrophages
The transfection of miR-25 mimics and/or si-HMGB1
significantly reduced HMGB1 mRNA expression (Fig. 4A) and attenuated protein expression in
macrophages (Fig. 4B), resulting in a
reduction in p-p65 protein expression (Fig. 4B). In addition, the levels of
inflammatory cytokines, HMGB1, TNF-α and IL-6, were significantly
decreased in culture supernatant (Fig.
4C), and the migration ability of macrophages was also
significantly attenuated (Fig. 4D) in
cells transfected with si-HMGB1 or miR-25 mimic + si-HMGB1 compared
with control.
Discussion
The causes of sepsis are diverse, the disease has a
complicated pathology and manifests as an acute and critical
condition (13). A large number of
pathogenic toxins activate the human immune system to produce
cytokines and inflammatory responses, leading to irreversible and
serious damage to cells, tissues and organs, and the immune system
(14). If sepsis is not treated in a
timely and effective manner, it is likely to develop into septic
shock, acute respiratory distress syndrome and multiple organ
dysfunction syndrome (15). Although
modern medical techniques have greatly improved, the mortality rate
of patients with sepsis remains high, and is the leading cause of
mortality in intensive care (16).
Therefore, it is of great importance to be able to diagnose sepsis
early, and make timely and effective interventions, so as to delay
the progression of disease and reduce the mortality rate of
patients with sepsis.
HMG (high mobility group protein) is a non-histone
chromatin-associated protein family, comprising HMGB1, HMGB2 and
HMGB3 (17). HMGB1 is the most
abundant and highly conserved HMG protein (18). It functions in a variety of biological
processes, including DNA replication, DNA damage repair,
transcription and translation (19).
It has been revealed that HMGB1 is an important inflammatory
cytokine, with an important role in mediation of inflammatory
responses (20). A number of studies
have demonstrated that HMGB1 serves a critical role in the
initiation of an inflammatory signaling cascade in sepsis, and that
it is the most important pro-inflammatory cytokine of the lethal
effect of sepsis (21). Compared with
inflammatory cytokines involved in early sepsis, including TNF-α,
HMGB1 is characterized by late elevated expression for a prolonged
duration, providing a wide window of time for the clinical
treatment of sepsis (22). As an
innate immune cell, macrophages serve a crucial role in fighting
against pathogenic microorganisms and initiating an immune response
(23). During sepsis, macrophages
synthesize and secrete HMGB1, which can promote the induction and
amplification of inflammatory cytokines through positive feedback,
and exacerbate and prolong the body inflammatory response process
(24). As important epigenetic
regulatory molecules, miRs serve an important role in immune cell
proliferation, differentiation, activation, release of inflammatory
cytokines and regulation of immune responses. The abnormal
expression of various miRs has been associated with the occurrence
of sepsis, and may be used as an important diagnostic and
prognostic marker of sepsis (25).
Studies have demonstrated that patients with sepsis exhibit
abnormal expression of miR-25 in their peripheral blood (1,2). In the
present study, bioinformatics analysis revealed a target binding
site between miR-25 and HMGB1 3′-UTR. It was also investigated
whether miR-25 serves a role in the targeted regulation of HMGB1
expression and release of macrophage inflammatory cytokines.
In the present study, it was demonstrated that the
serum HMGB1 content and the expression of HMGB1 protein in PBMCs of
patients with sepsis were significantly increased compared with
those of healthy controls. RT-qPCR demonstrated that the expression
of miR-25 in the serum and PBMCs of patients with sepsis was
significantly decreased compared with that of healthy controls.
These results suggest that the decreased expression of miR-25 may
be associated with the increased expression of HMGB1 and the
occurrence of sepsis. Studies by Huang et al (3) also indicated that the serum level of
HMGB1 in patients with sepsis was significantly elevated. Karlsson
et al (26) demonstrated that
increased HMGB1 of patients with sepsis was higher than that of
healthy controls by over 6 times. Yao et al (25) demonstrated that expression of miR-25
in the peripheral blood of patients with sepsis was significantly
decreased compared with healthy controls, and that the lower the
expression of miR-25, the lower the 28-day survival rate of
patients. Dual luciferase reporter assays demonstrated that
transfection of miR-25 mimics significantly reduced the relative
luciferase activity in 293 cells, indicating that miR-25 could
target the 3′-UTR region of HMGB1 and inhibit its expression.
The NF-κB signal transduction serves a key role in
the initiation of the immune response and the release of
inflammatory cytokines, as well as the occurrence of sepsis.
Toll-like receptors (TLRs) are an important family of receptors of
HMGB1. Toll-like receptors such as TLR2 and 4, which are expressed
on the surface of macrophages, interact with HMGB1 and activate the
NF-κB signaling pathway, to promote the transcription, synthesis
and secretion of inflammatory cytokines, including TNF-α, IL-1,
IL-6, and chemokines (27,28). In the present study, LPS treatment
significantly upregulated the expression of HMGB1 in macrophages,
enhanced the transcriptional activity of NF-κB and the release of
TNF-α and IL-6, and inhibited the expression of miR-25. Zhang
(24) indicated that LPS treatment
significantly induced the synthesis and secretion of HMGB1 by
macrophages. Furthermore, Zhou et al (8) demonstrated that LPS treatment
significantly increased the HMGB1 expression in macrophages. In
consistence with these results, the present study also demonstrated
that LPS treatment significantly upregulated the expression of
HMGB1 in macrophages. Transfection with miR-25 mimics and/or
si-HMGB1 significantly decreased the expression of HMGB1 in
macrophages, reduced the transcriptional activity of the NF-κB
signaling pathway, and reduced the HMGB1, TNF-α and IL-6 levels in
the culture supernatant. Decreased expression of miR-25 was
associated with the abnormal expression of HMGB1 following LPS
treatment. Elevated expression of miR-25 weakened the induction
effect of LPS on HMGB1, and attenuated the transcriptional activity
of NF-κB and the transcriptional activation of downstream
inflammatory cytokines, TNF-α and IL-6. Transfection with miR-25
mimics and/or si-HMGB1 also attenuated the migratory ability of
macrophages, which may be associated with decreased activation of
the NF-κB signaling pathway following reduction of HMGB1
expression. Furthermore, HMGB1 may bind C-X-C motif chemokine
ligand 12 and activate chemokine receptor C-X-C motif chemokine
receptor 4 to induce the proliferation and migration of immune
cells, thus amplifying the inflammatory response. However, this
hypothesis requires further investigation.
To conclude, the present study demonstrated that
miR-25 attenuated the induction of HMGB1 via LPS, decreased the
transcriptional activity of NF-κB and the transcriptional
activation of downstream inflammatory cytokines, TNF-α and IL-6,
and also suppressed macrophage migration.
References
|
1
|
Caserta S, Kern F, Cohen J, Drage S,
Newbury SF and Llewelyn MJ: Circulating plasma microRNAs can
differentiate human sepsis and systemic inflammatory response
syndrome (SIRS). Sci Rep. 6:280062016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Wang H, Ward MF and Sama AE: Targeting
HMGB1 in the treatment of sepsis. Expert Opin Ther Targets.
18:257–268. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Huang W, Tang Y and Li L: HMGB1, a potent
proinflammatory cytokine in sepsis. Cytokine. 51:119–126. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Huston JM, Wang H, Ochani M, Ochani K,
Rosas-Ballina M, Gallowitsch-Puerta M, Ashok M, Yang L, Tracey KJ
and Yang H: Splenectomy protects against sepsis lethality and
reduces serum HMGB1 levels. J Immunol. 181:3535–3539. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Wang SY, Li ZJ, Wang X, Li WF and Lin ZF:
Effect of ulinastatin on HMGB1 expression in rats with acute lung
injury induced by sepsis. Genet Mol Res. 14:4344–4353. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Bulun SE and Nezhat C: Aromatase,
microRNA, and inflammation: A complex relationship. Fertil Steril.
106:552–553. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Wang X, Guo Y, Wang C, Yu X and Yu H:
MicroRNA-142-3p inhibits chondrocyte apoptosis and inflammation in
osteoarthritis by targeting HMGB1. Inflammation. 39:1718–1728.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Zhou W, Wang J, Li Z, Li J and Sang M:
MicroRNA-2055b inhibits HMGB1 expression in LPS-induced sepsis. Int
J Mol Med. 38:312–318. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Benz F, Roy S, Trautwein C, Roderburg C
and Luedde T: Circulating microRNAs as biomarkers for sepsis. Int J
Mol Sci. 17:782016. View Article : Google Scholar
|
|
10
|
Weiss M, Huber-Lang M, Taenzer M, Traeger
K, Altherr J, Kron M, Hay B and Schneider M: Different patient case
mix by applying the 2003 SCCM/ESICM/ACCP/ATS/SIS sepsis definitions
instead of the 1992 ACCP/SCCM sepsis definitions in surgical
patients: A retrospective observational study. BMC Med Inform Decis
Mak. 9:252009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Ritchie W, Rasko JE and Flamant S:
MicroRNA target prediction and validation. Adv Exp Med Biol.
774:39–53. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2-ΔΔCT method. Methods. 25:402–408. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Boyd JH, Russell JA and Fjell CD: The
meta-genome of sepsis: Host genetics, pathogens and the acute
immune response. J Innate Immun. 6:272–283. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Czura CJ, Yang H, Amella CA and Tracey KJ:
HMGB1 in the immunology of sepsis (not septic shock) and arthritis.
Adv Immunol. 84:181–200. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Lin SM, Chung FT, Kuo CH, Chou PC, Wang
TY, Chang PJ, Lo YL, Huang CD, Lin HC, Wang CH and Kuo HP:
Circulating angiopopietin-1 correlates with the clinical course of
multiple organ dysfunction syndrome and mortality in patients with
severe sepsis. Medicine (Baltimore). 94:e8782015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Guirgis FW, Khadpe JD, Kuntz GM, Wears RL,
Kalynych CJ and Jones AE: Persistent organ dysfunction after severe
sepsis: A systematic review. J Crit Care. 29:320–326. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Chavan SS, Huerta PT, Robbiati S,
Valdes-Ferrer SI, Ochani M, Dancho M, Frankfurt M, Volpe BT, Tracey
KJ and Diamond B: HMGB1 mediates cognitive impairment in sepsis
survivors. Mol Med. 18:930–937. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Gentile LF and Moldawer LL: HMGB1 as a
therapeutic target for sepsis: It's all in the timing! Expert Opin
Ther Targets. 18:1–245. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Shao J, Zhao M, Tong M, Wei J, Wise MR,
Stone P, Chamley L and Chen Q: Increased levels of HMGB1 in
trophoblastic debris may contribute to preeclampsia. Reproduction.
152:775–784. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Chang KC, Ko YS, Kim HJ, Nam DY and Lee
DU: 13-Methylberberine reduces HMGB1 release in LPS-activated
RAW264.7 cells and increases the survival of septic mice through
AMPK/P38 MAPK activation. Int Immunopharmacol. 40:269–276. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zhao H, Liu Z, Liu W, Han X and Zhao M:
Betulin attenuates lung and liver injuries in sepsis. Int
Immunopharmacol. 30:50–56. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Gil M, Kim YK, Hong SB and Lee KJ:
Naringin decreases TNF-α and HMGB1 release from LPS-stimulated
macrophages and improves survival in a CLP-induced sepsis mice.
PLoS One. 11:e01641862016. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Kumar V: Targeting macrophage
immunometabolism: Dawn in the darkness of sepsis. Int
Immunopharmacol. 58:173–185. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Zhang Z, Zhang L, Zhou C and Wu H:
Ketamine inhibits LPS-induced HGMB1 release in vitro and in vivo.
Int Immunopharmacol. 23:14–26. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Yao L, Liu Z, Zhu J, Li B, Chai C and Tian
Y: Clinical evaluation of circulating microRNA-25 level change in
sepsis and its potential relationship with oxidative stress. Int J
Clin Exp Pathol. 8:7675–7684. 2015.PubMed/NCBI
|
|
26
|
Karlsson S, Pettila V, Tenhunen J,
Laru-Sompa R, Hynninen M and Ruokonen E: HMGB1 as a predictor of
organ dysfunction and outcome in patients with severe sepsis.
Intensive Care Med. 34:1046–1053. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Chen XL, Sun L, Guo F, Wang F, Liu S,
Liang X, Wang RS, Wang YJ and Sun YX: High-mobility group box-1
induces proinflammatory cytokines production of Kupffer cells
through TLRs-dependent signaling pathway after burn injury. PLoS
One. 7:e506682012. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Mudaliar H, Pollock C, Komala MG, Chadban
S, Wu H and Panchapakesan U: The role of toll-like receptor
proteins (TLR) 2 and 4 in mediating inflammation in proximal
tubules. Am J Physiol Renal Physiol. 305:F143–F154. 2013.
View Article : Google Scholar : PubMed/NCBI
|