Introduction
Morbidity rate and mortality rate of gastric cancer
are the highest for digestive tract tumors globally, and the
results indicate a younger trend (1,2). Drug
therapy is the main treatment option for intra-abdominal planting
transfer of gastric cancer; however, thus far the curative effect
is still not sufficient. Dextran sulfate (DS) is a macromolecule
Dextran with a molecular weight of 500,000. Previous studies have
demonstrated that DS can inhibit cancer cells adhesion, cell cycle
progression and gene expression (3,4), DS can
reduce of expression of gastric cancer cells in terms of vascular
endothelial growth factor (VEGF) and integrin β1 in vivo and
in vitro, and there was a significant inhibitory effect on
gastric cancer cell adhesion and angiogenesis (5), but the inhibiting mechanism of DS on the
metastasis of gastric cancer still requires investigating.
Peritoneal transfer of gastric cancer is a
multi-factor, multi-phase continuous complex process. In this
process, epithelial-mesenchymal transformation (EMT) serves a key
role in promoting the invasion and metastasis of tumor cells
(6). EMT mechanism is a complicated
process, and hypoxia is one of the most important initiating
factors to EMT (7), In a hypoxic
microenvironment, cancer cells adapt to the hypoxic
microenvironment through highly expressing hypoxia-inducible factor
1α (HIF-1α) (8), HIF-1α high
expression then promote: i) EMT markers, including Twist, E-cad and
N-cad; ii) the regulation of angiogenesis, including vascular
endothelial growth factor (VEGF); iii) cell adhesion, including
ITGβ1 (9,10). At present, the examination of EMT,
invasion and migration mechanisms is the notable basis for the
development in the diagnosis and treatment of gastric cancer, and
additionally helps to provide potential intervention targets for
the treatment of a malignant tumor (11). The present study investigated the
inhibiting effect of DS on EMT by measuring the expression of EMT
associated factors [Twist, E-cadherin (E-cad), N-cadherin (N-cad)
and β-catenin], proliferation, invasion and migration of 4 types of
different degree of differentiate gastric cancer cells in the
process of peritoneal metastasis, and then suggested a theoretical
basis for discovering a potential drug in the treatment of
peritoneal metastasis of gastric cancer.
Materials and methods
Gastric cancer cell lines
Poorly differentiation gastric cancer cells
(BGC-823) were purchased from Cobioer; Nanjing Kesheng
Biotechnology Co., Ltd. (Nanjing, China) High differentiation
gastric cancer cells AGS, undifferentiated gastric cancer cells
MGC-803, lymph node metastasis gastric cancer cells SGC-7901 and
normal gastric mucosa epithelial cells GES-1, were donated from The
Life Science Laboratory of Shanghai East China Normal University
(Shanghai, China).
The experimental drugs and
antibodies
DS, with a molecular weight ~500,000, was purchased
from Sigma-Aldrich (Merck KGaA, Darmstadt, Germany). A rabbit
anti-human anti-HIF-1α monoclonal antibody (Wuhan Sanying
Biotechnology, Wuhan, China; cat. no. 20960-1-AP; dilution,
1:1,500), rabbit anti-human E-cad Polyclonal Antibody (cat. no.
E-AB-31261; dilution, 1:200) and rabbit anti-human N-cad Polyclonal
Antibody (cat. no. E-AB-32170; dilution, 1:1,000), were purchased
from the Elabscience Biotechnology Co., Ltd., Wuhan, China.
Anti-Twist antibody (cat. no. ab50581; dilution, 1:1,000) was
purchased from Abcam (Cambridge, UK). A goat anti-rabbit (cat. no.
BA1003; dilution 1:5,000) biotin-conjugated immunoglobulin IgG
(Wuhan Boster Biological Technology Co., Ltd.) was used to stain
the primary antibody.
Cell culture
A total of five types of cells were cultured in
complete medium (Beijing Zhongshan Jinqiao Biotechnology Co., Ltd.,
Beijing, China) in a sterile conditions at a constant temperature
of 37°C and 5% CO2 until the logarithmic phase. Then,
cells were placed into a 60 mm petri dish. The control group and
experimental group were exposed to the same amount of PBS and DS
(final concentration of 0.3%), respectively. Cells were collected
for inspection at six time points (0, 6, 12, 24, 36 and 48 h after
culture). BGC-823 cells were cultured in a hypoxia incubator at
37°C with 5% CO2 and 1% O2, and cells collected for
inspection at six time points (0, 6, 12, 24, 36 and 48 h after
culture). The absence of a positive control group is a limitation
of the present study.
Cell Counting kit-8 (CCK-8) assays to
detect cell proliferation
Adjusting the cell suspension density to
2.5×104 /ml in RPMI-1,640 medium (Hyclone; GE Healthcare
Life Sciences, Logan, UT, USA), cells were placed in six 96-well
plates at 200 µl/well, and the culture was placed in the incubator
at 37°C and 5% CO2 for ~12 h. When the cells attached to
the side of the well, the experimental group was treated with the
culture medium containing 0.3% DS; furthermore, the control group
was treated with the culture medium containing the same amount of
PBS. Following 0, 6, 12, 24, 36 and 48 h, 10 µl CCK-8 fluid was
added into each well and then placed back into the incubator to
develop for 4 h at 37°C and 5% CO2. The absorbance (OD)
value at 450 nm for each well was tested by using a microplate
reader. Finally, an average value of the five complex wells for
each group.
Transwell cell invasion and migration
assays
Matrigel was placed into the upper layer of the
Transwell chamber and following solidification, the cell density
was adjusted to 1×105/ml. A total of 200 µl cells in
serum-free medium (Hangzhou Sijiqing Biological Engineering
Materials Co., Ltd., Hangzhou, China) were placed into the upper
chamber of an insert. Then, 500 µl medium containing FBS and DS was
added to the lower chamber of the experimental group, and 500 µl
medium containing FBS and PBS was added to the lower chamber of the
control group. After incubation for 24 h at 37°C, cells that had
invaded and migrated through the membrane could be observed by
staining with 0.1% crystal violet staining solution for 20 min at
37°C, and 5–10 visual fields were taken for counting the average
number of the cells using an inverted microscope (Olympus
Corporation, Tokyo, Japan).
Cell immunofluorescence staining
Treated cells were fixed for 14 min with cold 4%
polyphosphate formaldehyde, and the fixed cells were permeabilized
with 0.1% Triton X-100 (Beijing Zhongshan Jinqiao Biotechnology
Co., Ltd.) for 30 min, 3 times, and then blocked with 10% goat
serum (Beijing Zhongshan Jinqiao Biotechnology Co., Ltd.), for 30
min. Following incubation overnight at 4°C with primary antibodies
(twist, E-cad, N-cad; dilution 1:100), FITC marked fluorescence
second antibody (1:200) was added to the cells. Following an
incubation at 37°C for 0.5 h, DAPI was added in the dark, the film
was sealed and observed using a fluorescence microscope with a
magnification of ×100. Cumulative density demonstrated the
expression quantity of the factors, which was analyzed by Student's
t-test using GraphPad Prism 7 plotting software (GraphPad Software,
Inc., La Jolla, CA, USA).
Detection of HIF-1α and EMT associated
factors mRNA using reverse transcription-polymerase chain reaction
(RT-PCR)
The primers used were: HIF-1α forward,
5′-GAAAGCGCAAGTCTTCAAAG-3′ and reverse, 5′-TGGGTAGGAGATGGAGATGC-3′;
Twist forward, 5′-AATTGGGATGCATTCGAGTCTGTAA-3′ and reverse,
5′-TTCTGTCCGATGTCACTGCTGTC-3′; E-cad forward,
5′-TACACTGCCCAGGAGCCAGA-3′ and reverse, 5′-TGGCACCAGTGTCCGGATTA-3′;
N-cad forward, 5′-ACCTGAACGACTGGGGGCCA-3′ and reverse,
5′-TGCCAAAGCCTCCAGCAAGCA-3′; and GAPDH (internal control) forward,
5′-CAAGGTCATCCATGACAACTTTG-3′ and reverse,
5′-GTCCACCACCCTGTTGCTGTAG-3′. The primers were synthesized by
Sangon Biotech Co., Ltd., Shanghai, China. Treated cells at the
time points of 2, 8, 12 and 24 h were lysed, and total RNA was
extracted and reverse transcribed by using Total mRNA extraction
kit (Omega Bio-Tek, Inc., Norcross, GA, USA) and reverse
transcription kit (Fermentas; Thermo Fisher Scientific, Inc.,
Waltham, MA, USA) following the manufacturer's protocol. Following
the denaturation of cDNA (2 µl) at 94°C for 3 min, temperature
cycling (30 cycles) was performed as follows: 94°C for 45 sec, 30
sec annealing step at 58°C (for GAPDH, Twist, E-cad and N-cad) and
a 30 sec elongation step at 72°C. Temperature cycling was concluded
with a final elongation step for 5 min at 72°C. This procedure was
followed by grey value analysis using Quantity one version 4.0
(Bio-Rad Laboratories), the purpose/internal relative grayscale
value indicated the corresponding mRNA expression level. The mRNA
expression level of the cells in the experimental group was
compared with the control group at the time points of 2, 8, 12 and
24 h, respectively.
Western blot detection of HIF-1α and
EMT associated factor protein expression. Cultured BGC823 cells
were washed by ice-cold PBS three times
Cell lysis solution (Nanjing KeyGen Biotech Co.,
Ltd, Nanjing, China) was added, the dish agitated for 30 min,
centrifuged at 12,000 × g at 4°C for 15 min and the supernatant
removed. Detection of protein concentration was conducted using BCA
protein quantitative kits. Protein extracts (20 µg) were separated
by 8–10% SDS polyacrylamide gel electrophoresis, then transferred
to nitrocellulose membranes and blocked by 10% skim milk for 1.5 h
at 4°C, then incubated by the corresponding antibody at 4°C
overnight (HIF-1α, 1:1,000; Twist, 1:500; E-cad, 1:200; N-cad,
1:1,000; and β-actin, 1:1,500), β-actin was used as an internal
control. The second antibody incubation was at room temperature for
1 h, followed by ECL chemiluminescence reagent chromogenic (cat.
no. MA02454; Thermo Fisher Scientific, Inc.) for 1 min and exposure
in an Amersham Imager 600 instrument (GE Healthcare Life Sciences,
Little Chalfont, UK). This was followed with grey value analysis
with Quantity one, the relative grayscale value indicating the
corresponding protein expression level.
Statistical analysis
All the experiments were performed in triplicate.
SPSS version 17.0 (SPSS, Inc., Chicago, IL, USA) was used to
perform the statistical analyses. Data are expressed as the mean ±
standard deviation. The comparison of two groups was performed
using the Students' t-test. One-way analysis of variance was used
in the comparison of multiple groups followed by Dunnett's post-hoc
test. P<0.05 was considered to indicate a statistically
significant difference.
Results
DS impact on the proliferation of five
types of cells
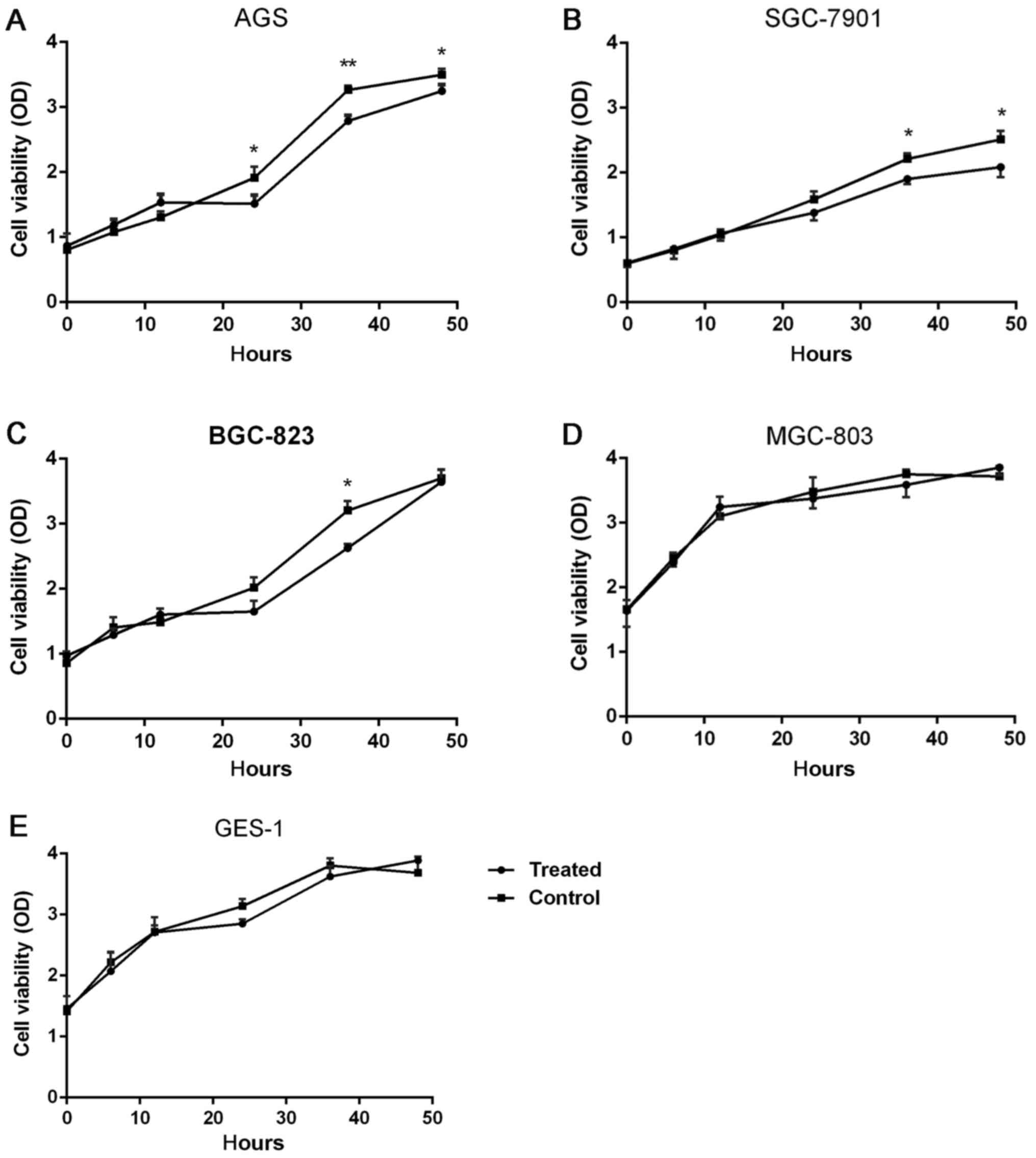
There were five types of cells examined at six time
points, 0, 6, 12, 24, 36 and 48 h. These results demonstrated that
for the AGS cells at 24 h (P=0.032, 21.1%), 36 h (P=0.0076, 15.4%)
and 48 h (P=0.041, 7.5%), the cell proliferation in the
experimental group was significantly reduced, compared with the
control group, as depicted in Fig.
1A. The proliferation of SGC-7901 cells was significantly
inhibited by DS, as depicted in Fig.
1B, at 36 h (P=0.036, 20%) and 48 h (P=0.027, 19.5%) For
BGC-823 cells, DS significantly reduced the proliferation of the
cells at 36 h, (P=0.043, 18.8%), as depicted in Fig. 1C. For MGC-803 and GES-1 cells, no
significant difference in cell proliferation was observed in the
experimental group and control group at 0, 6, 12, 24, 36 and 48 h,
as depicted in Fig. 1D and E.
DS inhibits the invasive ability and
migration ability of gastric cells
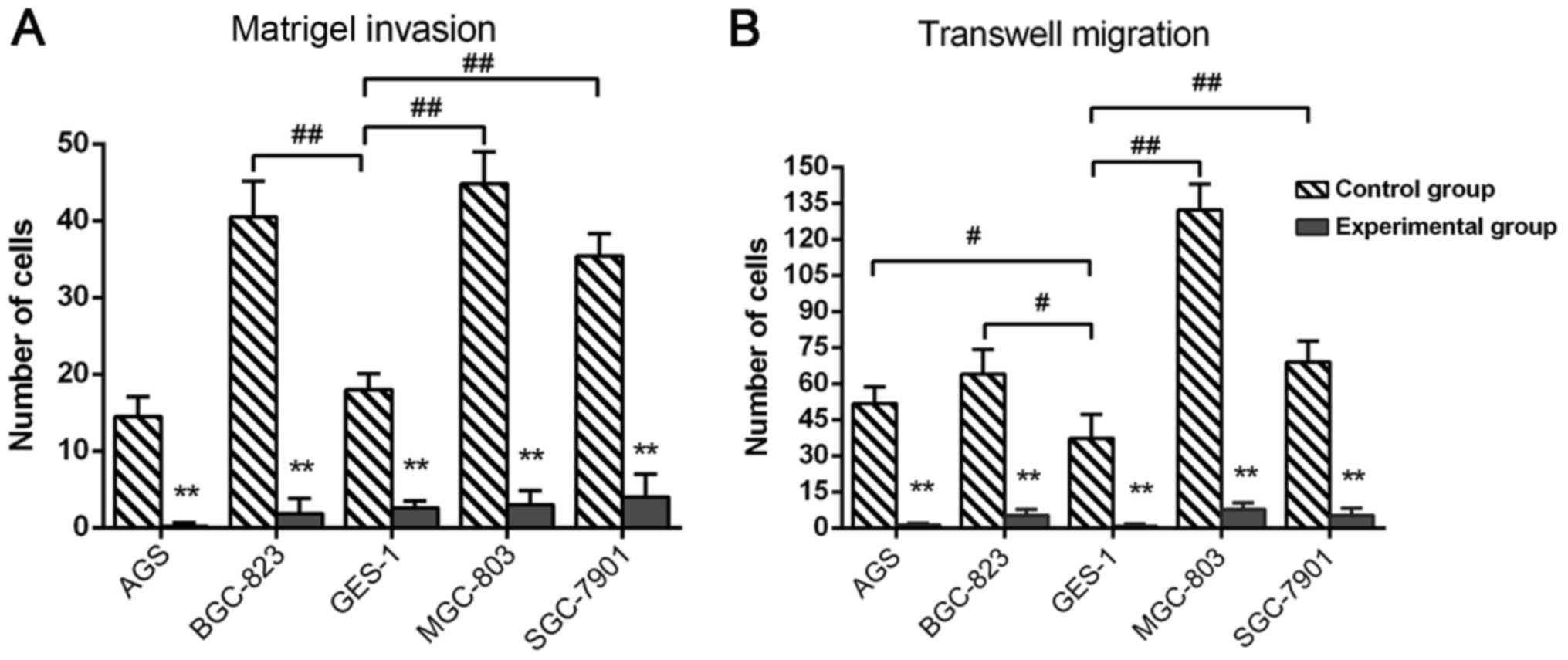
In the control group 24 h after growing on Matrigel
membrane, the number of the invaded cancer cells BGC-823
(P=0.0042), MGC-803 (P=0.0098) and SGC-7901 (P=0.0079) was
significantly increased compared with GES-1 cells, no significant
difference was observed between AGS and GES-1 cells; in the control
group, the average number of invaded cancer cells was highest in
the MGC-803 cell line, followed by BGC-823, SGC-7901 and AGS in
turn. Compared with the control group, the number of invasive
cancer cells in the experimental group was significantly reduced by
DS, and BGC-823 cells (inhibition rate 95%, P=0.0021) had the most
notable inhibition, followed by AGS (92.8%, P=0.0033), MGC-803
(90.1%, P=0.0042), SGC-7901 (87.8%, P=0.0057) and GES-1 cells
(72.3%, P=0.0076<0.01), as depicted in Fig. 2A. In the control group, following 24 h
growth on a Transwell membrane, the number of migrated gastric
cancer cell lines AGS (P=0.036), BGC-823 (P=0.29), MGC-803
(P=0.0075) and SGC-7901 (P=0.0053) was significantly greater,
compared with GES-1 cell; in the control group, the average number
of migrated cells was highest in MGC-803 cells, followed by
BGC-823, SGC-7901 and AGS. In the experimental group, DS
significantly reduced all the five cell numbers on the membrane
compared with control group, and the migration inhibition of AGS
cells (inhibition rate 96%, P=0.00062) is the most notable,
followed by SGC-7901 (94.1%, P=0.0074), MGC-803 (91.2%, P=0.0083),
BGC-823 (90.5%, P=0.0087) and GES-1 cells (84.3%, P=0.0091), as
depicted in Fig. 2B.
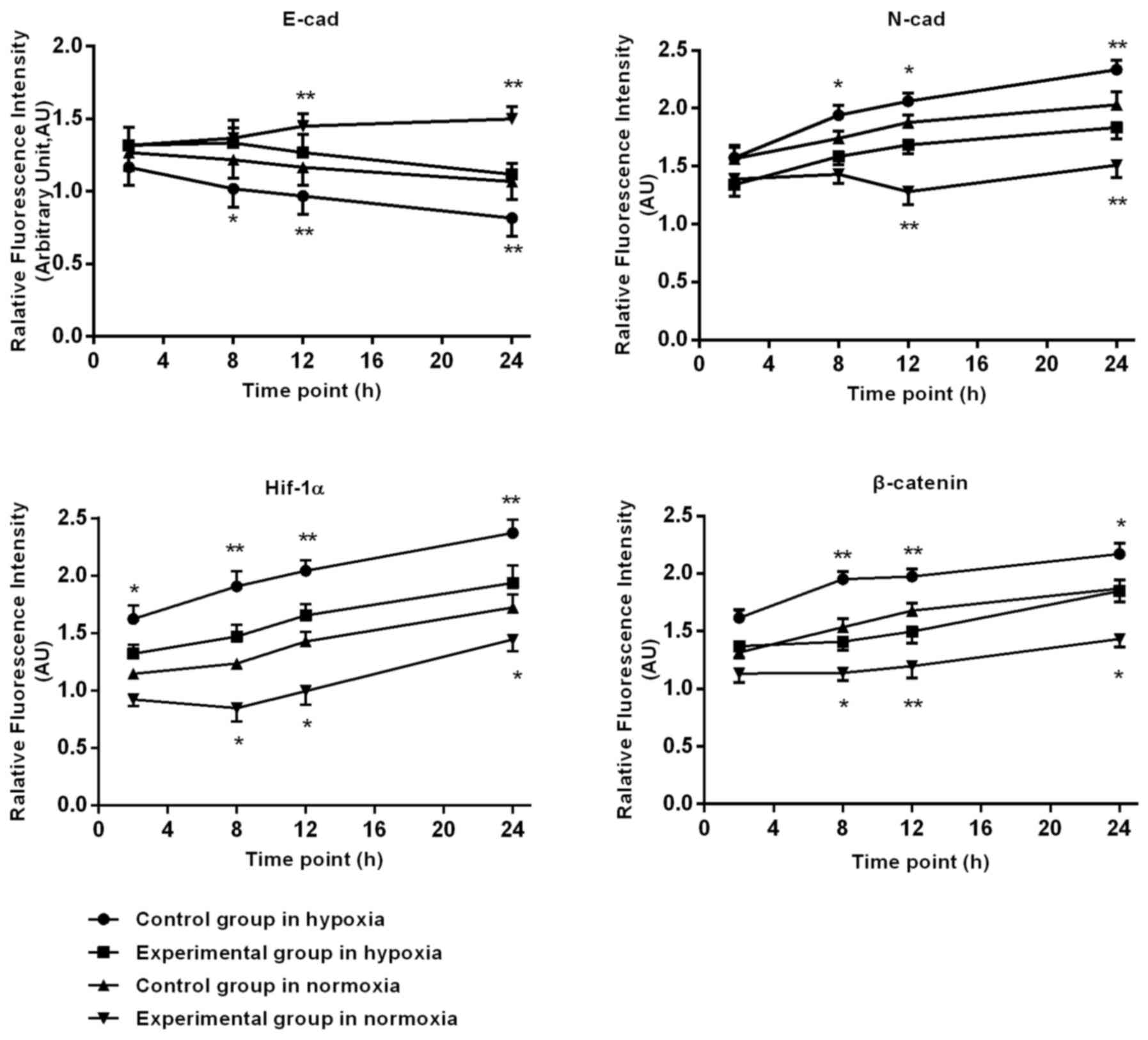
Immunofluorescence staining of HIF-1α
and EMT associated factors in BGC-823 cells
Following prolonged hypoxia, in the control group,
the expression of HIF-1α, N-cad gradually increased at 2, 8, 12 and
24 h. In the experimental group, the HIF-1α (P=0.022) and N-cad
(P=0.031) expression levels were significant reduced, compared with
the control group; E-cad expression in control group cells
gradually reduced, with a tendency to slow approaching 24 h. At 8,
12 and 24 h, the DS significantly slowed down in reducing E-cad
expression. β-catenin expression in the control group expression
gradually increased. In the cytoplasm and nuclei, the expression in
the experimental group in the nucleus and cytoplasm was less,
compared with the increase in the control group. The DS at 2, 8, 12
and 24 h significantly reduced the experimental β-catenin
expression.
Following prolonged culture in oxygen, the control
group HIF-1α expression increased gradually after 8 h, and at 8, 12
and 24 h, the expression significantly decreased in the
experimental group, compared with the control group. In the control
cells after 12 h, E-cad had a significantly lower expression, N-cad
was significantly upregulated, DS significantly slowed down in the
reduction of E-cad, and there was an increase of N-cad at 12 and 24
h. β-catenin expression in control cells increased gradually after
8 h, with a gradual increase in the cytoplasm and nuclei, and
relative decrease in the nucleus and cytoplasm of experimental
group, at 8, 12 and 24 h. Additionally, DS significantly reduced
the β-catenin expression of the BGC-823 cells, as depicted in
Fig. 3.
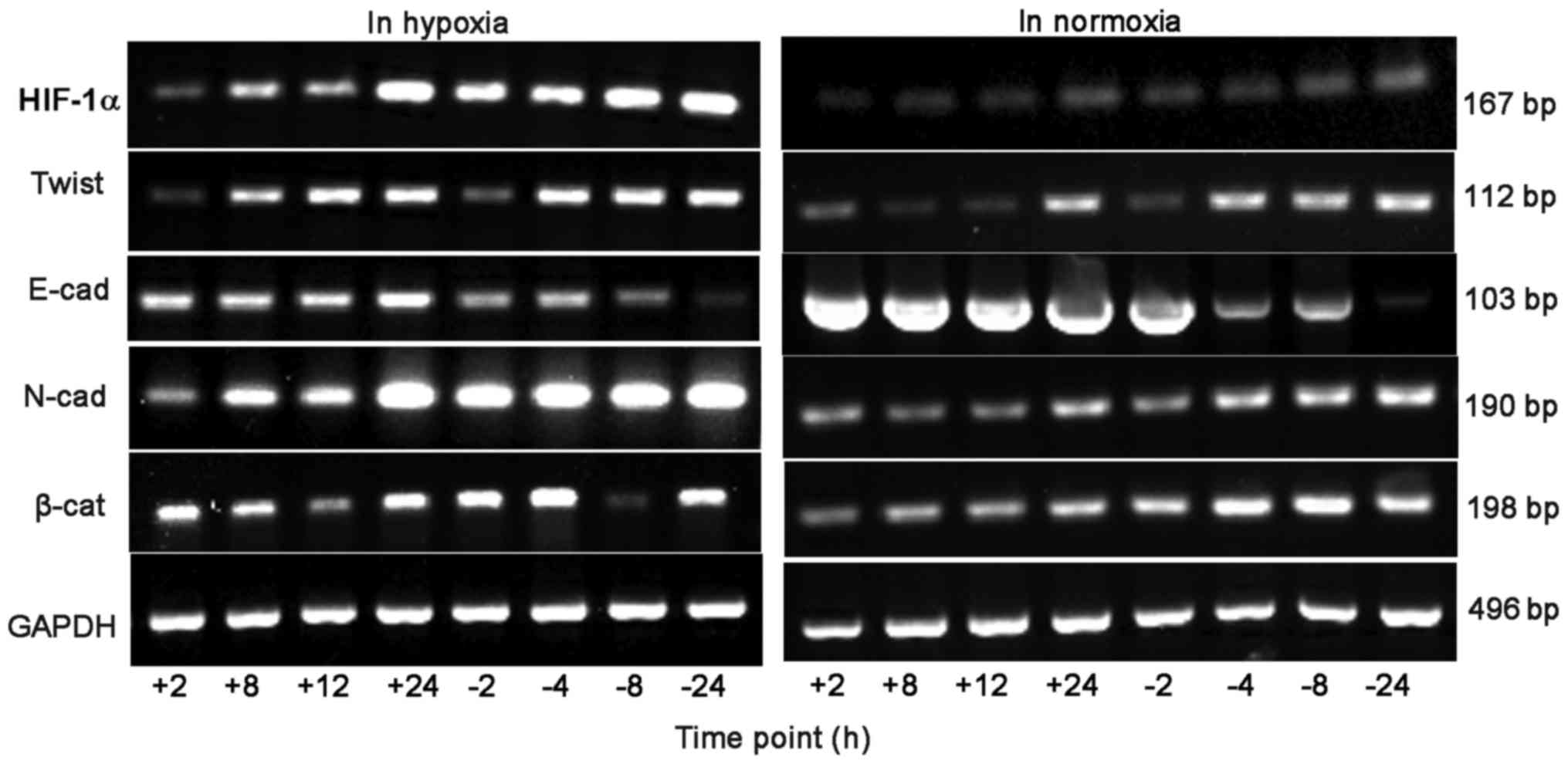
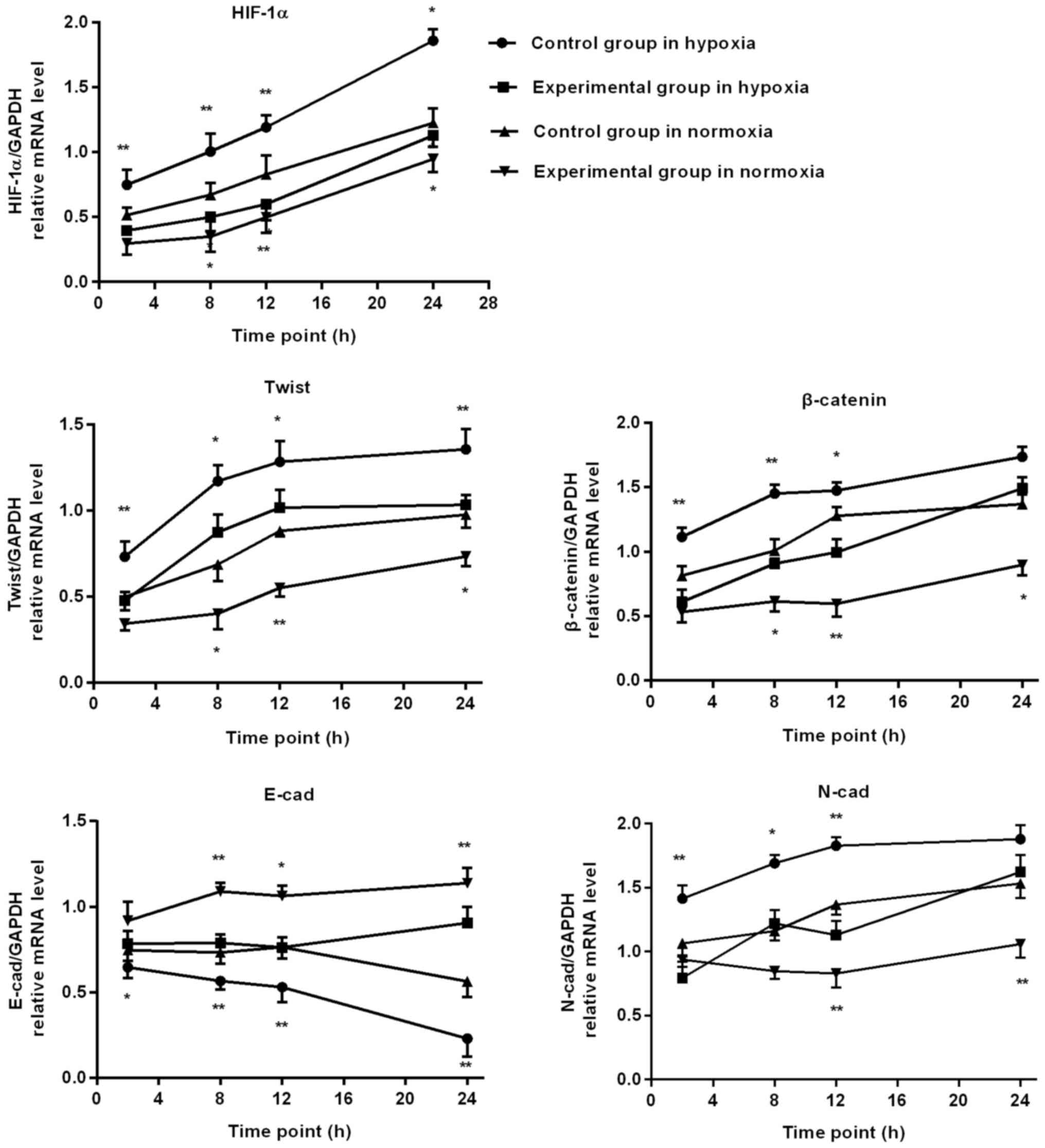
RT-PCR detection of HIF-1α and EMT
associated factors mRNA expression in BGC-823 cells
When hypoxia time was extended, in the control group
the cell HIF-1α, Twist and N-cad, β-catenin mRNA level gradually
increased, whereas E-cad mRNA level gradually reduced. At 2, 8, 12
and 24 h, the mRNA level of HIF-1α and Twist in the experimental
group was significantly lower, compared with the control group, and
the experimental group E-cad mRNA level was significantly
increased, compared with the control group. At 2, 8 and 12 h, the
N-cad and β-catenin mRNA level of the experimental group increased
significantly, compared with the control group.
Following prolonged culture in oxygen, in the
control group cells the mRNA level of HIF-1α, Twist, N-cad and
β-catenin all demonstrated an increasing trend, whereas the E-cad
mRNA level gradually reduced. At 8, 12 and 24 h, the mRNA levels of
HIF-1α, Twist and β-catenin in the experimental group were
significantly decreased, compared with the control group, and the
experimental group E-cad mRNA level was significantly increased,
compared with the control group. At 12 and 24 h, the N-cad mRNA
level of the experimental group was significantly increased,
compared with the control group, as depicted in Figs. 4 and 5.
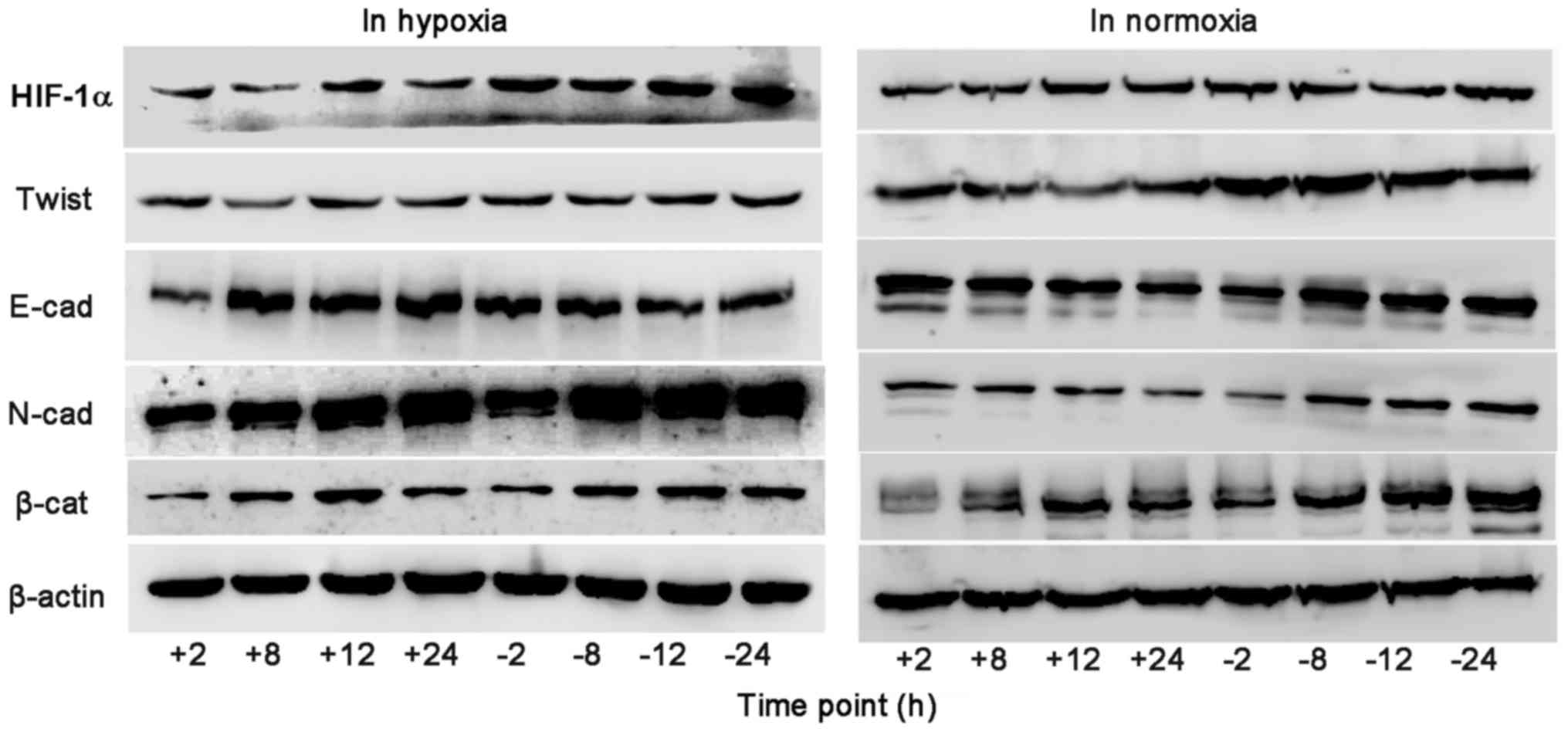
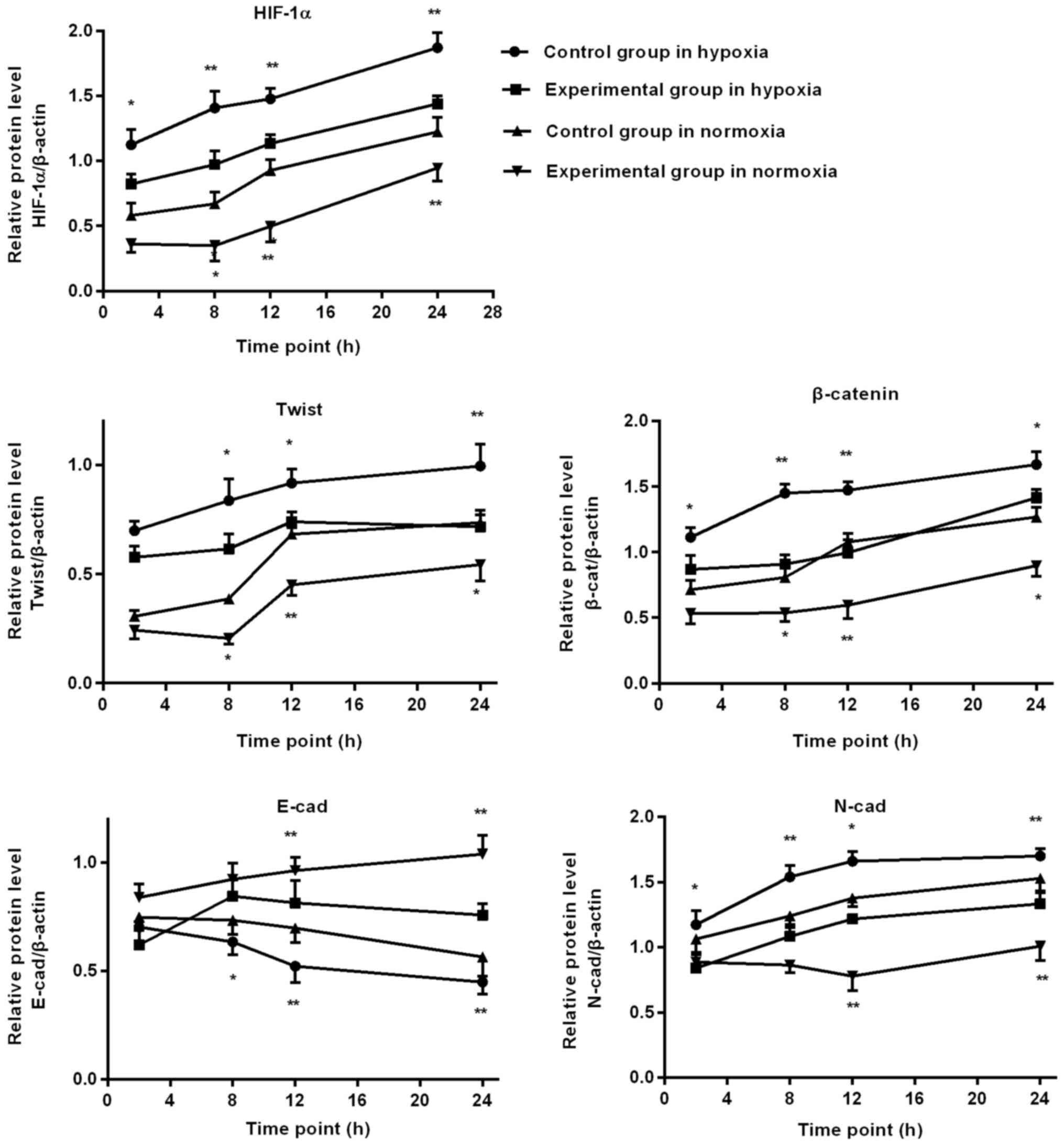
Western blot detection of EMT
associated factors protein expression in BGC-823 cells
In hypoxia environment, as the duration of hypoxia
increased, HIF-1α, Twist, N-cad and β-catenin expression gradually
increased in the control group, and HIF-1α, N-cad and β-catenin
expression of the experimental group was significantly lower,
compared with the control group, at 2, 8, 12 and 24 h. At 8, 12 and
24 h, Twist expression of the experimental group was significantly
lower, compared with the control group, and the expression of E-cad
increased significantly, compared with the control group.
In normoxia environment, as the duration of normoxia
increased, in the control group HIF-1α, Twist, β-catenin and N-cad
expression gradually increased, E-cad decreased at the time point
of 12 h. At 8, 12 and 24 h, HIF-1α, Twist and β-catenin expression
in the experimental group was significantly lower, compared with
the control group. At 12 and 24 h, the experimental group N-cad
expression was significantly lower than the control group, and the
expression of E-cad increased significantly, compared with the
control group, as depicted in Figs. 6
and 7.
Discussion
The prevention and treatment of proliferation,
invasion and metastasis of gastric cancer is one of the problems
that require further study.
The present study used the gastric cancer cell lines
AGS, BGC-823, MGC-803 and SGC-803 and normal gastric mucosa cells
GES-1 to detect the effects of DS on proliferation, invasion and
migration. The cell lines MGC-803 (undifferentiated), BGC-823 (low
differentiation) and AGS (high differentiated) are all derived from
gastric cancer tumors, and the SGC-7901 cell line was derived from
lymph node metastasis (moderately differentiated). GES-1 is derived
from human fetal gastric mucosa epithelial cells (immortalized)
(12).
One in vitro study has demonstrated that DS
can inhibit cancer cell adhesion, cell cycle progression and gene
expression (3). In the present study,
using a CCK-8 proliferation test, it was determined that at 6 and
12 h DS had no notable inhibitory effect on the proliferation of
five types of cells. At 24 h, DS inhibited AGS cell proliferation
by 21.1%, and DS reduced the proliferation of AGS, SGC7901 and
BGC-823 cells by 15.4, 18.8 and 20%, respectively at 36 h. At 48 h,
DS reduced the proliferation of AGS and SGC-7901 cells by 19.5 and
7.5%, respectively, and within 48 h, DS has no notable inhibitory
effect on MGC-803 cells. It may be safely concluded that DS
inhibited the highly differentiated gastric cancer cell AGS most,
as the reduction of the AGS cell number was the most significant,
whilst it had no significant proliferation inhibition effect on the
undifferentiated gastric cancer cell line MGC-803; Therefore, it
was speculated that gastric cancer cell proliferation inhibition by
DS may be associated with differentiation degree.
Tumor cells with different degrees of
differentiation have varying invasion and migration abilities
(13). Research has indicated that
gastric cancer cell line BGC-823 has an improved invasion ability,
compared with the cell lines AGS, SGC-7901 and GES-1 (14). The Transwell migration experiment in
the present study demonstrated that migrated numbers of the four
gastric cancer cell lines were notably more than the GES-1, the
four gastric cancer cells lines had an improved ability to migrate,
compared with the than normal gastric mucosa cells. Calculating the
average gastric cancer cells that migrated, the undifferentiated
gastric cancer cells MGC-803 had the highest, and then, in turn,
the low differentiated gastric cancer cells BGC-823, moderately
differentiated gastric cancer cells SGC-7901 and high
differentiated gastric cancer cell AGS. This demonstrated that the
lower degree of differentiation, the more cells that migrated
across the membrane, indicating an improved migration ability.
Significantly, DS reduced all five cell lines migration count,
compared with the control group. The cell line with the most
notable inhibitory effect was AGS, followed by SGC-7901, MGC-803,
BGC-823 and GES-1. So it was speculated that DS may have an
inhibitory effect on migration ability of all five types of cell
lines, which may be associated with the degree of
differentiation.
The primary difference between the invasion and
migration experiment was the layer of Matrigel. Cells must
penetrate the matrix firstly, then through the chamber on the
underside of the membrane filter. The invasion experiment indicated
that the average invaded cells was greatest for the
undifferentiated MGC-803 gastric cancer cells, and then, in turn,
the poorly differentiated gastric cancer cells BGC-823, moderately
differentiated gastric cancer cells SGC-7901 and high
differentiated gastric cancer cell AGS; therefore, the lower degree
of differentiation, the stronger the cell invasion ability.
Compared with control group, DS significantly reduced the invasion
of all five types of cells in the experimental group, and it has
the most notable inhibiting effect on BGC-823 cells, followed by
the AGS, MGC-803, SGC-7901 and GES-1. It was determined that the
strength of inhibition had no notable association with cell
differentiation. A previous study demonstrated that matrix
metalloproteinases secreted by tumor cells is the main component of
degrading basement membrane and mesenchyme (15). The strength of inhibition by DS had a
different order of cell lines for invasion and migration, which may
be due to different abilities of these cells secreting matrix
metalloproteinases and degrading extracellular matrix; in addition,
this process could be influenced by DS in some degree for different
cells, but the specific inhibition mechanism still requires further
study.
β-catenin serves an important role in the process of
invasion and migration of tumor cells, connecting adhesion
molecules outside the cell to the frame within cells, through
mediating the interaction of E-cad and β-catenin involved in the
cell adhesion, migration and transfer process (16). β-catenin is highly expressed in
gastric cancer cells AGS, HGC-27, MGC-803 and BGC-823. By contrast,
it has a lower expression in normal gastric mucosa cells (17). Abnormal expression of E-cad is closely
associated with tumor invasion, metastasis and diffusion (18). E-cad and β-catenin expression in
different degrees of differentiation of gastric cancer cells, to a
certain extent, could reflect the ability of invasion and
migration.
β-catenin signaling pathway, also known as the Wnt
signaling pathway, contains β-catenin, which has a key role in this
pathway. It combines with the E-cad intracellular area, producing
the β-catenin/E-cad complex, then connects to the actin
cytoskeleton, mediates adhesion between cells, thus regulating the
invasion and metastasis of tumor cells (19). The loss of combined E-cad leads to the
accumulation of intracellular β-catenin, causing an increasing in
β-catenin within nuclear localization in diffuse-gastric cancer,
and at the same time an immunohistochemical staining study has
demonstrated the reduction in E-cad on membrane (20). Accordingly, immunofluorescence
staining was conducted to observe the expression changes; the
effect of DS on MGC-803 is not particularly evident using CCK-8,
therefore DS effective cells (BGC-823) was selected for further
study. The results indicated that, with time, the expression of
HIF-1α, Twist and N-cad in the control group cells gradually
increased, whereas the E-cad expression gradually reduced.
Simultaneously, it was considered that the β-catenin expression
gradually increased, including in the nuclei; however, DS
significantly reduced the expression of β-catenin in cell nucleus.
As a necessary endogenous signal, Wnt signaling pathways can also
directly control the ability of HIF-1α to induce the occurrence of
EMT (21); therefore, it was
speculated that DS may use the Wnt pathway to inhibit the
expression of HIF-1α.
In a hypoxic microenvironment, HIF-1α overexpression
in cancer cells accelerates tumor growth and metastasis by inducing
tumor cells secretion of VEGF, regulating angiogenesis and
promoting EMT (22,23). A previous study confirmed that HIF-1α
induced tumor cell EMT through regulating the transcription factor
Twist, which inhibits the expression of E-cad and promotes the
expression of N-cad in hypoxia (24).
Another study demonstrated that DS can inhibit the HIF-1α
expression on the greater momentum of the mice (5). In the present study, gastric cancer cell
line BGC-823 was treated with DS and PBS, cultured in vitro
in normoxia and hypoxia conditions, checked at various time points,
observed for cell morphology changes. RT-PCR and western blot
analysis were also conducted for further detection, the results
demonstrated that DS have different degrees of inhibition on
HIF-1α, Twist and N-cad and β-catenin, as well as stimulation of
E-cad expression; therefore, DS may affect Twist and E-cad through
HIF-1α, inhibiting the occurrence of EMT.
In conclusion, DS has different degrees of
inhibition on the proliferation of cell lines AGS, BGC-823,
SGC-7901 and GES-1, which may be associated with the degree of
differentiation. DS has a different degree of inhibition effect on
migration and invasion ability of all five types of cells.
Furthermore, DS may inhibit HIF-1α through the Wnt signaling
pathway, reducing the gene and protein level of Twist, increasing
the epithelial marker E-cad and reducing the expression of
mesenchymal marker N-cad, thus inhibiting EMT of gastric cancer
cell line BGC-823. In order to further study the anticancer
mechanism of DS in human gastric cancer metastasis, experiments
in vivo will be carried out in the subsequent tests.
Acknowledgements
Not applicable.
Funding
This study was supported by Ningxia Science and
Technology Support Projects, West China First-Class Discipline
Project in Basic Medical funded by Ningxia Medical University, The
School of Basic Medicine at Ningxia Medical University and The
Institute of Basic Medicine at Ningxia Medical University (grant
no. 201-30181601).
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
YX designed the experiment, supervised the study and
confirmed the results. XJ performed the experiments and collected
and analyzed the data. YH designed the experiments, confirmed the
results and contributed to the writing of the manuscript. JW and XW
performed part of the experiments. HW analyzed part of the
data.
Ethics approval and consent to
participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Jemal A, Bray F, Center MM, Ferlay J, Ward
E and Forman D: Global cancer statistics. CA-Cancer J Clin.
61:69–90. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Shen L, Shan YS, Hu HM, Price TJ, Sirohi
B, Yeh KH, Yang YH, Sano T, Yang HK, Zhang X, et al: Management of
gastric cancer in Asia: Resource-stratified guidelines. Lancet
Oncol. 14:e535–e547. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Takagi T, Sakakura C, Kin S, Nakase Y,
Fukuda K, Shimomura K, Ito T, Fujiyama J, Yamasaki J, Tsujimoto H,
et al: Dextran sulfate suppresses cell adhesion and cell cycle
progression of melanoma cells. Anticancer Res. 25:895–902.
2005.PubMed/NCBI
|
|
4
|
Xu YY, Huang YN, Wang HH and Liu Y:
Inhibition of the peritoneal metastasis of human gastric cancer
cells by dextran sulphate in vivo and in vitro. Oncol Lett.
11:2384–2390. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Xu Y, Jin X, Huang Y, Dong J, Wang H, Wang
X and Cao X: Inhibition of peritoneal metastasis of human gastric
cancer cells by dextran sulphate through the reduction in HIF-1α
and ITGβ1 expression. Oncol Rep. 35:2624–2634. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Gurzu S, Turdean S, Kovecsi A, Contac AO
and Jung I: Epithelial-mesenchymal, mesenchymal-epithelial, and
endothelial-mesenchymal transitions in malignant tumors: An update.
World J Clin Cases. 3:393–404. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Daly CS, Flemban A, Shafei M, Conway ME,
Qualtrough D and Dean SJ: Hypoxia modulates the stem cell
population and induces EMT in the MCF-10A breast epithelial cell
line. Oncol Rep. 39:483–490. 2018.PubMed/NCBI
|
|
8
|
Noman MZ, Messai Y, Carré T, Akalay I,
Méron M, Janji B, Hasmim M and Chouaib S: Microenvironmental
hypoxia orchestrating the cell stroma cross talk, tumor progression
and antitumor response. Crit Rev Immunol. 31:357–377. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Bao B, Wang Z, Ali S, Kong D, Li Y, Ahmad
A, Banerjee S, Azmi AS, Miele L and Sarkar FH: Notch-1 induces
epithelial-mesenchymal transition consistent with cancer stem cell
phenotype in pancreatic cancer cells. Cancer Lett. 307:26–36. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Goggins BJ, Chaney C, Radford-Smith GL,
Horvat JC and Keely S: Hypoxia and integrin-mediated epithelial
restitution during mucosal inflammation. Front Immunol. 4:2722013.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Peng Z, Wang CX, Fang EH, Wang GB and Tong
Q: Role of epithelial-mesenchymal transition in gastric cancer
initiation and progression. World J Gastroenterol. 20:5403–5410.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Fu F, Hu H, Yang S and Liang X: Effects of
TIN2 on telomeres and chromosomes in the human gastric epithelial
cell line GES-1. Oncol Lett. 15:5161–5166. 2018.PubMed/NCBI
|
|
13
|
Goto A, Nishikawa J, Hideura E, Ogawa R,
Nagao M, Sasaki S, Kawasato R, Hashimoto S, Okamoto T, Ogihara H,
et al: Lymph node metastasis can be determined by just tumor depth
and lymphovascular invasion in early gastric cancer patients after
endoscopic submucosal dissection. Eur J Gastroenterol Hepatol.
29:1346–1350. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Hou XH: The expression of HORMAD2 and the
effects of it on proliferation and apoptosis on gastric cancer cell
(D). Lanzhou Univ. 2015.
|
|
15
|
Weili Xu: The effect of lysine
(K)-specific demethylase 1A on invasion and metastasis of gastric
cancer and its associated mechanism (D). Central South Univ.
2014.
|
|
16
|
Zheng L, Hu X, Huang Y, Xu G, Yang J and
Li L: In vivo bioengineered ovarian tumors based on collagen,
matrigel, alginate and agarose hydrogels: A comparative study.
Biomed Mater. 10:0150162015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Cheng XX, Wang ZC, Chen XY, Sun Y, Kong
QY, Liu J, Gao X, Guan HW and Li H: Frequent loss of membranous
E-cadherin in gastric cancers: A cross-talk with Wnt in determining
the fate of beta-catenin. Clin Exp Metastasis. 22:85–93. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Dong Li: Expression of β-catenin-the
Central Regulation Molecule of the Wnt Signaling Pathways in
gastric cancer and its significance (D). Nanchang Univ. 2014.
|
|
19
|
Zhang Z, Bu X, Chen H, Wang Q and Sha W:
Bmi-1 promotes the invasion and migration of colon cancer stem
cells through the downregulation of E-cadherin. Int J Mol Med.
38:1199–1207. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Huelsken J and Behrens J: The Wnt
signalling pathway. J Cell Sci. 115:3977–3978. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Kalluri R and Weinberg R: The basics of
epithelial-mesenchymal transition. J Clin Invest. 119:1420–1428.
2009. View
Article : Google Scholar : PubMed/NCBI
|
|
22
|
Ashaie MA and Chowdhury EH: Cadherins: The
superfamily critically involved in breast cancer. Curr Pharm Des.
22:616–638. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Zhang X, Liu G, Kang Y, Dong Z, Qian Q and
Ma X: N-cadherin expression is associated with acquisition of EMT
phenotype and with enhanced invasion in erlotinib-resistant lung
cancer cell lines. PLoS One. 8:e576922013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Wu S, Luo Z, Yu PJ, Xie H and He YW:
Suberoylanilide hydroxamic acid (SAHA) promotes the epithelial
mesenchymal transition of triple negative breast cancer cells via
HDAC8/FOXA1 signals. Biol Chem. 397:75–83. 2016. View Article : Google Scholar : PubMed/NCBI
|