Introduction
As the leading cause of cancer-associated mortality
worldwide (1), lung cancer imposes an
increasing burden on society and is a major challenge to
clinicians. The Global Cancer Statistics reported that an estimated
1.8 million novel cases of lung cancer occurred in 2012 worldwide,
accounting for ~13% of total cancer diagnoses (2). Non-small cell lung cancer (NSCLC) is the
major pathological type, consisting of 85% of all types of lung
cancer (3). In recent years, with the
development of molecular biotechnology techniques, drugs for the
treatment of NSCLC, particularly lung adenocarcinoma, targeting
signal transduction and angiogenesis have achieved certain effects;
however, patients with genetic disorders failed to benefit from
these drugs and they are not available to those of limited means in
certain countries (4).
Src family protein tyrosine kinases (SFKs), members
of the receptor tyrosine protein kinase family, include the
proto-oncogene ‘Scr’ was the first to be identified (5) and serves a key function in cellular
signal transduction pathways, immune responses and inflammatory
responses (6). Aberrant SFK activity
is observed in a number of types of human cancer, and is associated
with proliferation, invasion, apoptosis and migration of tumor
cells. There is evidence that expression of SFKs in gastric cancer
is associated with tumor invasion, and lymph node and distant
metastases (6). Overexpression of
SFKs was detected in ~80% of patients with colon cancer, and was
demonstrated to accelerate tumor metastasis and lead to
chemotherapeutic drug resistance via multiple downstream signaling
pathways (7). Furthermore, it has
been demonstrated that biological changes in breast cancer induced
by cluster of differentiation 44 silencing may be mediated by
cumulative downregulation of SFKs (8), and that knockdown of Lyn or other SFK
members may decrease proliferation, migration and invasion of human
pancreatic cancer cells (9).
A number of studies have focused on the association
between SFKs and NSCLC. One study identified sex-determining region
Y box 2 as a novel target of epidermal growth factor receptor
(EGFR)/Src/protein kinase B (Akt) signaling in NSCLC that modulates
self-renewal and expansion of stem-like cells from NSCLC (10). Furthermore, targeting SFKs is a
clinically applicable strategy to overcome resistance to
insulin-like growth factor 1 receptor tyrosine kinase inhibitors
(11). Lung cancer may be inhibited
by silencing Lyn kinase expression using small interfering RNA,
which decreased EGFR activation and cell viability (12). In addition, EGFR inhibition promotes
innate drug resistance and is associated with limited primary
responses (13). Furthermore,
resistance of NSCLC to anticancer treatment is also prevented by
mitochondrial changes and activation of caspases (14,15).
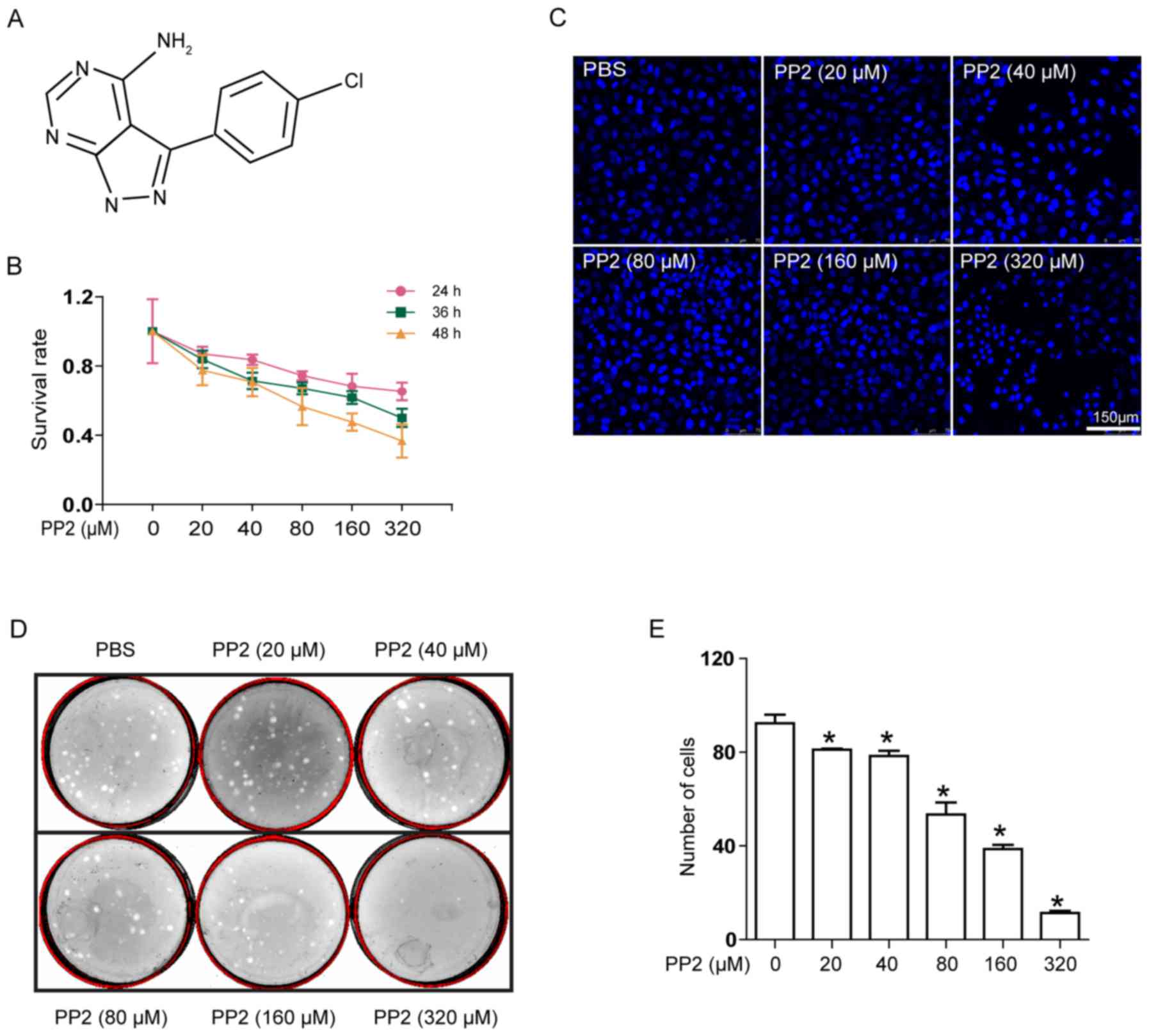
Pyrazolopyrimidine compound PP2 (Fig. 1A) is a selective inhibitor of SFKs.
EGFR mutants of the H1299 cell line exhibited different sensitivity
to PP2 (16). On this basis, we
hypothesize that there may be overexpression of Lyn kinase in
patients with lung cancer. This hypothesis was confirmed in our
previous study (unpublished data). The aim of the present study was
to determine whether PP2 is able to influence the biological
characteristics of A549 cells.
Materials and methods
Cell culture
The human NSCLC cell line A549 was obtained from the
American Type Culture Collection (Manassas, VA, USA). Cells were
cultured with RPMI-1640 medium (Gibco; Thermo Fisher Scientific,
Inc., Waltham, MA, USA) supplemented with 10% fetal bovine serum
(FBS; Gibco; Thermo Fisher Scientific, Inc.), penicillin (100 U/ml)
and streptomycin (100 µg/ml) (both Beyotime Institute of
Biotechnology, Haimen, China) at 37°C with 5% CO2.
MTT assay
Cells were seeded at 5×103 cells/well in
a 96-well plate and incubated overnight at 37°C to reach 85%
confluence. Cells were treated with different concentrations of PP2
(20, 40, 80, 160 and 320 µΜ; Abcam, Cambridge, MA, USA) which was
dissolved in Dulbecco's modified Eagle's medium (Sigma-Aldrich;
Merck KGaA, Darmstadt, Germany), for 24, 36 and 48 h. The control
group was treated with PBS. Following culture with PP2, 10 µl/well
MTT solution (5 mg/ml) was added prior to incubation for a further
4 h. Subsequently, 100 µl DMSO was added to each well prior to
incubation at 37°C for 3 h to solubilize the formazan crystals. The
absorbance of plates was determined at 570 nm using a microplate
reader (Tecan Group, Ltd., Männedorf, Switzerland).
Immunofluorescence microscopy
A549 cells treated with PP2 (0, 20, 40, 80, 160 and
320 µM) for 24 h were fixed with 4% paraformaldehyde for 30 min at
room temperature. The fixed A549 cells were washed with PBS three
times, prior to being permeabilized with methanol (Sigma-Aldrich;
Merck KGaA) for 5 min at room temperature. Subsequently, 100
µl/well DAPI (Beyotime Institute of Biotechnology) was used for
staining for 5 min in the dark at room temperature, prior to
washing with PBS three times. Images of all specimens were captured
using an SP5 Leica confocal microscope and analyzed with Leica
Application Suite software (Version number: 14.0.0162) (both Leica
Microsystems GmbH, Wetzlar, Germany).
Colony formation assay
Cells were seeded in dishes of 60 mm diameter with
500 cells/dish and cultured overnight. Cells were exposed to PP2 at
various concentrations (0, 20, 40, 80, 160 and 320 µΜ) for a total
of 10 days when the colonies were visible to the naked eye, and
medium was refreshed every 48 h. On the last day, the colonies were
washed with PBS and fixed with 4% paraformaldehyde, then washed by
PBS three times and stained with Wright-Giemsa stain (BaSO Biotech,
Taipei, Taiwan) at room temperature for 11 min. The number of
colonies formed in each group was determined, by counting with the
naked eye.
Cell invasion assay
Matrigel (3 mg/ml) was added at 40 µl/well to the
inner face of the membrane in the upper compartment of the
Transwell insert and 40 µl/well fibronectin (125 µg/ml;
Sigma-Aldrich; Merck KGaA) was added to the outer face of the
membrane, prior to drying overnight. Cells were resuspended in
FBS-free RPMI-1640 medium with 0.1% bovine serum albumin (BSA;
Sangon Biotech Co., Ltd., Shanghai, China). Subsequently,
2×105 cells were added into the upper compartment of the
Transwell insert with 300 µl/well FBS-free medium containing 0.1%
BSA. Cells were exposed to PP2 at various concentrations (0, 20,
40, 80, 160 and 320 µM). RPMI-1640 medium containing 10% FBS was
added to the lower compartment at 1 ml/well. Cells were incubated
at 37°C for 24 h. The insert was removed and cells on the outer
face were fixed with 4% paraformaldehyde for 30 min and stained
with Wright-Giemsa stain (BaSO Biotech, Taipei, Taiwan) at room
temperature for 11 min. Five random fields were selected from each
membrane with light microscope at ×100 magnification, and the
number of cells in each field was counted.
Immunofluorescence flow cytometry
Cells were seeded in 6-well plates and cultured in
RPMI-1640 medium containing 10% FBS overnight. Cells were treated
with PP2 at various concentrations (0, 20, 40, 80, 160 and 320 µM)
for 24 h, and then collected for propidium iodide (PI) and
fluorescein isothiocyanate/Annexin V (Annexin V-FITC) staining in
the dark at room temperature, the FITC Annexin V Apoptosis
Detection kit I (BD Biosciences, Franklin Lakes, NJ, USA) was used
according to the manufacturer's protocol. Apoptotic cells were
analyzed using flow cytometry with a FACSAria II instrument (BD
Biosciences) and data were analyzed by Cell Quest 5.1 (BD
Biosciences).
Western blot analysis
A549 cells were seeded in 6-well plates and cultured
overnight, prior to treatment with PP2 at various concentrations
(0, 20, 40, 80, 160 and 320 µM) for 24 h. Following incubation,
cells were lysed in radioimmunoprecipitation assay lysis buffer (20
mM Tris/HCl, pH 7.5, 150 mM NaCl, 1 mM EDTA, 1 mM EGTA, 1% NP-40
and 1% sodium deoxycholate) containing proteinase
(phenylmethylsulfonyl fluoride) and phosphatase inhibitors (NaF and
Na3VO4) and maintained on ice for 30 min. The
lysate was centrifuged at 11,113 × g for 10 min at 4°C on an
Allegra X-22R centrifuge (Beckman Coulter, Inc., Brea, CA, USA).
The protein concentration of each specimen was determined
quantitatively using a bicinchoninic acid protein concentration
assay kit (Beyotime Institute of Biotechnology). The suspension was
transferred to a new tube and kept on ice, then mixed with 5X
SDS-PAGE sample loading buffer (Beyotime Institute of
Biotechnology), and boiled at 100°C for 10 min. A 50 µg amount of
each protein sample was loaded per lane of an SDS-PAGE gel (10%
acrylamide) with two lanes of 2 µl protein molecular mass marker.
The gel was electrophoresed for 30 min at 80 V for stacking and 100
V for separation, then the protein was electrotransferred onto a
polyvinylidene fluoride membrane for 2.5 h at 300 mA. Non-specific
binding was blocked with 5% dried skimmed milk diluted in
Tris-buffered saline containing 0.1% Tween-20 (TBST) for 1 h and
washed with TBST three times. The membranes were incubated with
primary mouse monoclonal anti-human phosphoinositide 3-kinase
(PI3K; 1:1,000; cat. no. ab86714), rabbit polyclonal anti-human
phospho-PI3K (1:1,000; cat. no. ab182651), rabbit monoclonal
anti-human Akt (1:1,000; cat no. ab32505), rabbit monoclonal
anti-human phospho-Akt (1:1,000; cat. no. ab81283), mouse
monoclonal anti-human B-cell lymphoma 2 (Bcl-2; 1:500; cat. no.
ab692), rabbit polyclonal anti-human caspase-3 (1:1,000; cat. no.
ab2302), and mouse monoclonal anti-human GAPDH (1:1,000; cat. no.
ab8245) antibodies (all obtained from Abcam) at 4°C overnight.
Following removal of unbound antibodies and washing three times
with TBST, the membranes were incubated with secondary antibodies
(goat anti-rabbit polyclonal; 1:5,000; cat. no. ab6721) and goat
anti-mouse polyclonal (1:1,000; cat. no. ab6789) (both obtained
from Abcam) for 1 h at room temperature. The bands were visualized
using an enhanced chemiluminescence western blotting kit (Pierce;
Thermo Fisher Scientific, Inc.), according to the manufacturer's
protocol.
Statistical analysis
All data are expressed as the mean ± standard error
of the mean. The statistical significant differences were analyzed
using one-way analysis of variance followed by Bonferroni's
correction for comparison tests, using SPSS software (version 17.0;
SPSS, Inc., Chicago, IL, USA). P<0.05 was considered to indicate
a statistically significant difference.
Results
PP2 has a cytotoxic effect on A549
cells
The effect of PP2 on lung cancer remains unclear. In
order to elucidate this function, an MTT assay was used to
determine the effect of PP2 on the viability of A549 cells. Cells
were treated with various concentrations of PP2 (0, 20, 40, 80, 160
and 320 µM) at three different times (24, 36 and 48 h). The results
indicated that PP2 was cytotoxic towards A549 cells (Fig. 1B), with the survival rate decreasing
with increasing concentrations of PP2 and the extension of the
incubation time. Similarly, the morphological features of A549
cells treated with PP2 were also altered (Fig. 1C). The cell nuclei were irregular and
rather ambiguous at increased concentrations of PP2. These results
suggested that PP2 is able to decrease the viability of A549 cells
and alter the morphology of the cell nucleus.
PP2 suppresses the viability of A549
cells and decreases colony formation
In order to further verify the negative effect of
PP2 on the viability of A549 cells, a colony formation assay was
used to determine the effect of PP2 on cell viability. Following
treatment with PP2, the number of colonies formed decreased with
increasing concentrations of PP2 (Fig.
1D). Compared with the untreated control group, following
administration of PP2 at 320 µM, the number of A549 cell colonies
formed decreased significantly (Fig.
1E). These results suggested that PP2 decreased the viability
and colony formation ability of A549 cells effectively.
PP2 inhibits A549 cell invasion
A tumor invasion assay was used to detected the
effect of PP2 on the invasive ability of A549 cells in
vitro. Following treated with PP2 for 24 h, the number of
transmembrane cells at different concentrations were compared
(Fig. 2A). With increasing
concentration of PP2, the number of transmembrane cells decreased
(Fig. 2B). Compared with the control
group, there was a decrease of >50% in the number of
transmembrane cells treated with 80 µM PP2.
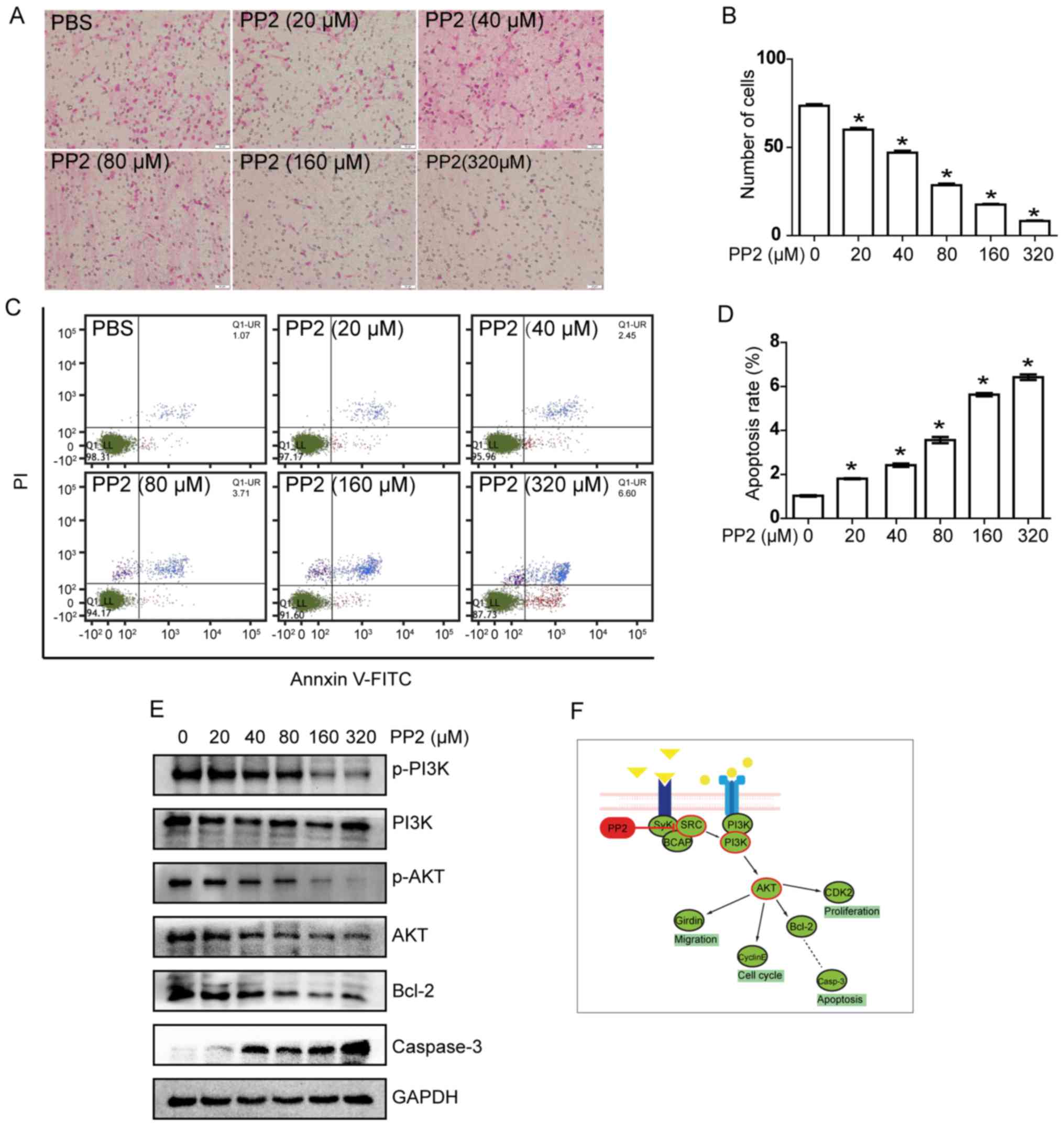 | Figure 2.PP2 inhibits invasiveness and induces
apoptosis of A549 cells in a dose-dependent manner by regulating
activation of the PI3K/Akt/Bcl-2/caspase-3 signaling pathway. (A)
Cell invasion assay. Representative images of cells treated with
various concentrations of PP2 (magnification, ×100). (B)
Quantification of invasion assay. (C) Flow cytometric analysis of
apoptotic cells treated with various concentrations of PP2. (D)
Quantification of apoptotic cells. (E) Protein expression of the
PI3K/Akt/Bcl-2/caspase-3 signaling pathway members in A549 cells
treated with various concentrations of PP2. (F) Schematic diagram
of the mechanism of action of PP2 on A549 cells. *P<0.05 vs.
control group (PBS/0 µM PP2). PI3K, phosphoinositide 3-kinase; Akt,
protein kinase B; Bcl-2, B-cell lymphoma 2; PE, phycoerythrin;
FITC, fluorescein isothiocyanate; Q, quadrant; UR, upper right; LL,
lower left; p-, phospho-; BCAP, B-cell adaptor for PI3K; CDK2,
cyclin-dependent kinase 2; Casp-3, caspase-3. |
PP2 induces apoptosis in A549
cells
To further elucidate the underlying molecular
mechanism by which PP2 induced the inhibition of cell viability, it
was investigated whether PP2 leads to induction of apoptosis in
A549 cells. A549 cells were treated with various concentrations of
PP2 for 24 h and analyzed using flow cytometry. The results
indicated that the percentage of apoptosis cells increased with
increasing concentrations of PP2 (Fig.
2C), the apoptosis rate increased with increasing
concentrations PP2 (Fig. 2D). These
results indicate that PP2 was able to induce apoptosis in A549
cells in a dose-dependent manner.
Effects of PP2 on
PI3K/Akt/Bcl-2/caspase-3 signaling pathway in A549 cells
The results of the present study indicated that PP2
is able to inhibit viability and induce apoptosis in A549 cells;
however, the underlying molecular mechanism remains unclear. To
elucidate the underlying mechanism of the effect of PP2 on A549
cells, the expression of associated proteins of the
PI3K/Akt/Bcl-2/caspase-3 signaling pathway was determined following
treatment with PP2. The results of the western blot assay are
presented in Fig. 2E. PP2 decreased
phospho-PI3K and phospho-Akt with increasing concentration of PP2.
These results suggest that PP2 was able to inhibit the protein
phosphorylation and activity of this signaling pathway, which are
consistent with the result of the present study.
Furthermore, the expression level of apoptotic
regulatory proteins was also determined. Compared with the control
group, the expression level of Bcl-2 was markedly downregulated,
whereas that of caspase-3 was increased following treatment with
PP2. These western blot results were consistent with those of the
flow cytometric analysis. Therefore, PP2 was identified to be able
to inhibit the viability and invasion of A549 cells, and also
induce apoptosis; effects achieved by regulating the activation of
the PI3K/Akt/Bcl-2/caspase-3 signaling pathway.
Discussion
Lung cancer is the leading cause of mortality from
cancer among males worldwide, with an increase in lung cancer
associated with increasing populations, aging populations and air
pollution (2). Although anticancer
therapies, including chemotherapy, radiation therapy and molecular
targeted therapy, are currently commonly used in the clinic, a
marked proportion of patients failed to benefit from these
treatments. In 2015 the five-year survival rate of patients with
lung cancer is only 16% (17).
Furthermore, the effects of drug resistance and genetic mutations
on lung cancer are becoming common problems for anticancer
therapies. In previous studies, SFKs have been recognized as having
a vital function in cancer cell proliferation, migration and
invasion (18,19). As a member of the SFK family, Lyn was
identified to be markedly expressed in lung tissue of patients with
lung cancer in our previous study (unpublished data). PP2 is a
selective inhibitor of SFKs, therefore PP2 was used to treat A549
cells in order to investigate its influence on biological
characteristics of cells and elucidate the underlying molecular
mechanisms.
Our previous study (unpublished data) identified
that PP2 was able to inhibit the viability of A549 cells and
decrease colony growth of cells, the morphological changes of
nucleus were the main characteristics in A549 cells, and the effect
was markedly dose- and time-dependent. Furthermore, PP2 was able to
markedly decrease the invasiveness, while promoting apoptosis, of
A549 cells. The underlying molecular mechanism was modulation of
the PI3K/Akt/Bcl-2/caspase-3 signaling pathway. These results
suggested that molecular targeted agents against SFKs may have
potential for anticancer therapies.
It has been identified that SFKs are translocated to
the sites of cell adhesion (18).
Owing to its particular localization, the catalytic activity of Src
initiates the intracellular signal transduction pathways that
influence cell proliferation and adhesive strength, the latter
contributing to regulation of cell migration. In addition, the
migration of cancer cells may be suppressed by PP2, because of its
function of activating the epithelial cadherin-mediated cell
adhesion system (19). In addition,
overexpression of c-Src and EGFR in fibroblast cells causes
synergistic increases in DNA synthesis, colony growth and tumor
formation in nude mice, whereas knockdown of Src may decrease human
pancreatic cancer cell proliferation, migration and invasion
(9,20). These results suggested that Src is
associated with proliferation, metastasis and invasion of cells, in
agreement with the results of the present study. Accordingly, it
was identified that the expression of PI3K/Akt and phosphorylated
PI3K/Akt was decreased. The PI3K/Akt/mammalian target of rapamycin
signaling pathway is an important intracellular signal transduction
pathway with an important function in cell viability and survival,
inhibition of apoptosis, angiogenesis, metastasis and resistance to
chemotherapy-radiotherapy (21,22). In a
previous study, activated Akt was traced in primary NSCLC tumors
and was suggested to be a poor prognostic factor for patients with
early-stage NSCLC (23).
Overexpression of the downstream kinase Akt may also result in
activation of the PI3K signaling pathway (21). These results indicated that PP2 is an
effective inhibitor to inhibit SFKs and suppress the downstream
PI3K/Akt signaling pathway, to inhibit cellular viability,
migration and invasion.
Additionally, from the flow cytometry data in the
present study, we hypothesize that PP2 is able to promote apoptosis
of A549 cells; the underlying molecular mechanism may be associated
with suppressing the expression of Bcl-2 and upregulating caspase-3
in the downstream pathway, as observed in the western blot analysis
of the present study. The Bcl-2 family of pro- and anti-apoptotic
proteins has been recognized as the important components of
regulating the mitochondrial pathway of apoptosis, with the major
anti-apoptotic proteins being Bcl-2, B-cell lymphoma extra-large
and myeloid cell leukemia-1. These proteins promote cellular
survival by sequestering pro-apoptotic proteins including
Bcl-2-interacting mediator of cell death and Bcl-2-associated death
promoter, which function as apoptotic ‘sensitizers’ or ‘effectors’
like Bcl-2-associated X protein or Bcl-2 homologous antagonist
killer (24). Therefore, we
hypothesize that Bcl-2 is a key protein in the apoptotic pathway
which is suppressed by PP2.
Joseph et al (15) reported that caspase-3 serves a vital
function in regulating nuclear changes during apoptosis. It was
identified that caspase-3 is associated with loss of the integrity
of the nuclear membrane, decreased synthesis of poly(ADP-ribose)
and DNA fragmentation (25). There is
evidence that effector caspases are responsible for initiating the
hallmarks of the degradation phase of apoptosis, including DNA
fragmentation, cell shrinkage and membrane blebbing (14), and poly(ADP-ribose) is critical for
DNA repair, regulation of chromosome structure, transcriptional
regulation, mitosis and apoptosis (26). Treatment with caspase-3 initiates
DNase activity and causes DNA fragmentation in nuclei (26,27). Nam
et al (19) identified that
PP2 is able to induce morphological changes in cancer cells. In the
present study, it was identified that the morphology of the nucleus
under light microscopy was irregular and ambiguous following
treatment with PP2. The alterations in nuclear characteristics
demonstrated that PP2 promotes the apoptosis pathway by
upregulating caspase-3. These results demonstrated the inhibition
of PP2 on A549 cell viability, and that the PP2-promoted apoptosis
in A549 cells occurred downstream of mitochondrial changes and
caspase activation, and upstream of nuclear events.
Acknowledgements
This work was supported by Inflammation and Allergic
Diseases Research Unit of Affiliated Hospital of Southwest Medical
University that provided the experimental site and all of the
instruments, and State Key laboratory of Quality Research in
Chinese Medicine/Macau Institute for Applied Research in Medicine
and Health of Macau University of Science and Technology that
provided technical guidance.
Funding
No funding was received.
Availability of data and materials
The datasets used or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
GPL and XQY conceived and designed the study. XD,
LJW, JW and YXS performed the experiments. XD and LJW wrote the
manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Chen W, Zheng R, Baade PD, Zhang S, Zeng
H, Bray F, Jemal A, Yu XQ and He J: Cancer statistics in China,
2015. CA Cancer J Clin. 66:115–132. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Torre LA, Bray F, Siegel RL, Ferlay J,
Lortet-Tieulent J and Jemal A: Global cancer statistics, 2012. CA
Cancer J Clin. 65:87–108. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Nur U, Quaresma M, De Stavola B, Peake M
and Rachet B: Inequalities in non-small cell lung cancer treatment
and mortality. J Epidemiol Community Health. 69:985–992. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Molina JR, Yang P, Cassivi SD, Schild SE
and Adjei AA: Non-small cell lung cancer: Epidemiology, risk
factors, treatment, and survivorship. Mayo Clin Proc. 83:584–594.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Martin GS: The hunting of the Src. Nat Rev
Mol Cell Biol. 2:467–475. 2001. View
Article : Google Scholar : PubMed/NCBI
|
|
6
|
Mello AA, Leal MF, Rey JA, Pinto GR,
Lamarão LM, Montenegro RC, Alves AP, Assumpção PP, Borges Bdo N,
Smith MC and Burbano RR: Deregulated expression of SRC, LYN and CKB
Kinases by DNA methylation and its potential role in gastric cancer
invasiveness and metastasis. PLos One. 10:e01404922015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Chen J, Elfiky A, Han M, Chen C and Saif
MW: The role of Src in colon cancer and its therapeutic
implications. Clin Colorectal Cancer. 13:5–13. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Nam K, Oh S, Lee KM, Yoo SA and Shin I:
CD44 regulates cell proliferation, migration, and invasion via
modulation of c-Src transcription in human breast cancer cells.
Cell Signal. 27:1882–1894. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Je DW, O YM, Ji YG, Cho Y and Lee DH: The
inhibition of SRC family kinase suppresses pancreatic cancer cell
proliferation, migration, and invasion. Pancreas. 43:768–776. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Singh S, Trevino J, Bora-Singhal N,
Coppola D, Haura E, Altiok S and Chellappan SP: EGFR/Src/Akt
signaling modulates Sox2 expression and self-renewal of stem-like
side-population cells in non-small cell lung cancer. Mol Cancer.
11:732012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Min HY, Yun HJ, Lee JS, Lee HJ, Cho J,
Jang HJ, Park SH, Liu D, Oh SH, Lee JJ, et al: Targeting the
insulin-like growth factor receptor and Src signaling network for
the treatment of non-small cell lung cancer. Mol Cancer.
14:1132015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Sutton P, Borgia JA, Bonomi P and Plate
JM: Lyn, a Src family kinase, regulates activation of epidermal
growth factor receptors in lung adenocarcinoma cells. Mol Cancer.
12:762013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Phuchareon J, McCormick F, Eisele DW and
Tetsu O: EGFR inhibition evokes innate drug resistance in lung
cancer cells by preventing Akt activity and thus inactivating Ets-1
function. Proc Natl Acad Sci USA. 112:E3855–E3863. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Shi Y: Mechanisms of caspase activation
and inhibition during apoptosis. Mol Cell. 9:459–470. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Joseph B, Ekedahl J, Lewensohn R,
Marchetti P, Formstecher P and Zhivotovsky B: Defective caspase-3
relocalization in non-small cell lung carcinoma. Oncogene.
20:2877–2888. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Fu YN, Yeh CL, Cheng HH, Yang CH, Tsai SF,
Huang SF and Chen YR: EGFR mutants found in non-small cell lung
cancer show different levels of sensitivity to suppression of Src:
Implications in targeting therapy. Oncogene. 27:957–965. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Zeng H, Zheng R, Guo Y, Zhang S, Zou X,
Wang N, Zhang L, Tang J, Chen J, Wei K, et al: Cancer survival in
China, 2003–2005: A population-based study. Int J Cancer.
136:1921–1930. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Frame MC: Src in cancer: Deregulation and
consequences for cell behaviour. Biochim Biophys Acta.
1602:114–130. 2002.PubMed/NCBI
|
|
19
|
Nam JS, Ino Y, Sakamoto M and Hirohashi S:
Src family kinase inhibitor PP2 restores the E-cadherin/catenin
cell adhesion system in human cancer cells and reduces cancer
metastasis. Clin Cancer Res. 8:2430–2436. 2002.PubMed/NCBI
|
|
20
|
Biscardi JS, Maa MC, Tice DA, Cox ME, Leu
TH and Parsons SJ: C-Src-mediated phosphorylation of the epidermal
growth factor receptor on Tyr845 and Tyr1101 is associated with
modulation of receptor function. J Biol Chem. 274:8335–8343. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Sarris EG, Saif MW and Syrigos KN: The
biological role of PI3K pathway in lung cancer. Pharmaceuticals
(Basel). 5:1236–1264. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Engelman JA, Luo J and Cantley LC: The
evolution of phosphatidylinositol 3-kinases as regulators of growth
and metabolism. Nat Rev Genet. 7:606–619. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Tsurutani J, Fukuoka J, Tsurutani H, Shih
JH, Hewitt SM, Travis WD, Jen J and Dennis PA: Evaluation of two
phosphorylation sites improves the prognostic significance of Akt
activation in non-small-cell lung cancer tumors. J Clin Oncol.
24:306–314. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Bose P, Rahmani M and Grant S: Coordinate
PI3K pathway and Bcl-2 family disruption in AML. Oncotarget.
3:1499–1500. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Elmore S: Apoptosis: A review of
programmed cell death. Toxicol Pathol. 35:495–516. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Ahel I, Ahel D, Matsusaka T, Clark AJ,
Pines J, Boulton SJ and West SC: Poly(ADP-ribose)-binding zinc
finger motifs in DNA repair/checkpoint proteins. Nature. 451:81–85.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Enari M, Sakahira H, Yokoyama H, Okawa K,
Iwamatsu A and Nagata S: A caspase-activated DNase that degrades
DNA during apoptosis, and its inhibitor ICAD. Nature. 391:43–50.
1998. View Article : Google Scholar : PubMed/NCBI
|