Introduction
Osteosarcoma is a malignant tumor, which caused the
most cancer-associated mortalities in Asia during the early 21st
century; however, the prognosis remains poorly understand (1–3). The
symptoms of osteosarcoma include: Tumor pain, caused by tumor
tissue erosion; and dissolved bone cortex (3). Increasing the apoptosis of osteosarcoma
cells induced by anticancer drugs has become a challenge in cancer
therapy due to tumor cell resistance via various signaling pathways
(4,5).
Recently, numerous strategies, with the aim of decreasing apoptotic
resistance, have been proposed and indicated that the overall
survival rate for patients with osteosarcoma can be improved, based
on clinical statistical analysis (6,7);
therefore, understanding the mechanism underlying apoptotic
resistance and efficacy target therapy is urgently required to
improve the overall survival rate for patients with
osteosarcoma.
Bone morphogenetic protein and activin
membrane-bound inhibitor (BAMBI) is a pseudo-receptor of SMAD7 and
is homologous to transforming growth factor-β receptor 1 (TGF-βR1),
which lacks the functional domain for an active kinase (8,9). BAMBI is
also regarded as a TGF-β pseudo-receptor and participates in the
regulation of the TGF-β-mediated signaling pathway in various
cancer types, including bladder, colorectal, ovarian, non-small
cell lung (NSCLC) and gastric cancer (10–13).
Additionally, BAMBI overexpression is beneficial for suppressing
the growth and metastasis of gastric cancer cells by inhibiting the
β-catenin and TGF-β signaling pathways (14). Furthermore, research has demonstrated
that downregulation of the TGF-β pseudo-receptor BAMBI in NSCLC
promotes the TGF-β signaling pathway, which further promotes the
growth and invasion of lung cancer tissues (15). These reports indicated that BAMBI may
be involved in the progression of human cancer.
In the present study, it was reported that BAMBI is
downregulated in osteosarcoma cells and upregulation of the TGF-β
pseudo-receptor BAMBI significantly inhibited the growth,
proliferation, migration, invasion and resistance to apoptosis of
osteosarcoma cells. The data indicated that BAMBI has critical
oncolytic effects on osteosarcoma progression and demonstrated the
therapeutic role for the treatment of osteosarcoma in vitro
and in vivo.
Materials and methods
Ethics statement
The present preclinical study was performed
according to the recommendations in the Guide for the Care and Use
of Laboratory Animals of China (16).
All experimental protocols and animals were approved by the
Committee on the Ethics of Animal Experiments Defence Research of
the Second Hospital of Tianjin Medical University (Tianjin, China).
Clinical samples were collected from The Second Hospital of Tianjin
Medical University (Tianjin, China). All patients were required to
write informed consent with a signature. The study was approved by
the Ethics Committee of The Second Hospital of Tianjin Medical
University (Tianjin, China). A total of 60 patients with
osteosarcoma (27 males, 33 females; age range, 22–61 years; median
age, 49 years) between July 2013 and March 2017 were recruited into
the present study.
Cells and reagents
Osteosarcoma cell lines SAOS2 and MG63 cells and
human normal osteoblast hFOB1.19 cells were purchased from American
Type Culture Collection (Manassas, VA, USA). SAOS2 and MG63 cells
were cultured in Dulbecco's modified Eagle's medium (Gibco; Thermo
Fisher Scientific, Inc., Waltham, MA, USA) supplemented with 10%
fetal bovine serum (FBS; Invitrogen; Thermo Fisher Scientific,
Inc.). hFOB1.19 cells were cultured in RPMI-1640 (Sigma-Aldrich;
Merck KGaA, Darmstadt, Germany) medium supplemented with 10% fetal
calf serum (Gibco; Thermo Fisher Scientific, Inc.). All cells were
cultured in a 37°C humidified atmosphere containing 5%
CO2.
Reverse transcription
quantitative-polymerase chain reaction (RT-qPCR)
Total RNA was extracted from SAOS2, MG63 and
hFOB1.19 cells using a RNAeasy Mini kit (Qiagen Sciences, Inc.,
Gaithersburg, MD, USA). A total of 1 µg total RNA was used to
transcribe into cDNA by using the PrimeScript RT Master Mix (Qiagen
Sciences, Inc.) according to the manufacturer's protocol. The cDNA
(10 ng) was subjected to RT-qPCR with SYBR Green Master Mix system
(Bio-Rad Laboratories, Inc., Hercules, CA, USA). All the forward
and reverse primers were synthesized by Invitrogen (Thermo Fisher
Scientific, Inc.). For the PCR experiments, the following forward
and reverse primers were used: Caspase-3, forward,
5′-TGGCAGCAGTGACAGCAGCA-3′ and reverse, 5′-TACGGAGGTGGAGTGGGTGT-3′;
caspase-8, forward, 5′-AGCCGAGGAAGAACTATGAAC-3′ and reverse,
5′-ATTTGAGGGTGAGGAATGGG-3′; P16, forward,
5′-GAGGGCAGAATCATCACGAAGT-3′ and reverse,
5′-TGAGAGATCTGGTTCCCGAAAC-3′; P21, forward,
5′-AGGCACGAGTAACAAGCTCAC-3′ and reverse,
5′-ATGAGGACATAACCAGCCACC-3′; Bcl-2, 5′-GTGGACATCCGCAAAGAC-3′ and
reverse, 5′-AAAGGGTGTAACGCAACTA-3′; BAMBI, forward,
5′-AGGCACGAGTAACAAGCTCAC-3′ and reverse,
5′-ATGAGGACATAACCAGCCACC-3′; Bcl-2, 5′-AAGGAATTTGTAACAAAGGT-3′ and
reverse, 5′-AGACCTGTGAGATGACCTCC-3′; and reference gene GAP DH,
forward 5′-GTGGGCGCCCAGGCACCA-3′ and reverse,
5′-CTCCTTAATGTCACGCACGATTT-3′. Amplification conditions consisted
of 5 sec of denaturation at 94°C, 9 sec of annealing at 55–60°C and
9 sec of extension at 72°C, for 45 cycles for each step. Relative
mRNA expression changes were calculated by 2−ΔΔCq
(17). The results are expressed as
the relative expression compared with control.
MTT assays
SAOS2 and MG63 cells were treated with BAMBI (0, 5,
10 and 15 mg/ml, Abcam, Cambridge, UK) or PBS and cultured in
96-well plates for 48 h. A total of 20 µl MTT (5 mg/ml) in PBS
solution was added to each well, and the plate was further
incubated for 4 h at 37°C. The medium was removed and 100 µl
dimethyl sulfoxide (Sigma-Aldrich; Merck KGaA) was added into the
wells to solubilize the crystals. The OD was measured by an iMark
microplate absorbance reader (Bio-Rad Laboratories, Inc.) at
wavelength of 450 nm.
TGF-β overexpression (pTGF-β)
TGF-β gene was cloned into a PMD-18-T vector and
sequenced to identify its sequence, according to a previous report
(18). The TGF-β gene was then cloned
into a eukaryotic expression vector pCMVp-NEO-BAN (pTGF-β; Takara
Biotechnology Co., Ltd, Dalian, China) to generate
TGF-β-overexpressed SAOS2 or MG63 cells as described previously
(18). SAOS2 and MG63 cells were
cultured in 6-well plates in RPMI-1640 medium containing 10% FBS at
37°C until 90% confluence and the media was then removed. SAOS2 and
MG63 (5×106) cells were transfected with pTGF-β (1.0 µg,
Takara Biotechnology Co.) or pvector (1.0 µg) using
Lipofectamine® 2000 (Thermo Fisher Scientific, Inc.),
according to the manufacturer's protocol. Stable
TGF-β-overexpression SAOS2 or MG63 cells were selected using the
dihydrofolate reductase/glutamine synthetase (Invitrogen; Thermo
Fisher Scientific, Inc.) screening system (19).
Terminal deoxynucleotidyl
transferase-mediated dUTP nick end labeling (TUNEL) assay
analysis
For analysis of the apoptosis of osteosarcoma, a
TUNEL assay (Beyotime Institute of Biotechnology, Haimen, China)
was used to detect TUNEL-positive cells. Tumor sections isolated
from xenografted mice were fixed with 4% paraformaldehyde solution
for 60 min at 4°C. The tumor tissues were washed with PBS three
times and then permeabilized by immersing cells slides in 0.2%
Triton X-100 solution in PBS for 30 min at 4°C. Subsequently, tumor
tissues were incubated with equilibration buffer (Beyotime
Institute of Biotechnology) for 30 min at 4°C. The tumor tissues
were then incubated with 50 µl reaction mixture (Beyotime Institute
of Biotechnology) at 37°C for 60 min and washed 3 times with PBS.
These sections were incubated in 5% bovine serum albumin (BSA) for
30 min and the fragmented DNA was labeled with the TUNEL reaction
solution at 37°C for 1 h. Hoechst 33258 (5 mg/l; H-33258;
Sigma-Aldrich; Merck KGaA) was used to stain the nuclei for 10 min
at room temperature. Converter-peroxidase was added to the sections
at 37°C for 30 min prior to the TUNEL-positive nuclei being
visualized by adding the DAB staining solution. Finally, tumor
tissues images were captured with a confocal microscope at 488 nm.
TUNEL-positive cells were counted in 5 randomly selected fields per
section. The apoptosis rate was expressed as the ratio of
TUNEL-positive cardiomyocytes to the total number of
cardiomyocytes.
Apoptotic detection
SAOS2 and MG63 cells were cultured in RPMI-1640
medium containing 10% FBS at 37°C until 90% confluence and then
treated with BAMBI (10 mg/ml). Cells were continually cultured for
48 h at 37°C and then trypsinized, collected and washed in cold PBS
3 times. Subsequently, the cells (1×106 cells/ml) were
mixed with PBS, labeled with Annexin V-fluorescein isothiocyanate
(FITC) and propidium iodide (Annexin V-FITC kit; BD, San Diego, CA,
USA) according to the manufacturers protocol and analyzed with a
FACScan flow cytometer (BD Biosciences, San Jose, CA, USA) and
Quantity One software (version 3.0; Bio-Rad Laboratories,
Inc.).
Cells invasion and migration
assays
SAOS2 and MG63 cells were treated with BAMBI (5
mg/ml) for 24 h at 37°C and used to analyze the cell invasion and
migration. SAOS2 and MG63 cells were placed in a 24-well culture
plate with chamber inserts (BD Biosciences). For migration assays,
5×104/well SAOS2 and MG63 cells in RPMI-1640 medium were
placed into the upper chamber with the non-coated membrane at 37°C
for 24 h. For invasion assays, the cells (5×104/well)
were placed into the upper chamber with the Matrigel-coated
membrane. In the invasion assay, cells were treated with BAMBI (5
mg/ml) for 24 h and subjected to the tops of BD BioCoat Invasion
Chambers (BD Biosciences), according to the manufacturer's
protocols. The medium and serum in the lower chamber was DMEM plus
20% FBS. The number of tumor cells that invaded and migrated
through the membrane were by stained with 0.5% crystal violet at
37°C for 30 min and counted at least three randomly selected fields
per membrane under a light microscope in five random visual fields
(magnification, ×200).
Western blot analysis
SAOS2 and MG63 cells were treated with SB431542 (5
ng/ml, cat. no 93-1674-1; Biovision Inc., Milpitas, CA, USA),
cisplatin (10 mg/ml, Takara Biotechnology Co., Ltd.) and BAMBI (10
mg/ml) and harvested by scraping and lysed in
radioimmunoprecipitation assay buffer (Invitrogen; Thermo Fisher
Scientific, Inc.) followed by homogenization at 4°C for 10 min.
Protein concentration was calculated using a BCA protein assay kit
(Thermo Fisher Scientific, Inc.). Proteins (10 µg) were analyzed
via 10% SDS-PAGE assays, followed by transferring onto
polyvinylidene fluoride membrane. Proteins were then blocked with
5% BSA (Sigma-Aldrich; Merck KGaA) for 2 h at 37°C and incubated
for 1 h at room temperature with primary rabbit anti-mouse
antibodies against: BAMBI (1:500; cat. no. AF2387; R&D Systems,
Inc., Minneapolis, USA); P21 (1:1,000; cat. no. ab109199; Abcam);
P16 (1:1,000; cat. no. ab51243; Abcam); B-cell lymphoma 2 (1:1,000;
Bcl-2; cat. no. ab692; Abcam); TGF-β (1:1,000; cat. no. AF532;
R&D Systems); epithelial (E)-cadherin (1:200; cat. no.
NBP238856; Novus Biologicals LLC, Littleton, CO, USA); vimentin
(1:500; cat. no. PAB24865; Abnova, Taipei, Taiwan); Twist (1:500;
cat. no. DR1088100UG; EMD Millipore, Billerica, MA, USA); Smad2
(1:500; cat. no. ab53110; Abcam); Smad3 (1:500; cat. no. ab40854;
Abcam); caspase-3 (1:500; cat. no. ab13847; Abcam); caspase-8
(1:500; cat. no. ab25901; Abcam); pSMAD2 (1:500; cat. no. ab53100;
Abcam); pSMAD3 (1:500; cat. no. ab63403; Abcam) and β-actin (1:500;
cat. no. ab8226; Abcam). Subsequently, proteins were inoculated
with rabbit horseradish peroxidase (HRP)-labeled IgG (1:10,000;
cat. no. ab6728; Abcam) for 12 h at 4°C. The proteins expression
levels were visualized using a chemiluminescence detection system
(Nikon Corporation, Tokyo, Japan). Expression levels were
determined relative to β-actin. The density of the bands was
analyzed using Quantity One software version 4.62 (Bio-Rad
Laboratories, Inc.). Protein expression was analyzed using BandScan
5.0 software (Glyko, Inc., Novato, CA, USA). All experiments were
repeated ≥3 times.
Immunohistochemistry
Tumors from Mg63-bearing xenograph mice were fixed
using 10% formaldehyde followed with being embedded in paraffin wax
and cut into serial sections of 4 µm thickness. Paraffin-embedded
tissue sections 4 µm thick were prepared and epitope retrieval was
performed for further analysis. The paraffin sections were
incubated with hydrogen peroxide (3%) for 10–15 min at 37°C and
were subsequently blocked with a regular blocking solution (normal
goat serum) for 10–15 min at 37°C. Tumor sections were incubated
with primary antibodies against: TGF-β; E-cadherin; vimentin; and
Twist. Subsequently, proteins were inoculated with rabbit
HRP-labeled IgG (1:5,000; cat. no. ab6728, Abcam) for 12 h at 4°C.
Specimens were visualized. Images were captured using fluorescence
video microscopy (BZ-9000; Keyence Corporation, Osaka, Japan) at
×400 magnification. A Benchmark automated staining system (Ventana
Medical Systems, Inc, Tucson, AZ USA) was used for observation of
integrin.
Animal study
Specific pathogen-free female nude (six-eight weeks
old, 25–32 g) C57BL/6 mice were purchased from Shanghai Slack
Experimental Animals Co., Ltd. (Shanghai, China). All mice were
housed at room temperature with a 12/12 h light/dark cycle and fed
ad libitum. Mouse breeding and experiments were carried out
under the Institutional Animal Care and Use Committee approved
protocols of Ethics Committee of Library Animals (16). Mg63 tumor cells (1×107)
were subcutaneously implanted into the right flank of C57BL/6 mice
(n=60). Mice bearing osteosarcoma were randomly divided into two
groups (n=30 in each group) and received treatment with BAMBI (10
mg/kg) or PBS. The treatments for tumor-bearing mice were initiated
when tumor diameters reached 5–7 mm on day 3 following tumor
inoculation. The detail procedures were referenced according to
previous report (20). The treatments
were continued seven times at intervals of every two days. Tumor
diameters were recorded every two days and tumor volume was
calculated using the formula: 0.52× smallest diameter2 ×
largest diameter (21). The
experimental mice were euthanized when tumor diameter reached 10 mm
with 1% pentobarbital (200 mg/kg) administered via intravenous
injection. On day 25, 10 mice in each group were sacrificed for
further analysis, including immunohistochemistry.
Statistical analysis
All data are presented as the mean ± standard error
of the mean of triplicate experiments. Unpaired data was determined
by Student's t-test and comparisons of data between multiple groups
were analyzed by analysis of one-way analysis of variance followed
by Fisher's Least Significant Difference post hoc test.
Kaplan-Meier analysis was used to estimate the risk of relapse and
re-treatment during the 100-day treatment. All data analysis was
performed using SPSS software (version 20.0; IBM Corp., Armonk, NY,
USA). P<0.05 was considered to indicate a statistically
significant difference.
Results
BAMBI expression is downregulated in
osteosarcoma cell lines
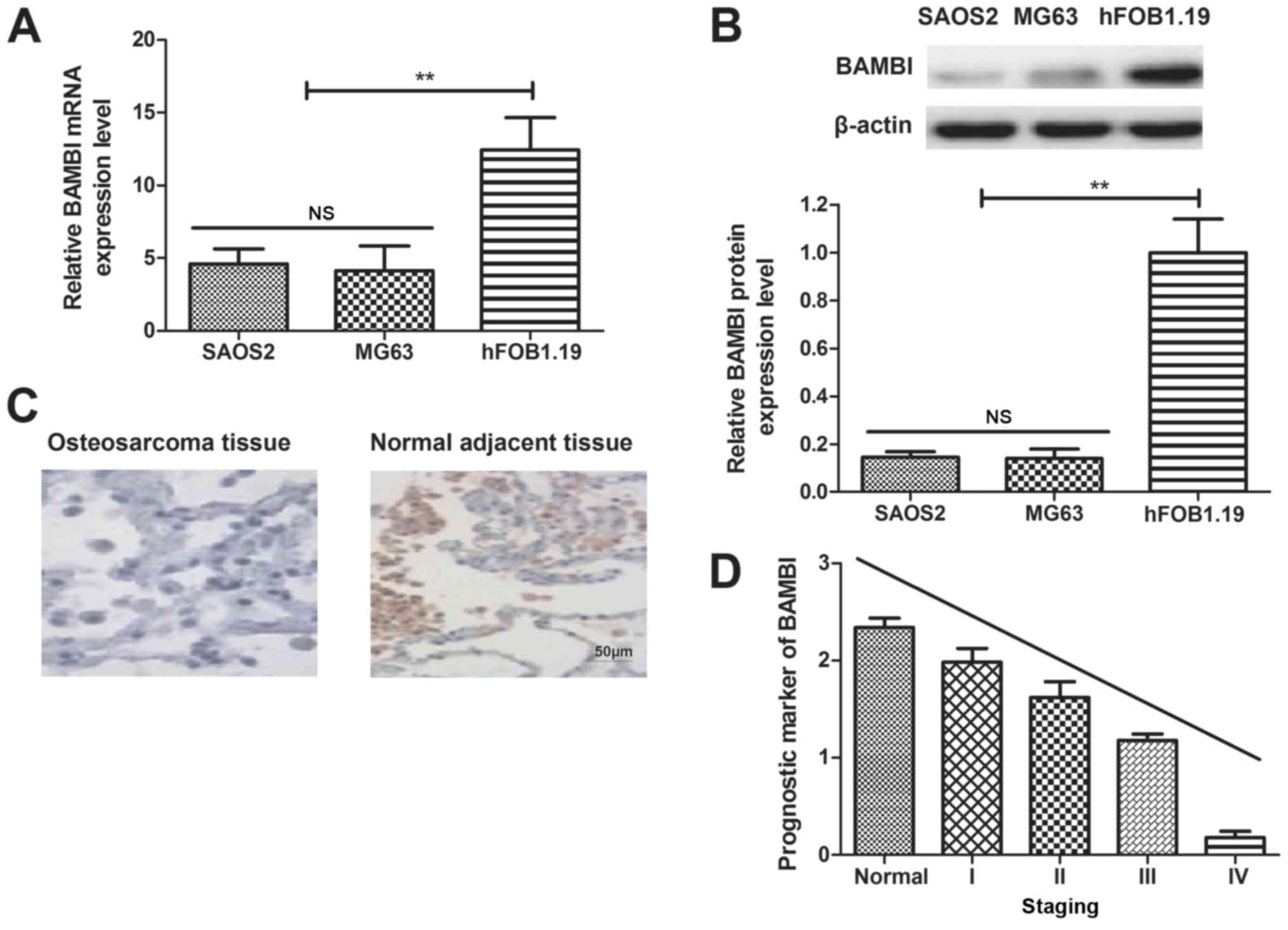
Expression levels of BAMBI in osteosarcoma cell
lines and clinical tumor tissues were analyzed. It was observed
that mRNA and protein expression levels of BAMBI were upregulated
in SAOS2 and MG63 cells, compared with normal cell line hFOB1.19
(Fig. 1A and B). It was also
determined that BAMBI expression levels were lower in osteosarcoma
tissues, compared with normal adjacent tissues, determined by
immunohistochemistry (Fig. 1C).
Results in Fig. 1D indicated that
BAMBI expression may be an independent prognostic marker in
osteosarcoma, as demonstrated by multivariate analyses.
Collectively, these results indicated that BAMBI downregulation may
be associated with osteosarcoma progression.
BAMBI reconstitution (10 mg/ml)
suppresses growth and aggressiveness of osteosarcoma cell
lines
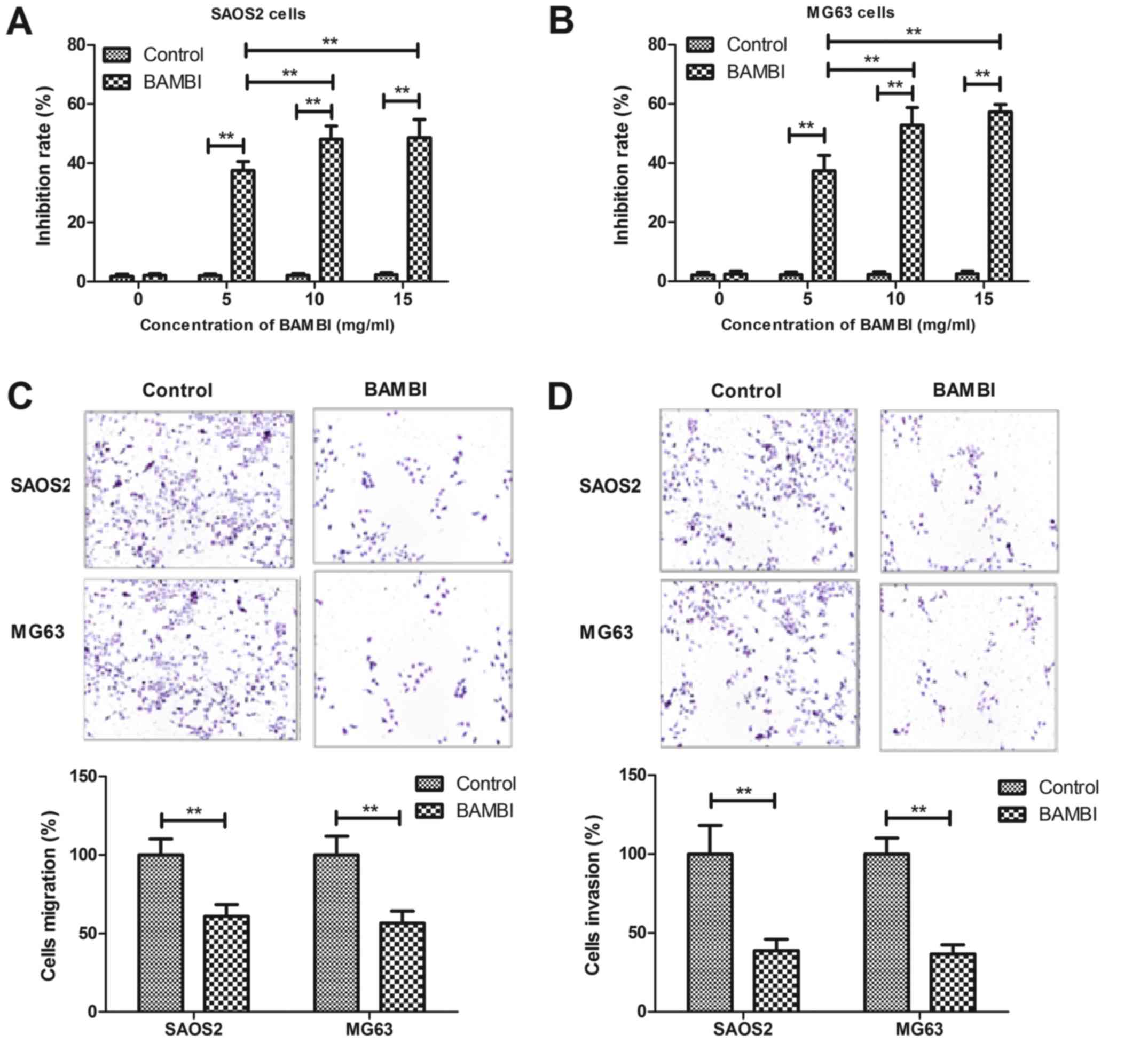
In order to analyze inhibitory effects of BAMBI on
osteosarcoma cell growth and aggressiveness of SAOS2 and MG63
cells, BAMBI was added into cultured osteosarcoma cells. As
depicted in Fig. 2A and B, BAMBI
treatment significantly inhibited SAOS2 and MG63 cells growth in a
dose-dependent manner (P<0.05). BAMBI (10 mg/ml) treatment was
observed to notably suppress the migration and invasion of SAOS2
and MG63 cells after 24 h incubation (Fig. 2C and D). Collectively, the data
indicated that BAMBI treatment inhibited the growth and
aggressiveness of osteosarcoma cells in vitro.
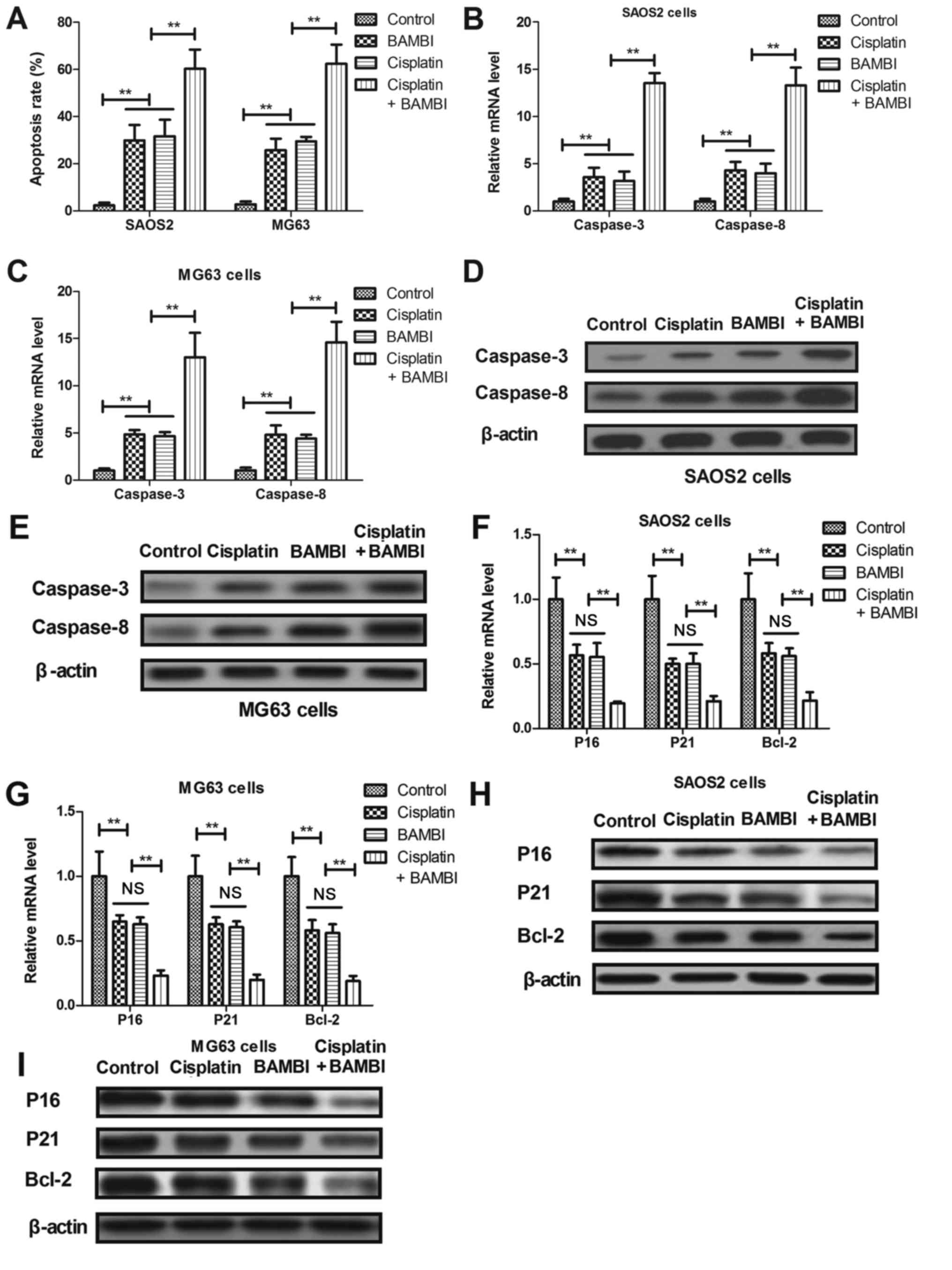
BAMBI reconstitution promoted
apoptosis of osteosarcoma cells induced by cisplatin
Apoptosis of osteosarcoma cell lines SAOS2 and MG63
was investigated following treatment with cisplatin (10 mg/ml). As
depicted in Fig. 3A, BAMBI (10 mg/ml)
promoted the apoptosis of SAOS2 and MG63 cells induced by
cisplatin, compared with non-treated cells. RT-qPCR demonstrated
that gene and protein expression levels of caspase-3 and caspase-8
were upregulated in BAMBI-treated SAOS2 and MG63 cells (Fig. 3B-E). It was demonstrated that
anti-apoptosis gene and protein expression levels of P16, P21 and
Bcl-2 were decreased in SAOS2 and MG63 following BAMBI treatment
(10 mg/ml), compared with the control (Fig. 3F-I). Collectively, these results
indicated that BAMBI promotes apoptosis of osteosarcoma cells
induced by cisplatin.
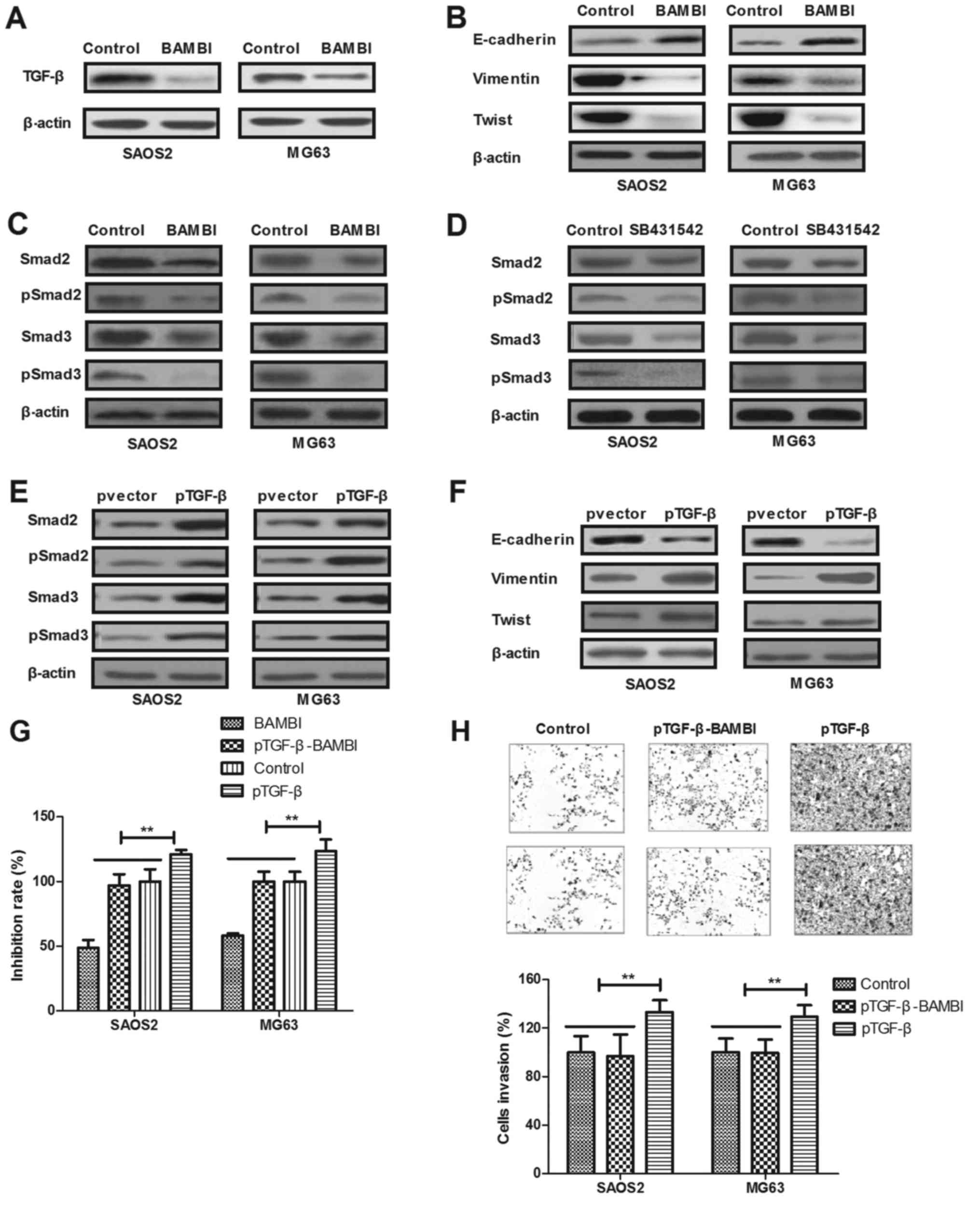
BAMBI inhibits osteosarcoma growth and
invasion via the TGF-β-induced EMT signaling pathway
In order to investigate the potential mechanism
underlying BAMBI-mediated inhibition of growth and aggressiveness
in osteosarcoma cell lines, the TGF-β-induced EMT signaling pathway
in SAOS2 and MG63 cells was analyzed. As depicted in Fig. 4A, BAMBI treatment suppressed TGF-β
protein expression in SAOS2 and MG63 cells. Additionally, BAMBI
treatment increased E-cadherin and inhibited vimentin and Twist
expression in SAOS2 and MG63 cells (Fig.
4B). Results demonstrated that expression and phosphorylation
levels of Smad2 and Smad3 were decreased by BAMBI treatment in
SAOS2 and MG63 cells (Fig. 4C). It
was determined that the blocked TGF-β receptor using SB431542 also
suppressed the expression and phosphorylation levels of Smad2 and
Smad3 in BAMBI-treated SAOS2 and MG63 cells (Fig. 4D); however, pTGF-β antagonized the
expression and phosphorylation of Smad2 and Smad3, as well as EMT
markers in BAMBI-treated SAOS2 and MG63 cells (Fig. 4E and F). Notably, it was determined
that pTGF-β inhibited BAMBI-inhibited growth and invasion of SAOS2
and MG63 cells (Fig. 4G and H).
Collectively, the results indicated that BAMBI reconstitution
inhibits osteosarcoma growth and invasion via inactivating the
TGF-β-induced EMT signaling pathway.
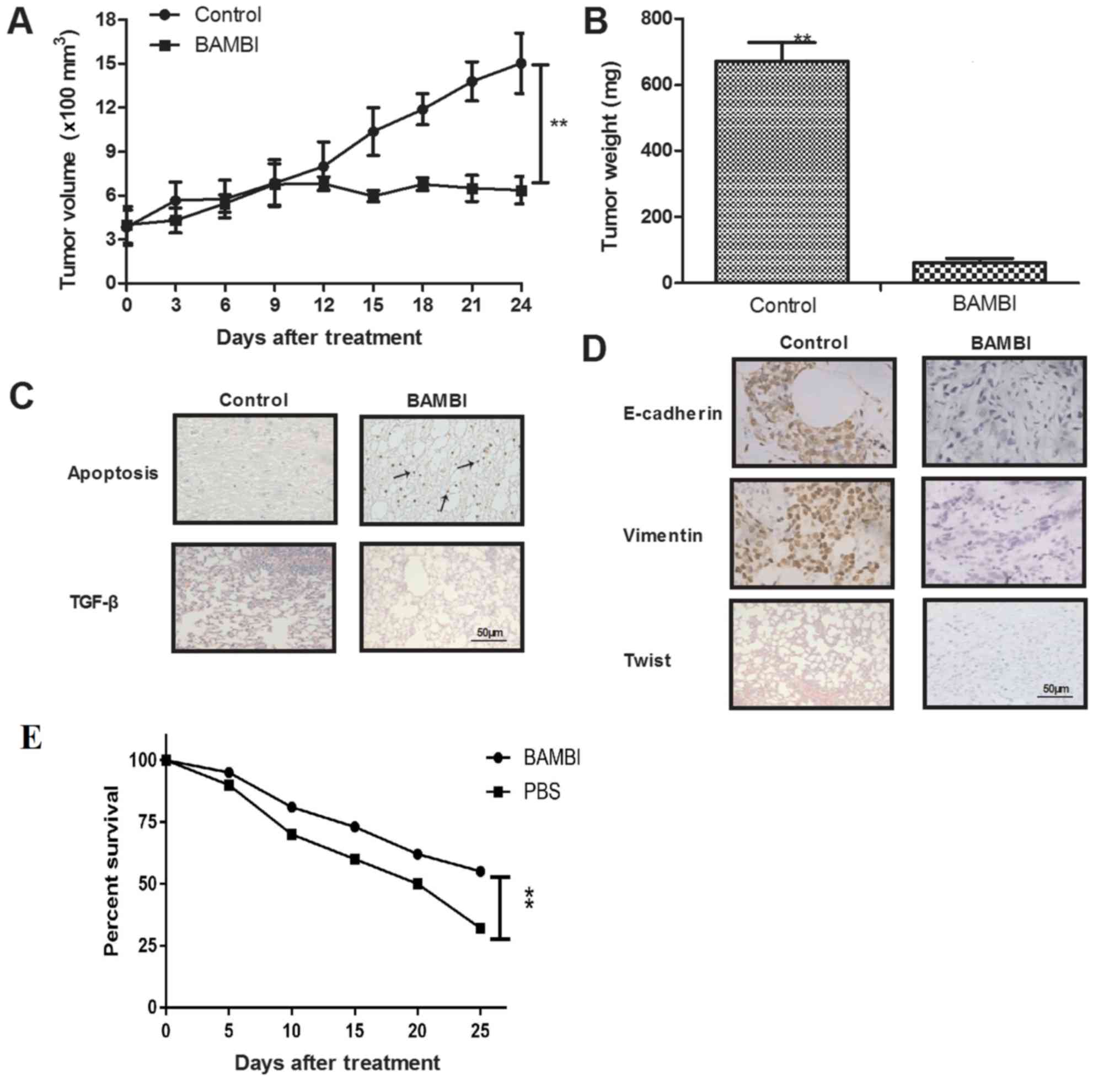
In vivo efficacy of BAMBI
To further identify the therapeutic efficacy of
BAMBI for osteosarcoma growth, osteosarcoma xenograft mice model
were established. The results demonstrated that the intratumor
injection of BAMBI (10 mg/ml) significantly inhibited tumor growth
and tumor weight, compared with PBS-treated group (Fig. 5A and B; P<0.05). The TUNEL assay
demonstrated that BAMBI increased apoptotic cells and decreased
TGF-β expression in the tumor sections, compared with control group
(Fig. 5C). Immunohistochemistry
indicated that EMT markers were decreased in BAMBI-treated tumor
tissues, compared with control (Fig.
5D). Collectively, these results indicated that BAMBI may be a
potential anticancer agent for osteosarcoma and improve the overall
survival rate of xenografted mice compared with control group
(Fig. 5E, P<0.05).
Discussion
Osteosarcoma occurs in bones and their affiliated
tissues; however, the mechanism underlying tumorigenesis remains
unclear (22). The common symptoms of
malignant osteosarcoma include: Bone pain; swelling; and fatigue
(23,24). Recently, numerous reports have
proposed strategies for the treatment of osteosarcoma (25–27);
however, the overall survival rate of patients with osteosarcoma
has not significantly improved. A previous study determined that
TGF-β1 inhibitory pseudo-receptor-BAMBI may be regarded as a target
in the β-catenin pathway of colorectal tumor cells, which further
results in the inhibition of tumor cells growth and metastasis
(28). Notably, the potential
molecular mechanism mediated by BAMBI in the progression of
osteosarcoma is not well understood. In the present study, the
inhibitory effects of BAMBI on osteosarcoma cell growth was
investigated in vitro and in vivo. The BAMBI-mediated
signaling pathway in osteosarcoma cell lines SAOS2 and MG63 was
analyzed. The data demonstrated that BAMBI expression is
downregulated in osteosarcoma cell lines and BAMBI suppresses the
growth and aggressiveness of osteosarcoma cell lines via the
TGF-β-induced EMT signaling pathway.
Although a previous study indicated the role of
BAMBI in gastric cells, very few studies focus on how
BAMBI-mediated growth and metastasis of osteosarcoma cells
(29). Notably, decreasing the BAMBI
expression enhanced TGF-β signaling and invasion in NSCLC cells
(15). In the present study, a lower
expression of BAMBI was observed in osteosarcoma cells following
TGF-β-induced aggression and EMT-dependent malignant processes
(30). Although Zhou et al
(31) indicated that BAMBI serves a
key role in the pathogenesis and progression of osteosarcoma by
regulating the expression of β-catenin and other signal molecules
via the pathways involved in the regulation of the cell cycle, the
results concluded conflicting results in human osteosarcoma. Our
hypothesis was further identified in xenografted mice and indicated
that BAMBI treatment significantly inhibited osteosarcoma cells
growth and promoted the apoptosis of tumor cells.
Previous reports have indicated that the TGF-β and
EMT signaling pathways are considered to be correlated with
malignancy of osteosarcoma and responsible for its growth,
migration and metastasis (32–35).
Tsubaki et al (36)
demonstrated that inhibition of the Ras/mitogen-activated protein
kinase kinase/extracellular signal-regulated kinase and
Ras/phosphoinositide 3-kinase/Akt pathways by reduction of the
expression of TGF-β could inhibit tumor growth in mouse
osteosarcoma. Studies have indicated that the EMT signaling pathway
serves a significant role in osteosarcoma and evidence indicated
that overexpression of EMT transcription factors, including Twist,
Snails and zinc finger E-box binding homeobox, is involved in the
complex pathogenesis of osteosarcoma (35,37).
Furthermore, Wendt et al (38)
indicated that deconstructing the mechanisms and consequences of
TGF-β-induced EMT exerted anticancer activities by prohibiting cell
proliferation. In the present study, the results indicated that
BAMBI treatment suppresses growth and aggressiveness of
osteosarcoma cell lines via the TGF-β-induced EMT signaling
pathway, whilst TGF-β overexpression antagonized the downregulated
expression and phosphorylation of Smad2 and Smad3, as well as EMT
markers caused by BAMBI treatment in SAOS2 and MG63 cells.
In conclusion, the data indicated that BAMBI
treatment may result in the inhibition of SAOS2 and MG63 cells via
regulation of the TGF-β-induced EMT signaling pathway, which
contributes to increasing the apoptosis of tumor cells (39). Outcomes demonstrated that BAMBI
treatment suppresses osteosarcoma cells in vitro and in
vivo, which may enhance the therapeutic effects of BAMBI in the
treatment of osteosarcoma. Notably, BAMBI treatment increases the
apoptosis of osteosarcoma cells induced by cisplatin via inhibition
of anti-apoptosis gene P16, P21 and Bcl-2 in SAOS2 and MG63 cells.
In combination, these investigations indicate that BAMBI may be a
potential agent for the treatment of osteosarcoma; however, future
studies are required to investigate and identify the therapeutic
effects of BAMBI in different osteosarcoma cell lines.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
FL and KW designed the study. LZ and YY performed
the experiments. YY analyzed the data.
Ethics approval and consent to
participate
All patients were required to provide written
informed consent prior to their inclusion. The study was approved
by the Ethics Committee of The Second Hospital of Tianjin Medical
University (Tianjin, China).
Consent for publication
All patients provided written informed consent for
the publication of their data.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Mathkour M, Garces J, Beard B, Bartholomew
A, Sulaiman OA and Ware ML: Primary high-grade osteosarcoma of the
clivus: A case report and literature review. World Neurosurg.
89:730.e9–730.e13. 2016. View Article : Google Scholar
|
|
2
|
Zheng YF, Lin J and Yang HL:
Chondroblastic osteosarcoma secondary to fibrosarcoma: A case
report and literature review. Oncol Lett. 10:3573–3576. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Friebele JC, Peck J, Pan X, Abdel-Rasoul M
and Mayerson JL: Osteosarcoma: A meta-analysis and review of the
literature. Am J Orthop (Belle Mead NJ). 44:547–553.
2015.PubMed/NCBI
|
|
4
|
Tsagaraki I, Tsilibary EC and Tzinia AK:
TIMP-1 interaction with alphavbeta3 integrin confers resistance to
human osteosarcoma cell line MG-63 against TNF-α-induced apoptosis.
Cell Tissue Res. 342:87–96. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Locklin RM, Federici E, Espina B, Hulley
PA, Russell RG and Edwards CM: Selective targeting of death
receptor 5 circumvents resistance of MG-63 osteosarcoma cells to
TRAIL-induced apoptosis. Mol Cancer Ther. 6:3219–3228. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Dell'Amore A, Asadi N, Caroli G, Dolci G,
Bini A and Stella F: Recurrent primary cardiac osteosarcoma: A case
report and literature review. General Thorac Cardiovasc Surg.
62:175–180. 2014. View Article : Google Scholar
|
|
7
|
Farcas N, Arzi B and Verstraete FJ: Oral
and maxillofacial osteosarcoma in dogs: A review. Vet Comp Oncol.
12:169–180. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Shangguan L, Ti X, Krause U, Hai B, Zhao
Y, Yang Z and Liu F: Inhibition of TGF-β/Smad signaling by BAMBI
blocks differentiation of human mesenchymal stem cells to
carcinoma-associated fibroblasts and abolishes their protumor
effects. Stem Cells. 30:2810–2819. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Guillot N, Kollins D, Gilbert V, Xavier S,
Chen J, Gentle M, Reddy A, Bottinger E, Jiang R, Rastaldi MP, et
al: BAMBI regulates angiogenesis and endothelial homeostasis
through modulation of alternative TGFβ signaling. PloS one.
7:e394062012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Fritzmann J, Morkel M, Besser D, Budczies
J, Kosel F, Brembeck FH, Stein U, Fichtner I, Schlag PM and
Birchmeier W: A colorectal cancer expression profile that includes
transforming growth factor beta inhibitor BAMBI predicts metastatic
potential. Gastroenterology. 137:165–175. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Khin SS, Kitazawa R, Win N, Aye TT, Mori
K, Kondo T and Kitazawa S: BAMBI gene is epigenetically silenced in
subset of high-grade bladder cancer. Int J Cancer. 125:328–338.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Miao S, Zhao L, Gao J, Wang H and Cui Z:
Distribution and mRNA expression of BAMBI in non-small-cell lung
cancer. Zhongguo Fei Ai Za Zhi. 12:203–207. 2009.(In Chinese).
PubMed/NCBI
|
|
13
|
Pils D, Wittinger M, Petz M, Gugerell A,
Gregor W, Alfanz A, Horvat R, Braicu EI, Sehouli J, Zeillinger R,
et al: BAMBI is overexpressed in ovarian cancer and co-translocates
with Smads into the nucleus upon TGF-beta treatment. Gynecol Oncol.
117:189–197. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Liu K, Song X, Ma H, Liu L, Wen X, Yu J,
Wang L and Hu S: Knockdown of BAMBI inhibits β-catenin and
transforming growth factor β to suppress metastasis of gastric
cancer cells. Mol Med Rep. 10:874–880. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Marwitz S, Depner S, Dvornikov D, Merkle
R, Szczygieł M, Müller-Decker K, Lucarelli P, Wäsch M, Mairbäurl H,
Rabe KF, et al: Downregulation of the TGFβ Pseudoreceptor BAMBI in
non-small cell lung cancer enhances tgfbeta signaling and invasion.
Cancer Res. 76:3785–3801. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Xing W, Zhigang W, Bing H, Haitao R, Pan
L, Chuanshan X, Yuanyi Z and Ao L: Targeting an ultrasound contrast
agent to folate receptors on ovarian cancer cells: Feasibility
research for ultrasonic molecular imaging of tumor cells. J
Ultrasound Med. 29:609–614. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Shi X, DiRenzo D, Guo LW, Franco SR, Wang
B, Seedial S and Kent KC: TGF-beta/Smad3 stimulates stem
cell/developmental gene expression and vascular smooth muscle cell
de-differentiation. PloS One. 9:e939952014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Renshaw A and Elsheikh TM: A validation
study of the Focalpoint GS imaging system for gynecologic cytology
screening. Cancer Cytopathol. 121:737–738. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Bai FL, Yu YH, Tian H, Ren GP, Wang H,
Zhou B, Han XH, Yu QZ and Li DS: Genetically engineered Newcastle
disease virus expressing interleukin-2 and TNF-related
apoptosis-inducing ligand for cancer therapy. Cancer Biol Ther.
15:1226–1238. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zhuang T, Djemil T, Qi P, Magnelli A,
Stephans K, Videtic G and Xia P: Dose calculation differences
between Monte Carlo and pencil beam depend on the tumor locations
and volumes for lung stereotactic body radiation therapy. J Appl
Clin Med Phys. 14:40112013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Durfee RA, Mohammed M and Luu HH: Review
of osteosarcoma and current management. Rheumatol Ther. 3:221–243.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
He F, Zhang W, Shen Y, Yu P, Bao Q, Wen J,
Hu C and Qiu S: Effects of resection margins on local recurrence of
osteosarcoma in extremity and pelvis: Systematic review and
meta-analysis. Int J Surg. 36:283–292. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Heaton TE, Hammond WJ, Farber BA, Pallos
V, Meyers PA, Chou AJ, Price AP and LaQuaglia MP: A 20-year
retrospective analysis of CT-based pre-operative identification of
pulmonary metastases in patients with osteosarcoma: A single-center
review. J Pediatr Surg. 52:115–119. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Angelini A, Mavrogenis AF, Trovarelli G,
Ferrari S, Picci P and Ruggieri P: Telangiectatic osteosarcoma: A
review of 87 cases. J Cancer Res Clin Oncol. 142:2197–2207. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Bilbao-Aldaiturriaga N, Askaiturrieta Z,
Granado-Tajada I, Goričar K, Dolžan V; For The Slovenian
Osteosarcoma Study Group, ; Garcia-Miguel P, Garcia de Andoin N,
Martin-Guerrero I and Garcia-Orad A: A systematic review and
meta-analysis of MDM2 polymorphisms in osteosarcoma susceptibility.
Pediatr Res. 80:472–479. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Hu K, Dai HB and Qiu ZL: mTOR signaling in
osteosarcoma: Oncogenesis and therapeutic aspects (Review). Oncol
Rep. 36:1219–1225. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Sekiya T, Adachi S, Kohu K, Yamada T,
Higuchi O, Furukawa Y, Nakamura Y, Nakamura T, Tashiro K, Kuhara S,
et al: Identification of BMP and activin membrane-bound inhibitor
(BAMBI), an inhibitor of transforming growth factor-beta signaling,
as a target of the beta-catenin pathway in colorectal tumor cells.
J Biol Chem. 279:6840–6846. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Pak KH, Kim DH, Kim H, Lee do H and Cheong
JH: Differences in TGF-β1 signaling and clinicopathologic
characteristics of histologic subtypes of gastric cancer. BMC
Cancer. 16:602016. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Cho Y, Cho EJ, Lee JH, Yu SJ, Kim YJ, Kim
CY and Yoon JH: Hypoxia enhances tumor-stroma crosstalk that drives
the progression of hepatocellular carcinoma. Dig Dis Sci.
61:2568–2577. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Zhou L, Park J, Jang KY, Park HS, Wagle S,
Yang KH, Lee KB, Park BH and Kim JR: The overexpression of BAMBI
and its involvement in the growth and invasion of human
osteosarcoma cells. Oncol Rep. 30:1315–1322. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Tu B, Peng ZX, Fan QM, Du L, Yan W and
Tang TT: Osteosarcoma cells promote the production of pro-tumor
cytokines in mesenchymal stem cells by inhibiting their osteogenic
differentiation through the TGF-β/Smad2/3 pathway. Exp Cell Res.
320:164–173. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Schedlich LJ, Yenson VM and Baxter RC:
TGF-β-induced expression of IGFBP-3 regulates IGF1R signaling in
human osteosarcoma cells. Mol Cell Endocrinol. 377:56–64. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Yu L, Liu S, Guo W, Zhang C, Zhang B, Yan
H and Wu Z: hTERT promoter activity identifies osteosarcoma cells
with increased EMT characteristics. Oncol Lett. 7:239–244. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Yang G, Yuan J and Li K: EMT transcription
factors: Implication in osteosarcoma. Med Oncol. 30:6972013.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Tsubaki M, Yamazoe Y, Yanae M, Satou T,
Itoh T, Kaneko J, Kidera Y, Moriyama K and Nishida S: Blockade of
the Ras/MEK/ERK and Ras/PI3K/Akt pathways by statins reduces the
expression of bFGF, HGF and TGF-β as angiogenic factors in mouse
osteosarcoma. Cytokine. 54:100–107. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Zhang D, Jiang F, Wang X and Li G:
Downregulation of ubiquitin-specific protease 22 inhibits
proliferation, invasion and EMT in osteosarcoma cells. Oncol Res.
25:743–751. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Wendt MK, Tian M and Schiemann WP:
Deconstructing the mechanisms and consequences of TGF-β-induced EMT
during cancer progression. Cell Tissue Res. 347:85–101. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Kitazawa S, Kitazawa R, Obayashi C and
Yamamoto T: Desmoid tumor with ossification in chest wall: Possible
involvement of BAMBI promoter hypermethylation in metaplastic bone
formation. J Bone Miner Res. 20:1472–1477. 2005. View Article : Google Scholar : PubMed/NCBI
|