Introduction
Breast cancer is the most common malignant tumor
type in women world wide (1).
Although several therapeutic advances have been achieved in
extending the disease-free survival and overall survival times of
patients, treatments for breast cancer remain far from
satisfactory. Identifying active components from natural plants has
become a novel treatment strategy (2,3).
Currently, numerous anti-cancer agents have been identified,
including Clematis ganpiniana (4).
Clematis ganpiniana, in the Ranunculaceae
family and Clematis genus, are widely used in traditional Chinese
medicine. Although the roots and rhizomes of Clematis
ganpiniana may be used as antibacterial and anticancer agents,
the chemical components require additional study (4). In a previous study, four types of
triterpenoid derivatives were identified and purified from
Clematis ganpiniana, which were demonstrated to induce
cytotoxicity in breast cancer cells (4). Furthermore, D Rhamnose β-hederin
(3β-[(α-L-arabinopyranosyl)-oxy] olean-12-en-28-oicacid) (DRβ-H),
one of the isolated oleanane-type triterpenoid saponins, was
investigated. The apoptotic activity and anticancer properties of
DRβ-H have been previously demonstrated, suggesting that DRβ-H may
be a potential treatment for breast cancer (5). However, the underlying molecular
mechanism by which DRβ-H inhibits malignant cells growth remains
unclear.
Exosomes have attracted interest since the
realization that these small vesicles are not merely cellular
debris, but rather important regulators in tumor biology processes,
including angiogenesis, invasiveness, metastasis and
chemoresistance (6). They contain a
wide variety of proteins, lipids and mRNAs, including microRNAs
(miRNAs) that maybe transferred from donor cells to recipient cells
inducing epigenetic changes (7,8). Exosome
secretion is one of the mechanisms by which breast cancer cells
communicate with surrounding cells and reprogram the tumor
microenvironment (9,10). By using established cell lines
(11), previous studies demonstrated
that drug-resistant breast cancer cells may spread chemoresistance
to target cells by releasing abundant exosomes and that such
modulatory effects may be partly attributed to the intercellular
transfer of specific miRNAs (12,13). In
the present study, the effects of DRβ-H on exosome secretion and
the functions of breast cancer-derived exosomes in cell growth were
evaluated.
Materials and methods
Cell culture and drug preparation
The cell line used in the present study was
wild-type drug-sensitive MCF-7 breast cancer cells (MCF-7/S)
purchased from the Cell Bank of the Chinese Academy of Sciences
(Shanghai, China). In selected experiments, MCF-7/S expressing
green fluorescent protein (GFP-S) was generated and characterized
as previously reported (14). Cells
were cultured in a 5% CO2 atmosphere at 37°C in
Dulbecco's modified Eagle's medium with a high glucose content
(HyClone; GE Healthcare Life Sciences, Logan, UT, USA),
supplemented with 10% fetal bovine serum (FBS) (Gibco; Thermo
Fisher Scientific, Inc., Waltham, MA, USA), 100 U/ml penicillin and
100 µg/ml streptomycin. For routine passage, cultures were split
1:3 when they reached between 70 and 80% confluence every 2–3 days.
To minimize the influence of exosomes in FBS, FBS was
ultracentrifuged at 100,000 × g at 4°C overnight to spin down any
potential vesicular content (Avanti J-30I; Beckman Coulter, Inc.,
Brea, CA, USA) and used for all studies. The protocols followed for
the extraction, purification and analysis of DRβ-H are described in
a previous study (4).
MTT assay
The inhibitory effect of DRβ-H on breast cancer
cells was determined usingan MTT assay as previously described with
minor modifications (5). Briefly,
5×103 cells were cultured in 96-well plates in
triplicate. After 8 h, DRβ-H was added at 0, 5, 10, 20, 40 or 80
µg/ml. As a negative control, cells were treated with PBS. The
cells were cultured for 0, 24, 48 and 72 h and then 20 µl MTT was
added into each well at 5 mg/ml final concentration for 4 h.
Following incubation, 150 µl dimethyl sulfoxide was added to
dissolve the formazan crystals for 20 min at room temperature. The
optical density value for each well was detected at a wavelength of
490 nm with a microplate reader (5082 Grodig; Tecan Group, Ltd.,
Mannedorf, Switzerland).
Flow cytometry analysis
Breast cancer cells exposed to the indicated
concentrations of DRβ-H (0, 10, 20 or 40 µg/ml) for 24 h were
harvested. Cells were washed twice with cold PBS, incubated with
Annexin-V-FITC (BDBiosciences, Franklin Lakes, NJ, USA) and
propidium iodide for 15 min at room temperature in the dark, and
analyzed using a flow cytometer (BD FACS Calibur; BD Biosciences,
Franklin Lakes, NJ, USA).
Exosome isolation and
characterization
Exosome isolation and characterization was performed
using protocols as previously described (12). Exosomes were lysed for protein/RNA
extraction, or diluted in PBS for incubation assay or labeled for
confocal observation (12). The
morphology and size of exosomes were examined by transmission
electron microscopy (JEM-1010; JEOL, Ltd., Tokyo, Japan) as
previously reported (15).
Exosome uptake
Exosomes were stained with a PKH26 Red Fluorescent
Cell Linker kit (Sigma-Aldrich; Merck KGaA, Darmstadt, Germany)
according to a previously published protocol (12). As the negative control, exosomes
without PKH26 labeling were also prepared. For observation of
exosome uptake, confocal laser scanning microscopy (LSM710; Zeiss
GmbH, Jena, Germany) was used (150,000 magnification).
RNA isolation and microarray
Exosomal RNA was isolated using the Total Exosome
RNA and Protein Isolation kit (Invitrogen; Thermo Fisher
Scientific, Inc., Waltham, MA, USA) in accordance with the
manufacturer's protocol. Cellular RNA was prepared using
TRIzol®reagent (Thermo Fisher Scientific, Inc.). miRNA
profiles were analyzed using an Affymetrix Gene Chip miRNA Array
3.0 (Capital Bio Corporation, Beijing, China) according to the
manufacturer's protocol. The levels of miRNAs between exosomes and
MCF-7/S were calculated as previously reported (16).
Transfection of miRNA inhibitors
miRNA inhibitors and the negative controls were
synthesized by GenePharma Co., Ltd. (Shanghai, China). Transfection
of miRNA inhibitors was performed using Lipofectamine 2000
(Invitrogen; Thermo Fisher Scientific, Inc.) according to the
manufacturer's protocol. Cells and exosomes were harvested 48 h
after transfection for subsequent analysis viapolymerase chain
reaction (PCR).
Western blot analysis
Proteins were extracted from exosomes using the
Total Exosome RNA and Protein Isolation kit (Invitrogen; Thermo
Fisher Scientific, Inc.) in accordance with the manufacturer's
protocols. Equal amounts of proteins were subjected to SDS-PAGE and
transferred to polyvinylidenedi fluoride membranes. The CD63
protein level was measured by western blotting analysis using
anantibody against CD63 (Santa Cruz, USA). β-actin (Sigma, Germany)
was used as an internal control for normalization. Then, bound
proteins were visualized using the ECL Plus kit (EMD Millipore,
Billerica, MA, USA) with Image Lab Software version 5.2.1 (Bio-Rad,
USA).
Reverse transcription-quantitative
(RT-q) PCR
The RT-qPCR was run in triplicate using the SYBR
green (Biouniquer Technology, Nanjing, China) technique. Briefly,
cDNA for miRNA was synthesized using the BU-Script RT kit
(Biouniquer Technology, Nanjing, China). Specific stem-loop primers
(Springen Biotechnology, Nanjing, China) were designed for the
selected miRNAs, and U6 was used as an internal control.
Expressions of miRNAs and U6 were evaluated using following
primers: miR-130a forward, 5′-TTCACATTGTGCTACTGTCTGC-3′;
miR-183 forward, 5′-TATGGCACTGGTAGAATTCACT-3′;
miR-20b forward, 5′-TGTCAACGATACGCTACGA-3′; miR-25
forward, 5′-TCTGGTCTCCCTCACAGGAC-3′; and miR-452 forward,
5′-GCGAACTGTTTGCAGAGG-3′. Gene amplifications were performed on a
Light Cycler 480 (Roche, Australia) as follows: 91°C for 5 min
followed by 45 cycles (91°Cf or 15 sec, and 60°C for 30 sec),
followed by melting curve detection. The relative miRNA expression
was calculated using the 2−ΔΔCq method.
Target gene prediction
The TargetScan (http://www.targetscan.org/) and miRDB (http://www.mirdb.org/miRDB/) databases were employed
to predict miRNA targets (17,18). Only
the genes predicted by these two independent tools were considered.
The Kyoto Encyclopedia of Genes and Genomes (KEGG) pathways were
analyzed by the online DAVID program (http://david.abcc.ncifcrf.gov/) (19,20).
Statistical analysis
Statistical analysis was performed using SPSS 20.0
statistical software (IBM Corp., Armonk, NY, USA). All experiments
were performed in triplicate and the representative data are
presented as the mean ± standard deviation. Differences were
determined using the Student's t-test or using a one-way ANOVA with
Student-Newman-Keuls post-hoc test. P<0.05 was considered to
indicate a statistically significant difference.
Results
DRβ-H inhibits the proliferation of
MCF-7/S cells
The chemical structure of DRβ-H is presented in
Fig. 1A. MCF-7/S cells were treated
with different concentrations of DRβ-H (0, 5, 10, 20, 40 or 80
µg/ml) or for different times (0, 24, 48 or 72 h) and the rate of
inhibition of growth was measured using an MTT assay. DRβ-H exerted
a significant inhibitory effect on MCF-7/S cells in a dose- and
time-dependent manner compared with the negative control (Fig. 1B and C).
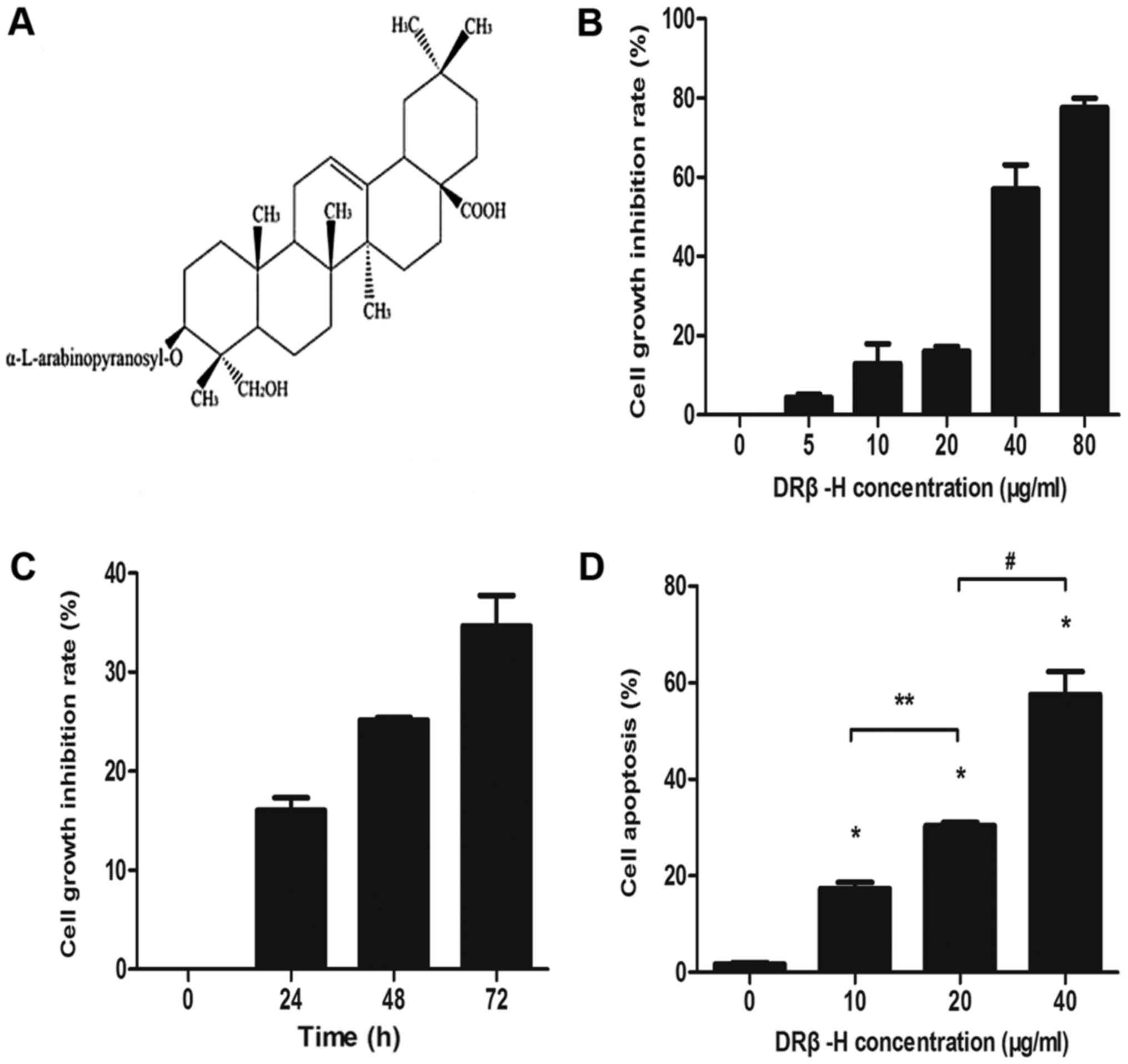 | Figure 1.Effects of DRβ-H on the proliferation
and apoptosis of MCF-7/S cells. (A) The chemical structure of
DRβ-H. (B) MTT assay of MCF-7/S treated by different concentrations
of DRβ-H (0, 5, 10, 20, 40 and 80 µg/ml) for 24 h. DRβ-H inhibited
cell proliferation in a dose-dependent manner. (C) MTT assay of
MCF-7/S treated by 20 µg/ml DRβ-H for different times (0, 24, 48
and 72 h). DRβ-H inhibited cell proliferation in a time-dependent
manner. (D) Flow cytometry analysis of MCF-7/S treated by different
concentrations of DRβ-H (0, 10, 20 and 40 µg/ml) for 24 h. DRβ-H
induced cell apoptosis in a dose-dependent manner. Data are
presented as the mean ± the standard deviation of three independent
experiments. *P<0.05 vs. DRβ-H-untreated group. The ** and
# shows that the two groups had statistically
significant difference (P<0.05). MCF-7/s, wild-type
drug-sensitive MCF-7 breast cancer cells; DRβ-H, DRhamnose
β-hederin. |
DRβ-H induces apoptosis in MCF-7/S
cells
To explore whether the anti-tumor effect of DRβ-H
was also associated with apoptosis, MCF-7/S cells were treated with
different concentrations of DRβ-H (0, 10, 20 or 40 µg/ml) for 24 h
and the rate of apoptosis was determined by flow cytometry.
Incubation of MCF-7/S cells with DRβ-H increased the apoptotic rate
compared with control cells treated without DRβ-H (Fig. 1D). Furthermore, it was identified that
increased DRβ-H concentrations increased the apoptotic effect,
demonstrating a dose-dependent association.
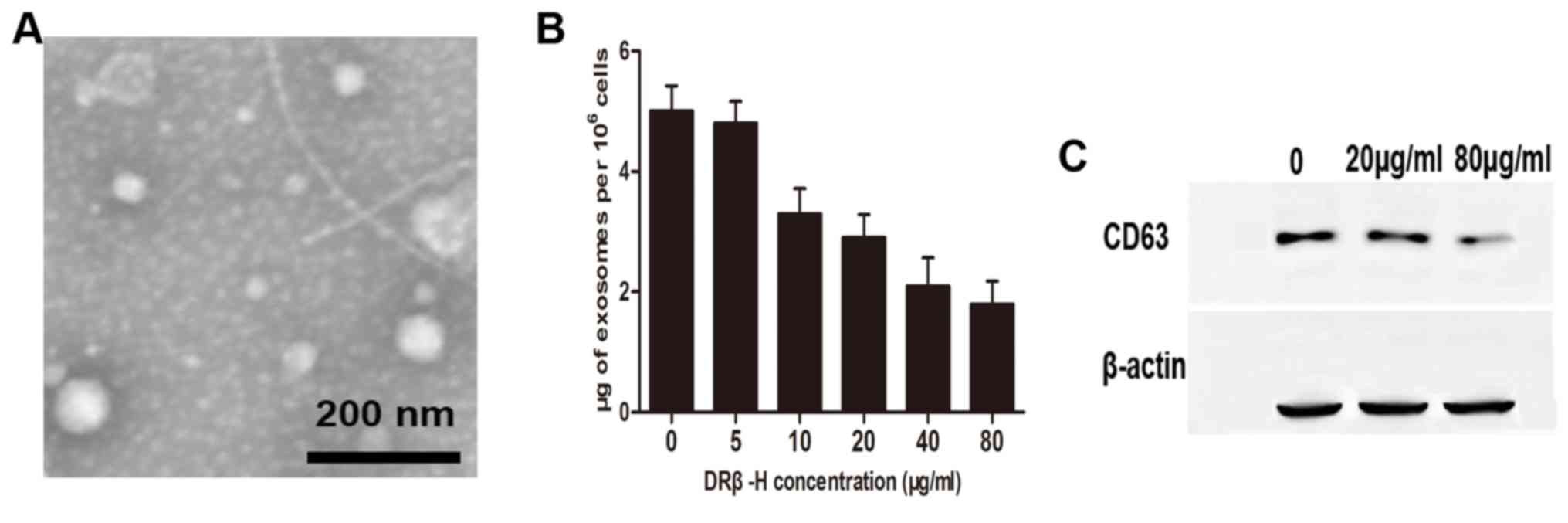
DRβ-H reduces exosome release in
MCF-7/S cells
The protocol of exosome isolation was based on
differential sedimentation properties, and used a series of
centrifugation and ultracentrifugation steps as previously
published (12). Exosomes were
homogeneous in morphology, and exhibited round vesicles measuring
between 50 and 100 nm in diameter as determined by transmission
electron microscopy (Fig. 2A). The
exosome quantity was evaluated by measuring protein content. The
authors' previous study demonstrated that exosomes derived from
cancer cells were responsible for drug efficacy during toxic insult
(13). Thus, it was hypothesized
exosome release is involved in DRβ-H-mediated growth inhibition.
MCF-7/S cells were treated with different concentrations of DRβ-H
and it was revealed that the quantity of exosomes was decreased in
a dose-dependent manner (Fig. 2B).
Additionally, treatment of cells with high concentration of DRβ-H
reduced exosome marker CD63 compared with the control cells treated
with lower concentration of DRβ-H (Fig.
2C), indicating that DRβ-H may inhibit exosome secretion.
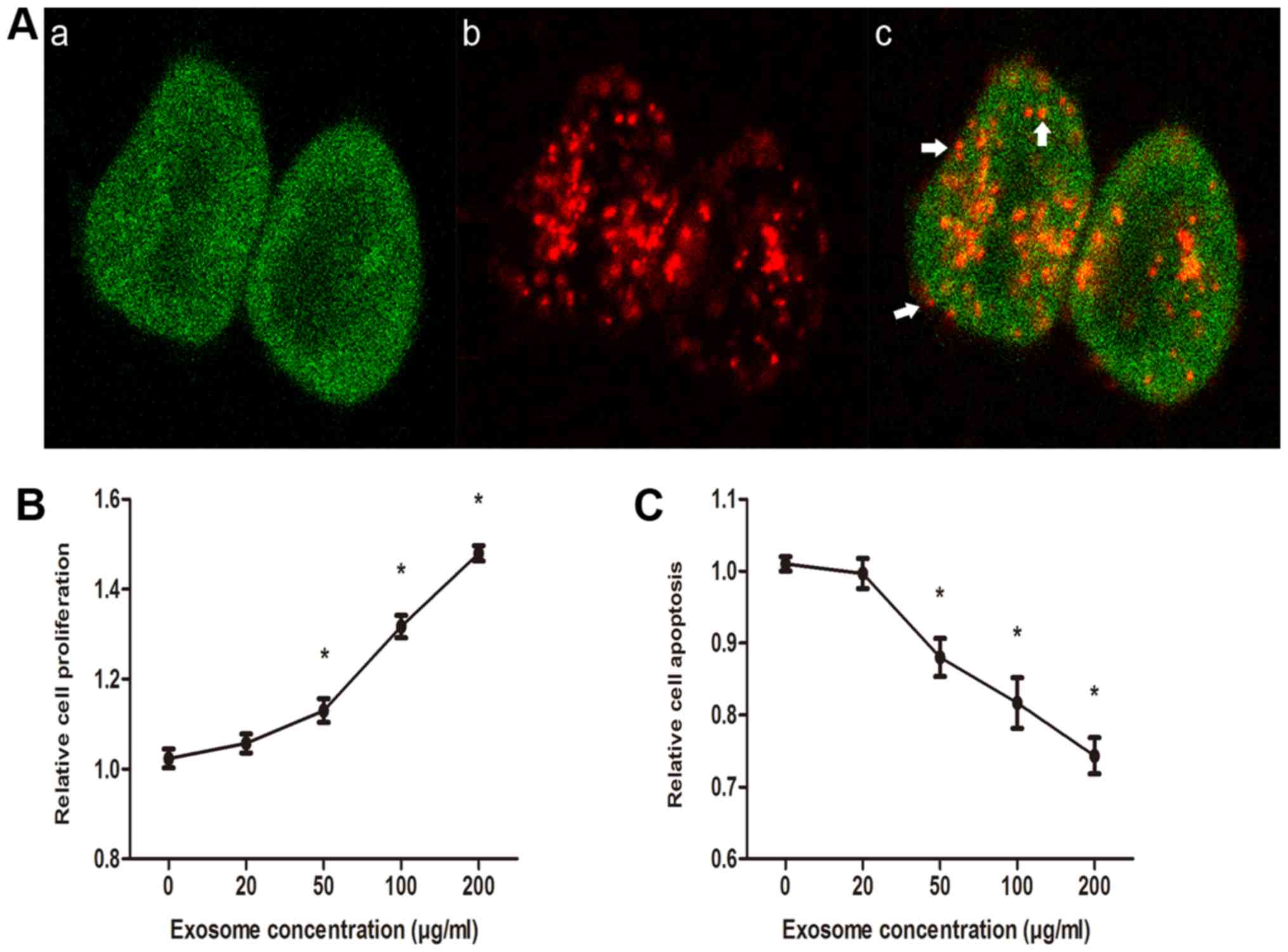
DRβ-H suppresses MCF-7/S growth by
inhibiting exosome release
To visualize exosome uptake by recipient cells,
exosomes were labeled by PKH26 red dye and incubated with GFP-S for
24 h, after which confocal laser scanning microscopy was performed.
The internalization of exosomes was indicated by several red
fluorescent punctuated signals on the cell membranes and inside the
cytoplasm of GFP-S (Fig. 3A; white
arrows indicate red fluorescent punctuated signals). The effects of
exosomes on cell growth were assessed in DRβ-H-treated MCF-7/S
cells. As presented in Fig. 3, the
proliferation rate modulated by DRβ-H was relatively lower in
MCF-7/S cells without exosomes, whereas a significant increase in
proliferation rate was identified in MCF-7/S cells treated with
exosomes (Fig. 3B). Furthermore,
incubation of MCF-7/S cells with exosomes significantly decreased
apoptosis when compared with control cells incubated without
exosomes (Fig. 3C). These data, along
with the observations in the aforementioned section, collectively
suggested that exosomes were able to promote recipient MCF-7/S
cells growth and DRβ-H suppressed MCF-7/S cells growth by
inhibiting exosome release.
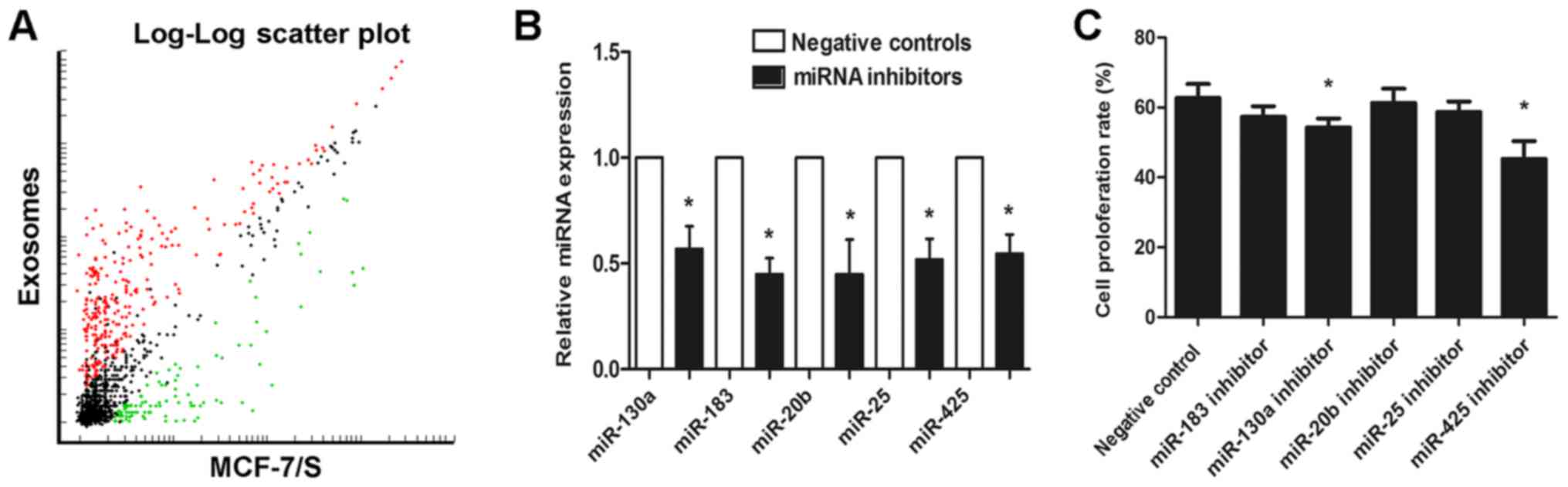
DRβ-H decreases exosomal miRNAs to
regulate MCF-7/S proliferation
Given that exosomes contain cellular information, an
attempt was made to explore whether exosomal miRNAs affect MCF-7/S
proliferation. By comparing the miRNA profiles of exosomes and
their cells of origin, it was identified that there are numerous
miRNAs shared by exosomes and MCF-7/S. Furthermore, a number of
miRNAs were detected exclusively in exosomes compared with those in
the parental cells, whereas a group of miRNAs expressed by cells
were absent in the corresponding exosomes (Fig. 4A). Next, the functions of the top five
miRNAs (miR-130a, miR-183, miR-20b, miR-25 and
miR-425) more localized in exosomes compared with that
incells were examined by transfecting with miRNA inhibitors. The
successful knockdown of the five exosomal miRNAs was subsequently
validated by RT-qPCR (Fig. 4B). miRNA
knockdown exosomes were added into recipient MCF-7/S cells and cell
viability was detected by analyzing the proliferation rate. As
presented in Fig. 4, of the five
miRNAs tested, only miR-130a and miR-425 knockdown
exosomes were able to significantly decrease cell proliferation
(Fig. 4C), indicating that exosomal
miR-130a and miR-425 could enhance MCF-7/s cell
viability.
Target prediction and pathway analysis
of miRNAs
To evaluate the biological processes regulated by
miR-130a and miR-425, their targets were investigated
using TargetScan and miRDB. Only the genes listed by these two
independent algorithms were chosen for further study. A total of
443 genes were detected and a KEGG analysis was performed using the
DAVID program. As presented in Table
I, the predicted genes were suggested to participate in several
signaling pathways, including mammalian target of rapamycin (mTOR),
ErbB, mitogen activated protein kinase (MAPK) and transforming
growth factor (TGF)-β signaling pathways. Furthermore, a number of
pathways associated with the tumor metabolic process were also
detected, in particular choline metabolism and proteoglycansin
cancer.
 | Table I.KEGG pathway analysis with DAVID
tool. |
Table I.
KEGG pathway analysis with DAVID
tool.
| Term | Gene count | Percentage | P-value |
|---|
| Protein processing
in endoplasmic reticulum | 12 | 2.8 |
3.5×10−3 |
| ErbB signaling
pathway | 8 | 1.8 |
6.1×10−3 |
| MAPK signaling
pathway | 14 | 3.2 |
1.2×10−2 |
| Choline metabolism
in cancer | 8 | 1.8 |
1.3×10−2 |
| TNF signaling
pathway | 8 | 1.8 |
1.7×10−2 |
| TGF-β signaling
pathway | 7 | 1.6 |
1.9×10−2 |
| Adipocytokine
signaling pathway | 6 | 1.4 |
3.1×10−2 |
| AMPK signaling
pathway | 8 | 1.8 |
3.4×10−2 |
| Phagosome | 9 | 2.1 |
3.9×10−2 |
| Oxytocin signaling
pathway | 9 | 2.1 |
4.6×10−2 |
| Osteoclast
differentiation | 8 | 1.8 |
4.7×10−2 |
| mTOR signaling
pathway | 5 | 1.1 |
5.8×10−2 |
| Insulin signaling
pathway | 8 | 1.8 |
5.9×10−2 |
| Signaling pathways
regulating pluripotency of stem cells | 8 | 1.8 |
6.3×10−2 |
| Proteoglycans in
cancer | 10 | 2.3 |
6.5×10−2 |
| ECM-receptor
interaction | 6 | 1.4 |
6.8×10−2 |
| Calcium signaling
pathway | 9 | 2.1 |
8.2×10−2 |
| Circadian
entrainment | 6 | 1.4 |
9.1×10−2 |
| Fc epsilon RI
signaling pathway | 5 | 1.1 |
9.2×10−2 |
Discussion
A large number of active components purified from
natural plants have been used to treatvarious tumors including
breast cancer (2,3). The authors' previous study demonstrated
that DRβ-H, a noveloleanane-type triterpenoid saponin derived from
Clematis ganpiniana, exhibited apotentinhibitory effecton
breastcancer cells (5). In the
present study, it was reported that DRβ-H may inhibit breast cancer
cell growth by remodeling the tumor microenvironment via reducing
exosome release.
DRβ-Hwasable to reduce proliferation and induce
apoptosis of breast cancer cells. This was based on MTT assay
results and confirmed with flow cytometry analysis of cancer cells
incubated with different concentrations of DRβ-H. It was previously
reported that DRβ-H regulated the phosphoinositide 3-kinase/AKT
serine threonine kinase signaling pathway and activated
pro-apoptotic proteins of the B cell lymphoma-2 family (5). No apoptosis-associated signaling
pathways were examined in the present study, and it will be
necessary to investigate the concrete pathways involved in
proliferation inhibition.
Exosomes are small vesicles between 50 and 100 nm in
diameter that are implicated with several key functions during
tumor progression, including angiogenesis, immunosuppression,
invasion and metastasis (9).
Intercellular communication is also one such function, via
exosomes' ability to internalize into surrounding cellsand
facilitate constant transfer of active miRNAs (10,21). The
authors previous study demonstrated that drug-resistant breast
cancer cells were able to spread resistance capacity to sensitive
cells viaexo somes and that such effects were partially attributed
to the cell-to-cell shuttle of specific miRNAs (12,13). In
the present study, it was identified that DRβ-H downregulated the
production of tumor-derived exosomes. It was further assessed
whether exosomes affected the growth of breast cancer cells. The
results indicated that exosomes enhanced the proliferation and
attenuated apoptosis following absorption and internalization by
target breast cancer cells. The observation of exosomes moving from
donor cells to the same type of recipient cells opens up an
intriguing possibility that malignant capacity maybe transferred
via exosomes. However, the exposure of breast cancer cells to DRβ-H
may trigger cells to release less exosomes compared with that in
normal conditions, leading to reduced cell growth.
By comparing the miRNA microarray of exosomes and
their cells of origin (data not shown), it was identified that
exosomes contained a series of miRNAs shared with their parental
cells. Additionally, several specific miRNAs were present
dominantly or at higher levels in exosomes compared with that in
the donor cells. These results are consistent with certain other
studies, suggesting that loading of miRNAs into exosomes may not be
a random event, but instead is regulated by a selective process
(22,23). Asit is difficult to determine the
functions of all the exosomal miRNAs, the focus of the present
study was shifted to explore the roles of the five miRNAs most
strongly localized in exosomes in breast cancer cells. In the
present study, only exosomal miR-130a and miR-425
significantly increased cell viability. KEGG pathway analysis of
the predicted targets of the two miRNAs demonstrated that the
target genes are associated with them TOR, ErbB, MAPK and TGF-β
signaling pathways. It is notable that all these signaling pathways
are important for cell proliferation and survival (24–27).
There are certain limitations of the present study.
It has not been demonstrated that exosomal miR-130a and
miR-425 were shuttled into recipient cells and serveas
functional molecules to participate a certain signaling pathway.
Nevertheless, as confirmed by a number of studies, exosomal miRNAs
exert gene silencing to fine-tune target expression through the
same mechanism as endogenous miRNAs (13,28,29).
Future studies may attempt to separately investigate the role of
exosomal miRNAs, and assess the biological significance of secreted
miR-130a and miR-425 in the regulation of malignant
progression.
In conclusion, the present study expands on previous
findings and, to the best of our knowledge, provides the first
evidence suggesting that DRβ-H may suppress breast cancer cell
growth by manipulating the tumor microenvironment via inhibition of
exosome release.
Acknowledgements
Not applicable.
Funding
The present study was supported by the Natural
Science Foundation of China (grant nos. 81702591 and 81502294), the
Natural Science Foundation of Jiangsu Province (grant no.
BK20170294) and Science Foundation of Changzhou (grant no.
CJ20159044).
Availability of data and materials
The datasets analyzed during the current study are
available from the corresponding author on reasonable request.
Authors' contributions
Conceived and designed the experiments: WC, LC and
QD. WC, LC and MP performed the experiments. WC, LC, QQ, YZ, and LX
analyzed the data. WC and LC wrote the manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
DeSantis CE, Ma J, Goding Sauer A, Newman
LA and Jemal A: Breast cancer statistics, 2017, racial disparity in
mortality by state. CA Cancer J Clin. 67:439–448. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Wang CY, Bai XY and Wang CH: Traditional
Chinese medicine: A treasured natural resource of anticancer drug
research and development. Am J Chin Med. 42:543–559. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Cragg GM and Newman DJ: Natural products:
A continuing source of novel drug leads. Biochim Biophys Acta.
1830:3670–3695. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ding Q, Yang LX, Yang HW, Jiang C, Wang YF
and Wang S: Cytotoxic and antibacterial triterpenoids derivatives
from Clematis ganpiniana. J Ethnopharmacol. 126:382–385. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Cheng L, Xia TS, Wang YF, Zhou W, Liang
XQ, Xue JQ, Shi L, Wang Y and Ding Q: The apoptotic effect of D
Rhamnose β-hederin, a novel oleanane-type triterpenoid saponin on
breast cancer cells. PLoS One. 9:e908482014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Chin AR and Wang SE: Cancer-derived
extracellular vesicles: The ‘soil conditioner’ in breast cancer
metastasis? Cancer Metastasis Rev. 35:669–676. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Valadi H, Ekström K, Bossiös A, Sjostrand
M, Lee JJ and Lötvall JO: Exosome-mediated transfer of mRNAs and
microRNAs is a novel mechanism of genetic exchange between cells.
Nat Cell Biol. 9:654–659. 2007. View
Article : Google Scholar : PubMed/NCBI
|
|
8
|
Yu X, Odenthal M and Fries JW: Exosomes as
miRNA carriers: Formation-function-future. Int J Mol Sci. 17(pii):
E20282016. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Yu DD, Wu Y, Shen HY, Lv MM, Chen WX,
Zhang XH, Zhong SL, Tang JH and Zhao JH: Exosomes in development,
metastasis and drug resistance of breast cancer. Cancer Sci.
106:959–964. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Chen X, Liang H, Zhang J, Zen K and Zhang
CY: Secreted microRNAs: A new form of intercellular communication.
Trends Cell Biol. 22:125–132. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zhong S, Li W, Chen Z, Xu J and Zhao J:
MiR-222 and miR-29a contribute to the drug-resistance of breast
cancer cells. Gene. 531:8–14. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Chen WX, Liu XM, Lv MM, Chen L, Zhao JH,
Zhong SL, Ji MH, Hu Q, Luo Z, Wu JZ and Tang JH: Exosomes from
drug-resistant breast cancer cells transmit chemoresistance by a
horizontal transfer of microRNAs. PLoS One. 9:e952402014.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Chen WX, Cai YQ, Lv MM, Chen L, Zhong SL,
Ma TF, Zhao JH and Tang JH: Exosomes from docetaxel-resistant
breast cancer cells alter chemosensitivity by delivering microRNAs.
Tumour Biol. 35:9649–9659. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Miot S, Gianni-Barrera R, Pelttari K,
Acharya C, Mainil-Varlet P, Juelke H, Jaquiery C, Candrian C,
Barbero A and Martin I: In vitro and in vivo validation of human
and goat chondrocyte labeling by green fluorescent protein
lentivirus transduction. Tissue Eng Part C Methods. 16:11–21. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Corcoran C, Rani S, O'Brien K, O'Neill A,
Prencipe M, Sheikh R, Webb G, McDermott R, Watson W, Crown J and
O'Driscoll L: Docetaxel-resistance in prostate cancer: Evaluating
associated phenotypic changes and potential for resistance transfer
via exosomes. PLoS One. 7:e509992012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Jaiswal R, Luk F, Gong J, Mathys JM, Grau
GE and Bebawy M: Microparticle conferred microRNA
profiles-implications in the transfer and dominance of cancer
traits. Mol Cancer. 11:372012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Lewis BP, Burge CB and Bartel DP:
Conserved seed pairing, often flanked by adenosines, indicates that
thousands of human genes are microRNA targets. Cell. 120:15–20.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wang X: Computational prediction of
microRNA targets. Methods Mol Biol. 667:283–295. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Kanehisa M, Araki M, Goto S, Hattori M,
Hirakawa M, Itoh M, Katayama T, Kawashima S, Okuda S, Tokimatsu T
and Yamanishi Y: KEGG for linking genomes to life and the
environment. Nucleic Acids Res. 36(Database issue): D480–D484.
2008.PubMed/NCBI
|
|
20
|
Huang DW, Sherman BT, Tan Q, Kir J, Liu D,
Bryant D, Guo Y, Stephens R, Baseler MW, Lane HC and Lempicki RA:
DAVID bioinformatics resources: Expanded annotation database and
novel algorithms to better extract biology from large gene lists.
Nucleic Acids Res. 35(Web Server Issue): W169–W175. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Chen WX, Zhong SL, Ji MH, Pan M, Hu Q, Lv
MM, Luo Z, Zhao JH and Tang JH: MicroRNAs delivered by
extracellular vesicles: An emerging resistance mechanism for breast
cancer. Tumour Biol. 35:2883–2892. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Palma J, Yaddanapudi SC, Pigati L, Havens
MA, Jeong S, Weiner GA, Weimer KM, Stern B, Hastings ML and Duelli
DM: MicroRNAs are exported from malignant cells in customized
particles. Nucleic Acids Res. 40:9125–9138. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Gibbings DJ, Ciaudo C, Erhardt M and
Voinnet O: Multivesicular bodies associate with components of miRNA
effector complexes and modulate miRNA activity. Nat Cell Biol.
11:1143–1149. 2009. View
Article : Google Scholar : PubMed/NCBI
|
|
24
|
Dey N, De P and Leyland-Jones B:
PI3K-AKT-mTOR inhibitors in breast cancers: From tumor cell
signaling to clinical trials. Pharmacol Ther. 175:91–106. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Zhou BP and Hung MC: Dysregulation of
cellular signaling by HER2/neu in breast cancer. Semin Oncol.
30:38–48. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Wang J, Shao N, Ding X, Tan B, Song Q,
Wang N, Jia Y, Ling H and Cheng Y: Crosstalk between transforming
growth factor-beta signaling pathway and long non-coding RNAs in
cancer. Cancer Lett. 370:296–301. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Miyamoto Y, Suyama K and Baba H: Recent
advances in targeting the EGFR signaling pathway for the treatment
of metastatic colorectal cancer. Int J Mol Sci. 18(pii): E7522017.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Kosaka N, Iguchi H, Hagiwara K, Yoshioka
Y, Takeshita F and Ochiya T: Neutral sphingomyelinase 2
(nSMase2)-dependent exosomal transfer of angiogenic microRNAs
regulate cancer cell metastasis. J Biol Chem. 288:10849–10859.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Morel L, Regan M, Higashimori H, Ng SK,
Esau C, Vidensky S, Rothstein J and Yang Y: Neuronal exosomal
miRNA-dependent translational regulation of astroglial glutamate
transporter GLT1. J Biol Chem. 288:7105–7116. 2013. View Article : Google Scholar : PubMed/NCBI
|