Introduction
With progression in cancer treatment, the survival
rate of patients has increased globally in 2012 (1,2). In the
USA, the number of people with a cancer diagnosis was ~14.5 million
in 2014 (3). Based on the
Surveillance, Epidemiology and End Results (SEER) program, cancer
survivors have a higher risk of second primary cancer (SPC)
compared with the general population (4). Despite the medical progression achieved
and the advances in locoregional control, continual SPC is one of
the factors that impede the improvement of survival rate of
patients with cancer (5); therefore,
SPC is an important prognostic factor for patients with head and
neck cancer (HNC) (6). Furthermore,
SPC more frequently develops following HNC, compared with the
general population (5,7). 2-[18F]-fluoro-2-deoxy-D-glucose-positron
emission tomography (FDG-PET) is primarily used to examine lymph
nodes, the initial stage of HNC, treatment responses, unknown
primary cancer (PCs) types and relapses or metastases (8–11);
however, there is no accurate FDG-PET protocol to detect SPC over a
long-term follow-up period subsequent to HNC treatment. In the
present study, a rare case of triple PC, including well to
moderately differentiated squamous cell carcinoma (SCC) of the
mandible, axillary cutaneous poorly differentiated SCC and
adenocarcinoma (AC) of the prostate, was described. The prostate
tumor was incidentally detected by FDG-PET. The aim of the present
study was to discuss the necessity of FDG-PET as a protocol of
postoperative follow-up.
Case report
Written informed consent was obtained from the
patient for the publication of this case report and the
accompanying images. The report was submitted for ethical review to
the Ethics Committee of the University of the Ryukyus (Okinawa,
Japan), which waived the requirement for review per institutional
protocol due to the study not containing content that requires
ethical approval. The Ethics Committee approved the submission and
publication of the manuscript.
A 70-year-old man was evaluated and treated for
cancer of the right mandible in September 2012 at the Department of
Oral and Maxillofacial Surgery, University Hospital of the Ryukyus
(Okinawa, Japan). The disease was initially considered to be benign
or an inflammation in the radiolucent area around the wisdom teeth,
and the patient underwent extraction of the right third molar and
curettage (with histological test) at a different clinic (Nanbu
Tokushukai Hospital, Okinawa, Japan); however, the
histopathological examination at this clinic demonstrated well to
moderately differentiated SCC, according to the World Health
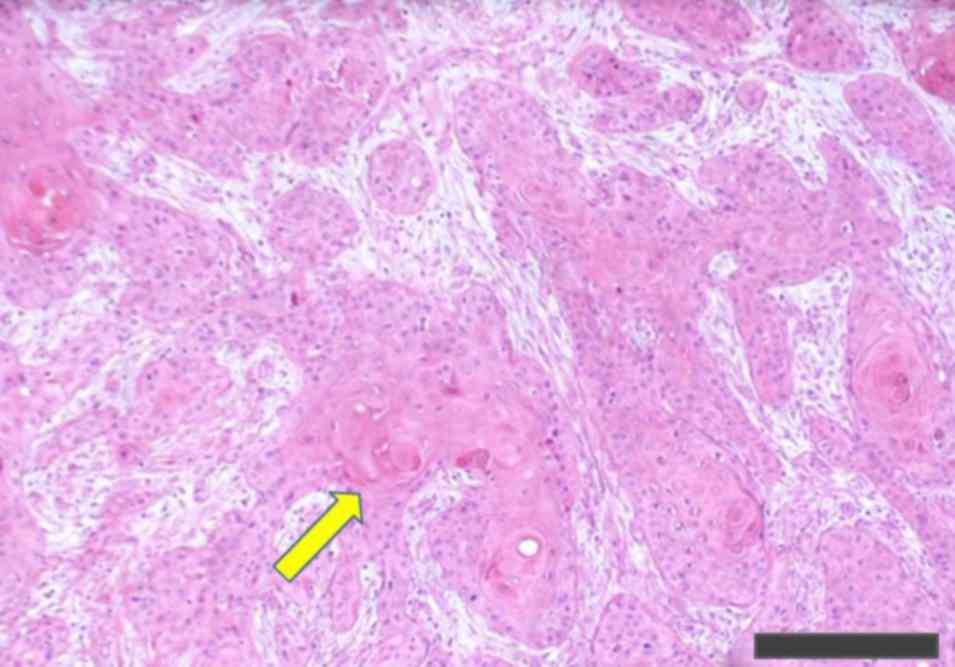
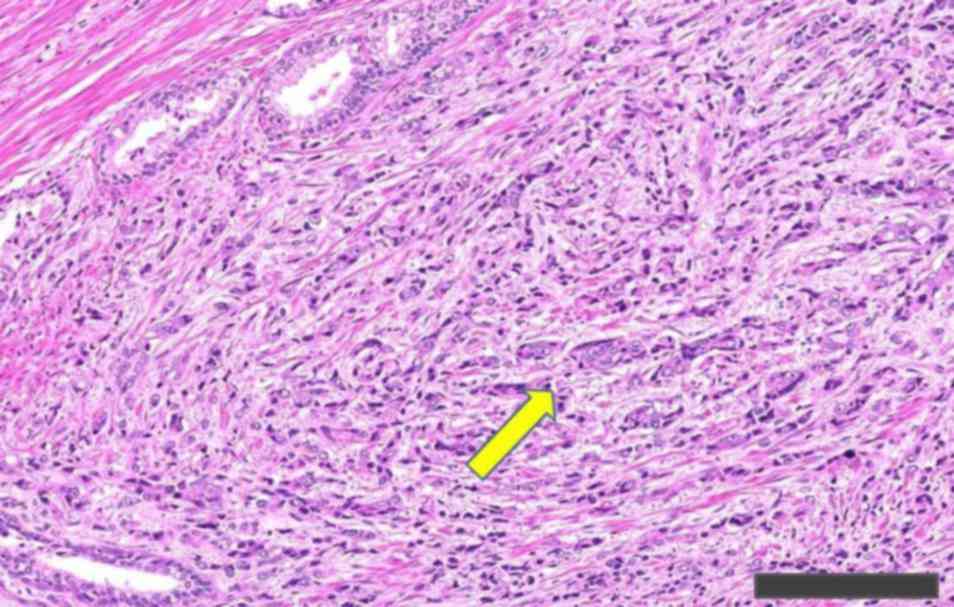
Organization Classification of Tumours (12) (Fig. 1).
Histopathological examination was conducted with hematoxylin and
eosin staining. In brief, resected tissues were fixed in 20%
formalin for ~24 h at room temperature. Subsequently, slides were
washed with xylene for 9 min, then dexylened with 100, 95, 80 and
70% ethanol for 20, 10, 10 and 10 times, respectively. Slides were
then stained with hematoxylin for 10 min, and washed with tap water
for 3 min. Subsequently, slides were washed with 0.5% hydrochloric
acid alcohol once and washed with tap water for 5 min. Following
this, slides were stained with eosin for 6 min and slides were
dehydrated with 95 and 100% ethanol for 10 and 40 times,
respectively. Additionally, slides were immersed in xylene 30 times
and cover glass was placed on the slides. All of the methods were
at room temperature, and then examined using a light microscope at
×200 magnification. Subsequently, the patient was referred to the
University Hospital of the Ryukyus. Physical examination indicated
slight swelling of the gum around the extraction socket. No
specific lesion was located in the mouth, and no palpable lymph
node enlargement was evident in the head or neck region. The
patient had no history of cancer and had never undergone radiation
therapy or chemotherapy. Additionally, the patient had been a
moderate smoker in his 20s and was a moderate drinker. The family
history included gastric cancer in two brothers. The patient also
suffered from well-controlled hypertension, reflux esophagitis,
hyperuricemia, sensorineural hearing loss, cerebral infarction and
dyslipidemia.
Contrast-enhanced computed tomography (CT),
contrast-enhanced magnetic resonance imaging (MRI) and FDG-PET were
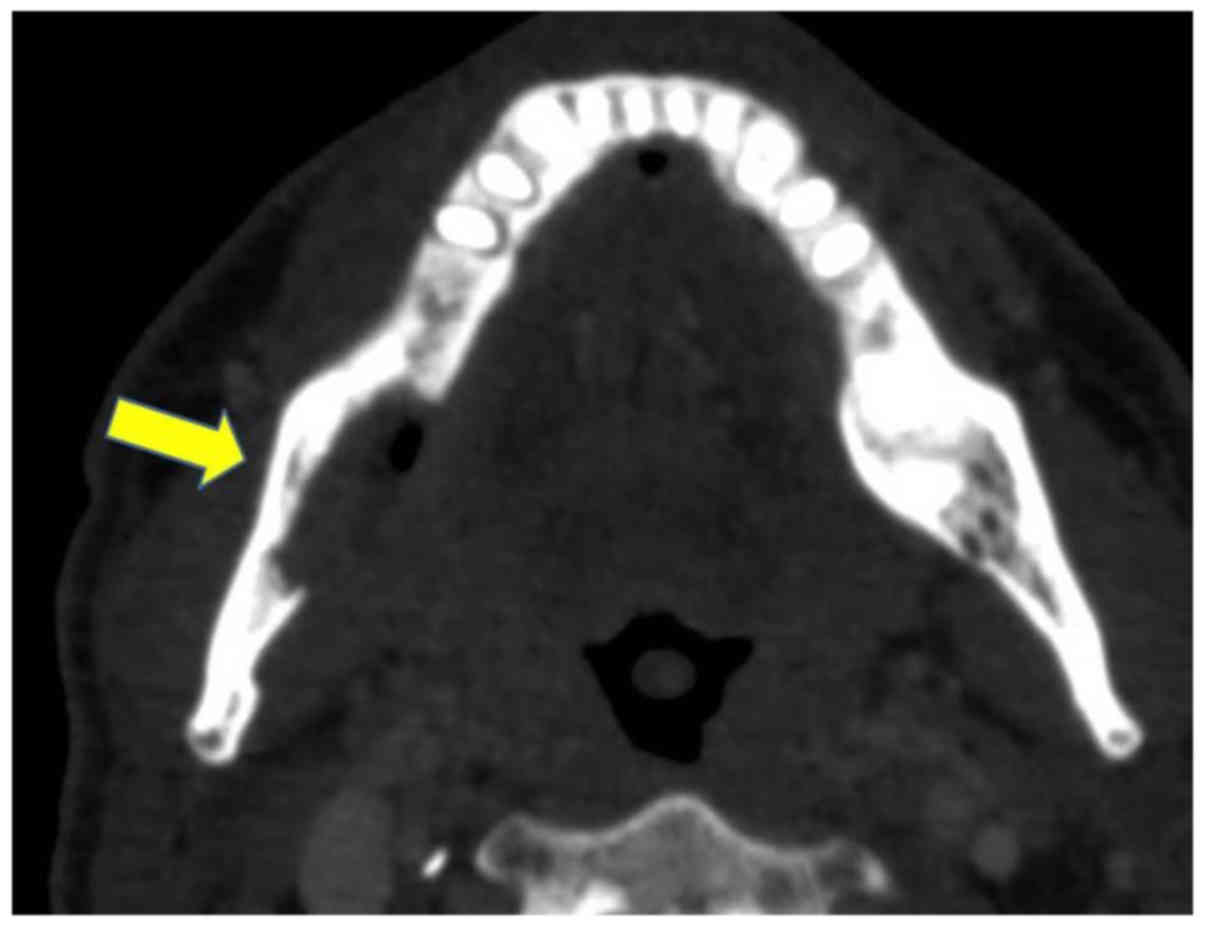
used to examine the mandible cancer stage. The CT results
demonstrated bone resorption in the right mandible and tumor
invasion was suspected (Fig. 2),
whereas no lesions were observed in the cervical lymph nodes,
lungs, bones or liver. The MRI results indicated high-signal
intensity around the margins of the curettaged area; however, no
other lesions were located in the head and neck region. FDG-PET was
used to examine the stage of SCC and revealed increased FDG uptake
[maximum standardized uptake value (SUVmax), 11.9] in the right
mandible. Additionally, no other potential cancer areas were
located in the rest of the body. An upper gastrointestinal
endoscopy demonstrated no abnormal data. As a result, the patient
was diagnosed with mandibular SCC (T4N0M0, stage IVa), according to
the Union for International Cancer Control Tumor-Node-Metastasis
classification, 7th edition (13).
The patient received 6 cycles of bleomycin
chemotherapy preoperatively (total dose, 90 mg), as described
previously (14,15). In October 2012, the patient underwent
segmental mandibulotomy and reconstructive grafting (non
vascularized bone grafting), ipsilateral supraomohyoid neck
dissection and tracheotomy. A pathological examination demonstrated
SCC of the submaxilla, with venous invasion but no lymphatic
invasion. Furthermore, bone invasion was present, but the surgical
margins were negative. Cervical lymph nodes exhibited no evidence
of cancer. Following surgery, a salivary fistula of the parotid
region persisted. The patient then underwent postoperative
radiation (total dose, 20 Gy in 10 fractions over ~3 weeks).
Resolution of the salivary fistula using low-dose radiation therapy
was attempted (16,17), and notably, the fistula was
successfully resolved. Although a number of studies have reported a
lower success rate of radiotherapy following the reconstruction of
a non-vascularized bone graft (18,19), the
radiotherapy for those patients is not contraindicated in the
National Comprehensive Cancer Network guideline (20); therefore, a low-dose radiation was
selected to resolve the salivary fistula of the parotid region
(17). Surgical site infection did
occur, and the grafted bone and titan plate were surgically removed
4 months after the implantation. Subsequently, no clinical or
radiological signs of mandible cancer recurrence/metastasis or
infection in the head and neck region or the lungs were
observed.
The patient developed skin cancer (second type) and
prostate cancer (PRC) (third type) 3 years after the mandible
(first type) cancer treatment. A nodule on the left axillary
cutaneous lesion was noted by the patient in July 2015. In April
2016, the nodule (18×10×7 mm in size) was resected at a different
hospital (Chibana Clinic, Okinawa, Japan) and pathologically
diagnosed as poorly differentiated SCC of the skin, according to
the World Health Organization Classification of Tumours (21). A pathological examination demonstrated
poorly differentiated SCC, with positive margins (data not shown).
Pathological examination was conducted with hematoxylin and eosin
staining. In brief, resected tissues were fixed in 10% formalin for
~24 h at room temperature. Subsequently, slides were washed with
xylene for 15 min, then dexylened with 100, 80 and 50% ethanol for
2, 1 and 1 min, respectively. Subsequently, slides were washed with
tap water for 2 min. Following this, slides were washed with
distilled water for 30 sec. Slides were then stained with
hematoxylin for 4 min and washed with tap water for 6 min.
Additionally, slides were washed with distilled water for 30 sec.
Subsequently, slides were stained with eosin for 3 min and slides
were dehydrated with 100% ethanol for 4 min. Following this, slides
were immersed in xylene for 8 min and cover glass was placed on the
slides. All of the methods were at room temperature, and then
examined using a light microscope at ×200 magnification.
Subsequently, the patient was referred to University Hospital of
the Ryukyus for further examination and treatment.
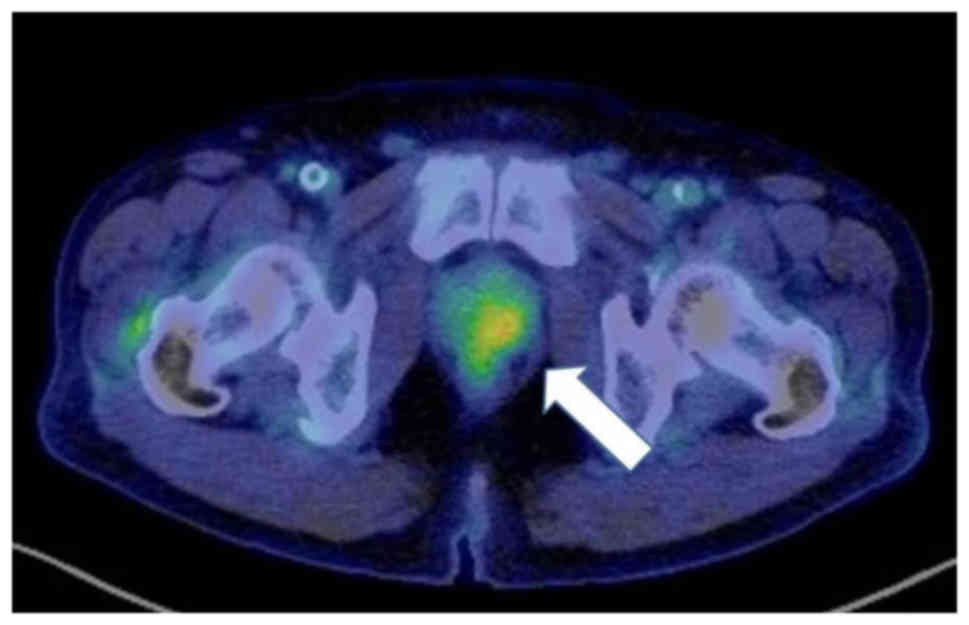
Contrast-enhanced CT and FDG-PET were performed to evaluate the
skin cancer stage. There was no accumulation in the axillary skin,
axillary lymph nodes, lungs, and head and neck region; however, FDG
uptake was observed in the prostate (SUVmax, 4.61) (Fig. 3). The patient had a prostate-specific
antigen (PSA) level of 19.87 ng/ml (normal range, 0–3.53 ng/ml).
Due to the absence of clinical symptoms of ‘non-head and neck, lung
and esophagus cancer’, the PSA or carcinoembryonic antigen test was
not performed prior to the PET examination of the skin cancer.
Notably, the SPC incidence of this cancer type (non-head and neck,
lung and esophagus cancer) is low in patients with HNC (22). Additional resection of skin (second)
cancer was first performed in June 2016 at the University Hospital
of the Ryukyus and poorly differentiated SCC was resected.
Pathological examination was conducted with hematoxylin and eosin
staining. In brief, resected tissues were fixed in 10% formalin for
~24 h at room temperature. Subsequently, slides were washed with
xylene for 9 min, then dexylened with 100, 95 and 70% ethanol for
3, 1 and 1 min, respectively. Subsequently, slides were washed with
tap water for 3 min. Slides were then stained with hematoxylin for
4 min, and washed with tap water for 3 min. Following this, slides
were dehydrated with 95% ethanol for 1 min. Subsequently, slides
were stained with eosin for 3 min and slides were washed with 95
and 100% ethanol for 2 and 8 min, respectively. Subsequently,
slides were immersed in xylene for 12 min and cover glass was
placed on the slides. All of the methods were at room temperature,
and then examined using a light microscope at ×100 magnification
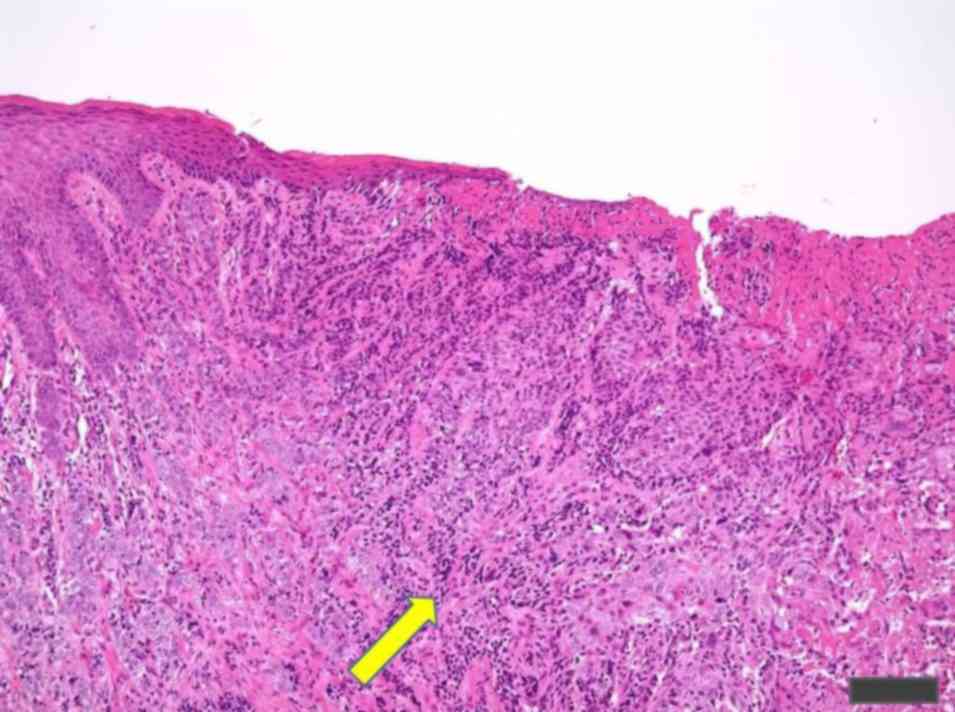
(Fig. 4). Following the resection of
the second cancer, a guided percutaneous needle biopsy of the
prostate lesion was performed in August 2016 at the University
Hospital of the Ryukyus, which indicated a diagnosis of AC (Gleason
score 4+5), according to Gleason and Mellinger (23). Laparoscopic prostatectomy and pelvic
lymphadenectomy was then performed for the PRC (third type) in
October 2016 at the University Hospital of the Ryukyus.
Pathological data demonstrated positive pT4 (bladder) invasion
(Fig. 5). Pathological examination
was conducted with hematoxylin and eosin staining. In brief,
resected tissues were fixed in 10% formalin for ~24 h at room
temperature. Subsequently, slides were washed with xylene for 9
min, then dexylened with 100, 95 and 70% ethanol for 3, 1 and 1
min, respectively. Subsequently, slides were washed with tap water
for 3 min. Slides were then stained with hematoxylin for 4 min and
washed with tap water for 3 min. Following this, slides were
dehydrated with 95% ethanol for 1 min. Subsequently, slides were
stained with eosin for 3 min and slides were washed with 95 and
100% ethanol for 2 and 8 min, respectively. Following this, slides
were immersed in xylene for 12 min and cover glass was placed on
the slides. All of the methods were at room temperature, and then
examined using a light microscope at ×200 magnification. Negative
lymphatic (D2-40) and vascular (by Victoria blue-hematoxylin and
eosin staining) invasion, positive perineural (S-100) invasion and
positive margins, but no lymph node metastasis was found. Victoria
blue-hematoxylin and eosin staining was conducted. In brief,
resected tissues were fixed in 10% formalin for ~24 h at room
temperature. Subsequently, slides were washed with xylene for 9
min, and then dexylened with 100, 95 and 70% ethanol for 6, 3 and 3
min, respectively. Slides were then stained with Victoria blue
overnight at room temperature, the remainder of Victoria blue was
removed with 70% ethanol and then washed with tap water.
Subsequently, slides were washed with xylene for 3 min, and then
washed with tap water for 3 min. Following this, slides were
stained with hematoxylin for 3 min. Subsequently, washed with tap
water for 3 min and dehydrated with 95% ethanol 20 times. Following
this, slides were stained with eosin for 3 min, and then dehydrated
with 100% ethanol for 4 min. Slides were then immersed in xylene
for 12 min, and cover glass was placed on the slides. All of the
methods were at room temperature, and then examined using a light
microscope at ×200 magnification. However, in February 2017,
follow-up PSA indicated the high PSA level of 35.67 ng/ml (normal
range, 0–3.53 ng/ml) was elevated, and subsequent CT and FDG-PET
demonstrated recurrence of the PRC, accompanied with lymph node
metastasis of the left axillary and pelvic regions, and suspected
multiple liver metastases (the liver lesion was then examined by
fine-needle biopsy in April 2017 and no cancerous lesions were
histologically located). Axillary lymph node dissection was
performed in July 2017, and the lymph nodes were histologically
diagnosed as SCC metastases. The patient was treated with
chemotherapy [from March 2017, docetaxel (60 mg/m2) was
intravenously administered 10 times, and degarelix were
administered subcutaneous injections into the abdominal region 14
times (240 mg once in March 2017 and then 80 mg 13 times)] for the
liver, pelvic and prostate regions. The time between administration
of the two drugs was 4 or 5 weeks. The lesions gradually diminished
at the time of writing, therefore his prognosis cannot be stated,
and further treatment of the chemotherapy (identical dose of
docetaxel and degarelix) are planned. Based on the aforementioned
lesions, the patient was diagnosed with ‘triple PCs’, according to
the criteria by Warren and Gates (24), and the skin and PRCs were synchronous,
according to the criteria by Moertel et al (25). The skin cancer (poorly differentiated
SCC) was not considered the metastasis of the mandible cancer (well
to moderately differentiated SCC), according to the criteria by
Warren and Gates (24).
Discussion
There are two important points in the present
report: Firstly, to the best of our knowledge, the combination of
triple PCs (well-differentiated SCC of the mandible, axillary
cutaneous poorly differentiated SCC and prostate AC) has not been
previously reported; secondly, to detect SPC, we suggest that
FDG-PET should be used for the long-term follow-up of patients with
HNC.
Based on the aforementioned lesions, the patient in
the present case was diagnosed with triple PCs according to the
criteria suggested by Warren and Gates in 1932 (24), and the skin and PRCs were synchronous
according to the criteria defined by Moertel et al in 1961
(25). A systematic literature search
of PubMed (https://www.ncbi.nlm.nih.gov/pubmed/) and Google
Scholar (https://scholar.google.co.jp/) articles published
between 1932, when the SPC criteria was firstly defined (24), and 2017 was performed. The databases
were searched using the following terminological combinations (one
term used from each category): i) Any region of the head and neck,
including oral cavity, oral, mouth, oral floor, tongue, lip, soft
plate, gingiva, buccal, maxillary, mandibular, tonsil, neck, face,
cheek, salivary grand, parotid gland, sublingual gland,
submandibular gland, nose, nasal cavity, paranasal sinus,
nasopharynx, larynx, oropharynx, mesopharynx, hypopharynx, glottis,
thyroid, ear; ii) prostate; and iii) SPC terms, including second
primary cancer, second primary malignancy, second primary tumor,
multiple primary cancer, multiple primary neoplasm, multiple
primary malignancies, multiple primary malignant, triple primary,
triple cancer, quadruple primary, quadruple cancer, quintuple
primary, quintuple cancer, sextuple primary, sextuple cancer,
septuple primary, septuple cancer, octuple primary, octuple cancer,
nonuple primary, or nonuple cancer. A report containing more than
decuple primary cancer types, including PRC, could not be located.
English articles were then searched using the aforementioned terms.
This resulted in the identification of 23 cases of patients with
multiple (triple or more) cancer types, including HNC and PRC
(25–39) (Table I).
The study by Gordon (26) was located
by non-systematic literature research, using Google Scholar and the
term ‘synchronous primary carcinoma.’ However, the current
combination was not found. The most frequent sites of incident of
SPC following HNC are the head and neck again, the lungs and the
esophagus (7,22); while SPC of the skin or prostate is
uncommon. Although a number of risks of SPC have been suggested to
date, the risk of SPC is unclear (40). As the combination of triple PCs in the
present study was unique, the risk factors may have included the
administration of preoperative chemotherapy (41) and radiation postoperatively for index
mandible cancer (41,42), the fact that the patient was a current
drinker (41,43), a family history of gastric cancer in
two brothers (44) and finally, the
patient being a former smoker (43,45). The
association between being a former smoker and secondary cancer risk
in cancer survivors is unclear in the present case due to the
cessation of smoking more than 40 years previously. Shiels et
al (45) demonstrated that being
a former smoker resulted in a higher SPC risk compared with the
risk for those who had never smoked. Based on the aforementioned
data, all factors for SPC risk in the current case should be
carefully considered. Rose et al (6) analyzed 34,568 patients with
non-metastatic SCC of HNC, based on SEER data, and reported that
patients with HNC are at a high risk of SPC. The 5-year cumulative
all-cause fatality rate, HNC-specific fatality rate, SPC fatality
rate and non-cancer fatality rate were 51.3, 23.8, 14.6 and 13.0%,
respectively. Additionally, the 10-year cumulative rate of SPC
mortality reached 20% (6). As a
result, mortality due to SPC is high and persistent over a long
term, and contributes to the poor prognosis of SCC in patients with
HNC (6). Other previous studies have
also indicated that even in long-term cancer survivors, SPC
following HNC poses a high risk (39,42,46).
Furthermore, SPC more frequently develops in various sites in
patients with HNC, compared with the SPC occurrence of the general
population (5,7); therefore, clinicians should give more
focus to SPC following HNC treatment, and a more accurate protocol
should be established.
 | Table I.Cases exhibiting triple or greater
primary cancer of multiple types, including head and neck, and
prostate cancer. |
Table I.
Cases exhibiting triple or greater
primary cancer of multiple types, including head and neck, and
prostate cancer.
|
| Order of sites
exhibiting the occurrence of multiple primary cancer |
|---|
|
|
|
|---|
| Author (year) | First | Second | Third | Fourth | Fifth | Sixth | (Refs.) |
|---|
| Gordon (1948) | PRC | Lung | Thyroid |
|
|
| (26) |
| Goodner and Watson
(1956) | Soft plate | Esophagus | PRC |
|
|
| (27) |
| Moertel et
ala (1961) | Colon | Lip | PRC |
|
|
| (25) |
|
| Kidney | Mouth | PRC |
|
|
| (25) |
|
| Lip | Lung | PRC |
|
|
| (25) |
|
| Lung | PRC | Thyroid |
|
|
| (25) |
|
| Lip | Skin | PRC |
|
|
| (25) |
|
| Mouth | Mouth | PRC |
|
|
| (25) |
|
| Lip | Mouth | PRC |
|
|
| (25) |
| Bittorf et
al (2001) | PRC | Colorectum | Oral cavity |
|
|
| (28) |
|
| Thyroid | UB | PRC |
|
|
| (28) |
| Rho et al
(2002) | Vocal cord | Bowen's
disease | PRC | Laryngeal |
|
| (29) |
| Rai et al
(2007) | PRC | Kidney | Thyroid |
|
|
| (30) |
| Jaudah et al
(2008) | Thyroid | PRC | Renal |
|
|
| (31) |
| Yamashita et
al (2010) | PRC | Gastric | Laryngeal |
|
|
| (32) |
| Salem et al
(2012) | PRC | Nasopharyngeal | Lung |
|
|
| (33) |
|
| Renal | Nose | Auricle | PRC | Colon |
| (33) |
| Guven et al
(2014) | Bladder | PRC | Thyroid |
|
|
| (34) |
| Mukaiyama et
al (2014) | Glottis | Renal pelvis | UB | Oral floor | PRC | Esophagus | (35) |
| Mohammed et
al (2015) | PRC | UB | Thyroid |
|
|
| (36) |
| Testori et
al (2015) | Lung | Oropharynx | Large bowel | PRC |
|
| (37) |
| Pastore et
al (2015) | Kidney | Oropharynx | PRC |
|
|
| (38) |
| Adel et al
(2016) | Buccal | Lip | Gum | PRC |
|
| (39) |
| Present case | Mandible | Axillary skin | PRC |
|
|
| – |
We suggest that FDG-PET could be used for detecting
SPCs in the long-term follow-up of patients with HNC. To date,
there is no accurate protocol of FDG-PET for the follow-up of
patients with HNC. In the present case, PRC (third type) was
determined during the preoperative FDG-PET for skin (second type)
cancer. The PRC (third type) was not determined during the first
follow-up period of 3 years, between the mandible (first type)
cancer treatment and the skin (second type) cancer occurrence.
Contrast-enhanced CT was performed from the head to the lungs
routinely during the follow-up subsequent to the HNC treatment; if
FDG-PET had been performed as a routine follow-up tool of mandible
cancer, the PRC may have been detected earlier. Cancer of the oral
cavity and skin are relatively simple to detect due to the lesions
being observed directly. Conversely, as PRC is asymptomatic in the
early stages (47) and is an internal
disease, it is frequently incidentally detected. In order to
confirm the diagnosis method of PRC of those patients, the cases in
Table I were further reviewed and the
manner in which the subsequent PRC was detected is indicated
(28,29,31,33–35,37,38)
(Table II). Of the 9 patients in
which PRC was diagnosed, including the present case, PRC was
detected by a serum test in 2, incidentally by surgery of the other
tumor in 2, by FDG-PET in 2 and by clinical symptoms in 1 (PRC
detection in 2 patients was not described). Similar to the present
case, Testori et al (37)
incidentally detected lung cancer using FDG-PET (37). Notably, among the cases in Table II, 5/7 patients presented with no
subjective symptoms of PRC and were incidentally diagnosed with
cancer. Additionally, in the SEER study, the incidence of secondary
PRC within 1 year of HNC was only 0.3% among 26,258 male patients
with oral and pharyngeal (tongue, mouth, tonsil, oropharyngeal and
hypopharyngeal) cancer (4).
 | Table II.Method of detecting subsequent PRC
from cases of Table I. |
Table II.
Method of detecting subsequent PRC
from cases of Table I.
| Author (year) | Interval between
first cancer and subsequent PRC | Method of detecting
PRC | Subjective symptoms
of PRC | (Refs.) |
|---|
| Bittorf et
al (2001) | 11 years | NA | NA | (28) |
| Rho et al
(2002) | 3 years | Serum tumor marker
test to rule out hidden cancer following the diagnosis of Bowen's
disease | None | (29) |
| Jaudah et al
(2008) | 10 years | Urinary
symptom | Urinary
symptom | (31) |
| Salem et al
(2012) | 5 years | NA | NA | (33) |
| Guven et al
(2014) | Simultaneous | Incidentally
determined in BC surgery | Hematuria and
frequent urination, which were considered to be a result of BC | (34) |
| Mukaiyama et
al (2014) | 3 years 9
months | Incidentally
determined in recurrent BC surgery | None | (35) |
| Testori et
al (2015) | Simultaneous | Incidentally
detected by FDG-PET for suspected lung cancer | None | (37) |
| Pastore et
al (2015) | 7 months | Serum tumor marker
test for follow-up after kidney cancer treatment | None | (38) |
| Present case | 3 years 8
months | Incidentally
detected by FDG-PET to determine the preoperative axillary cancer
staging | None | – |
Patients with cancer should be carefully followed up
in order to detect SPC for the following reasons: i) patients with
cancer have a higher risk for SPC a long time period after PC
compared with the general population (42); ii) as aforementioned, SPC more
frequently develops in various sites in the body following HNC
(5,7);
iii) PRC should be detected at an early stage when it is
asymptomatic, due to this condition demonstrating a poor prognosis
at advanced stages (48) [for
patients with localized stage PRC, the 5-year relative survival
rate is ~100%; by contrast, for patients with advanced (distant)
stage PRC, the rate declines to 28% (49)]; and iv) PRC accounted for ~20% of new
cancer cases in males in the USA in 2016 (50). In the USA, among males, the most
prevalent cancer in 2016 was PRC, which was recorded in 3.3 million
cases (49); therefore, long-term
follow-up to detect PRC following HNC treatment is required.
Yamashita et al (43)
routinely performed FDG-PET/CT scans once every 6–12 months for 5
years in the follow-up period following the initial cancer
treatment of 434 patients with newly diagnosed HNC, and determined
that 12% of patients had synchronous SPC and 24% of patients had
metachronous SPC. It is important to utilize FDG-PET for screening
SPC during follow-up of HNC, as well as during preoperative cancer
staging (43,51). Compared with CT or MRI, PET/CT has the
advantage of evaluating SPC not only at initial staging of first
HNC (51), but also postoperative
follow-up, similar to the present case, due to PET/CT can evaluate
the entire body range; however, there is no accurate protocol for
detecting SPC for the long-term follow-up of HNC to date.
FDG-PET/CT has an important role in the management of patients with
HNC, in order to diagnose long-term surveillance of recurrence or
metastasis (11). For patients with
HNC, FDG-PET/CT is generally performed at ≥6 months after the
initial therapy (11); however, there
are numerous studies regarding PET/CT that have reported a range of
follow-up periods (9,36,43,52),
indicating that the optimal follow-up period has yet to be defined.
In the present case, second primary PRC was detected 3 years after
the initial HNC. Although PET/CT can be performed for extended
follow-ups, it has certain disadvantages and risks, including the
following: The scan may provide false-positive results (9); patients with diabetes mellitus cannot
undergo PET/CT (53); PET/CT
sensitivities depend on the body site (54); and finally, inflammatory lesions,
metal artifacts or benign lesions can cause difficulties in
performing PET/CT (55). Furthermore,
the complications caused by the exposure to X-rays whilst
performing PET/CT should be considered (53). Additionally, the decision to perform
the examination differs among nations, indicating that clinicians
should consider the characteristics of the health care system of
each country.
There are several limitations in the present study,
including the fact that the conclusions are based on a single case
report, which limits the generalizability, and that the present
combination of triple cancer was researched using PubMed and Google
scholar, which are major search services, but other search engines
were not used. For the present case, further studies may provide
beneficial information for detecting SPCs, including PRC, following
the treatment of HNC.
In conclusion, a rare case of triple PCs was
described in the present study. We suggest that FDG-PET should be
performed to detect hidden SPC, such as PRC, for the long-term
follow-up of patients with HNC, particularly in cases where risk
factors are present. SPC should be detected early in order to
maximize positive patient outcomes.
Acknowledgements
The authors would like to thank Professor Kenzo
Takahashi from the Department of Dermatology, Graduate School of
Medicine, University of the Ryukyus (Okinawa, Japan) for his
advice.
Funding
No funding was received.
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
NM and TM acquired the data, performed the
literature review and edited the manuscript. AA substantially
contributed to the concept and design of the study. TN, OA, AM, TG,
SS and KN acquired the data and contributed clinical advice. HM and
AA revised the manuscript. HM and NY evaluated the specimens and
gave histopathological advice. TM had a major role in writing the
manuscript.
Ethics approval and consent to
participate
The report was submitted for ethical review to the
Ethics Committee of the University of the Ryukyus (Okinawa, Japan),
which waived the requirement for review per institutional protocol
due to the study not containing content that requires ethical
approval. The Ethics Committee approved the submission and
publication of the manuscript. Written informed consent was
obtained from the patient for the publication of this case report
and the accompanying images.
Patient consent for publication
Written informed consent was obtained from the
patient for the publication of this case report and the
accompanying images.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
SPC
|
second primary cancer
|
|
FDG-PET
|
2-[18F]-fluoro-2-deoxy-D-glucose-positron emission tomography
|
|
SEER
|
Surveillance, Epidemiology and End
Results
|
|
HNC
|
head and neck cancer
|
|
PC
|
primary cancer
|
|
SCC
|
squamous cell carcinoma
|
|
AC
|
adenocarcinoma
|
|
CT
|
computed tomography
|
|
MRI
|
magnetic resonance imaging
|
|
SUVmax
|
maximum standardized uptake value
|
|
PSA
|
prostate-specific antigen
|
|
PRC
|
prostate cancer
|
References
|
1
|
Cakir A, Akgun Z, Fayda M and Agaoglu F:
Comparison of three dimensional conformal radiation therapy,
intensity modulated radiation therapy and volumetric modulated arc
therapy for low radiation exposure of normal tissue in patients
with prostate cancer. Asian Pac J Cancer Prev. 16:3365–3370. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
IARC, . GLOBOCAN 2012: Estimated Cancer
Incidence, Mortality and Prevalence Worldwide in 2012. http://globocan.iarc.fr/Pages/fact_sheets_cancer.aspxJune
16–2018
|
|
3
|
Choi Y, Kim SY, Kim SH, Yang J, Park K and
Byun Y: Inhibition of tumor growth by biodegradable microspheres
containing all-trans-retinoic acid in a human head-and-neck cancer
xenograft. Int J Cancer. 107:145–148. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Curtis RE, Freedman DM, Ron E, Ries LAG,
Hacker DG, Edwards BK, Tucker MA and Fraumeni JF Jr: New
malignancies among cancer survivors: SEER Cancer Registries,
1973–2000. National Cancer Institute; https://seer.cancer.gov/archive/publications/mpmono/MPMonograph_complete.pdfSeptember
18–2017
|
|
5
|
Morris LG, Sikora AG, Hayes RB, Patel SG
and Ganly I: Anatomic sites at elevated risk of second primary
cancer after an index head and neck cancer. Cancer Causes Control.
22:671–679. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Rose BS, Jeong JH, Nath SK, Lu SM and Mell
LK: Population-based study of competing mortality in head and neck
cancer. J Clin Oncol. 29:3503–3509. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Chuang SC, Scelo G, Tonita JM, Tamaro S,
Jonasson JG, Kliewer EV, Hemminki K, Weiderpass E, Pukkala E,
Tracey E, et al: Risk of second primary cancer among patients with
head and neck cancers: A pooled analysis of 13 cancer registries.
Int J Cancer. 123:2390–2396. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Plaxton NA, Brandon DC, Corey AS, Harrison
CE, Karagulle Kendi AT, Halkar RK and Barron BJ: Characteristics
and limitations of FDG PET/CT for imaging of squamous cell
carcinoma of the head and neck: A comprehensive review of anatomy,
metastatic pathways, and image findings. AJR Am J Roentgenol.
205:W519–W531. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Al-Ibraheem A, Buck A, Krause BJ,
Scheidhauer K and Schwaiger M: Clinical applications of FDG PET and
PET/CT in head and neck cancer. J Oncol. 2009:2087252009.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Lonneux M, Hamoir M, Reychler H, Maingon
P, Duvillard C, Calais G, Bridji B, Digue L, Toubeau M and Grégoire
V: Positron emission tomography with [18F]fluorodeoxyglucose
improves staging and patient management in patients with head and
neck squamous cell carcinoma: A multicenter prospective study. J
Clin Oncol. 28:1190–1195. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Manca G, Vanzi E, Rubello D, Giammarile F,
Grassetto G, Wong KK, Perkins AC, Colletti PM and Volterrani D:
(18)F-FDG PET/CT quantification in head and neck squamous cell
cancer: Principles, technical issues and clinical applications. Eur
J Nucl Med Mol Imaging. 43:1360–1375. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Takata T and Slootweg PJ: Tumours of the
oral cavity and mobile tongueWorld Health Organization (WHO)
classification of head and neck tumours. El-Naggar AK, Chan JKC,
Grandis JR, Takata T and Slootweg PJ: 4th. IARC Press; Lyon: pp.
108–111. 2017
|
|
13
|
Sobin LH, Gospodarowicz MK and Wittekind
C: International Union Against CancerTNM classification of
malignant tumors. 7th. Wiley-Blackwell; New York, NY: 2009
|
|
14
|
Licitra L, Grandi C, Guzzo M, Mariani L,
Lo Vullo S, Valvo F, Quattrone P, Valagussa P, Bonadonna G,
Molinari R and Cantù G: Primary chemotherapy in resectable oral
cavity squamous cell cancer: A randomized controlled trial. J Clin
Oncol. 21:327–333. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Yamamoto E, Kohama G, Sunakawa H, Iwai M
and Hiratsuka H: Mode of invasion, bleomycin sensitivity, and
clinical course in squamous cell carcinoma of the oral cavity.
Cancer. 51:2175–2180. 1983. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Mantsopoulos K, Goncalves M and Iro H:
Transdermal scopolamine for the prevention of a salivary fistula
after parotidectomy. Br J Oral Maxillofac Surg. 56:212–215. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Shimm DS, Berk FK, Tilsner TJ and
Coulthard SW: Low-dose radiation therapy for benign salivary
disorders. Am J Clin Oncol. 15:76–78. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Maurer P, Eckert AW, Kriwalsky MS and
Schubert J: Scope and limitations of methods of mandibular
reconstruction: A long-term follow-up. Br J Oral Maxillofac Surg.
48:100–104. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Moura LB, Carvalho PH, Xavier CB, Post LK,
Torriani MA, Santagata M and Chagas Júnior OL: Autogenous
non-vascularized bone graft in segmental mandibular reconstruction:
A systematic review. Int J Oral Maxillofac Surg. 45:1388–1394.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
National Comprehensive Cancer Network, .
Head and Neck Cancers Version 1. 2018.https://www.nccn.org/professionals/physician_gls/pdf/head-and-neck.pdfApril
18–2018
|
|
21
|
Weedon D, Morgan MB, Gross C, Nagore E and
Yu LL: Squamous cell carcinomaPathology and Genetics of Skin
Tumours. LeBoit PE, Burg G and Weedon DAS: IARC Press; Lyon: pp.
20–25. 2006
|
|
22
|
Jain KS, Sikora AG, Baxi SS and Morris LG:
Synchronous cancers in patients with head and neck cancer: Risks in
the era of human papillomavirus-associated oropharyngeal cancer.
Cancer. 119:1832–1837. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Gleason DF and Mellinger GT: Prediction of
prognosis for prostatic adenocarcinoma by combined histological
grading and clinical staging. J Urol. 111:58–64. 1974. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Warren S and Gates O: Multiple primary
malignant tumors: A survey of the literature and a statistical
study. Am J Cancer. 16:1358–1414. 1932.
|
|
25
|
Moertel CG, Dockerty MB and Baggenstoss
AH: Multiple primary malignant neoplasms. I. Introduction and
presentation of data. Cancer. 14:221–230. 1961. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Gordon BS: Triple synchronous primary
carcinoma. Arch Pathol (Chic). 45:56–64. 1948.PubMed/NCBI
|
|
27
|
Goodner JT and Watson WL: Cancer of the
esophagus; its association with other primary cancers. Cancer.
9:1248–1252. 1956. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Bittorf B, Kessler H, Merkel S, Brückl W,
Wein A, Ballhausen WG, Hohenberger W and Günther K: Multiple
primary malignancies: An epidemiological and pedigree analysis of
57 patients with at least three tumours. Eur J Surg Oncol.
27:302–313. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Rho NK, Choi SJ and Lee ES: A case of
multiple Bowen's disease with squamous cell carcinoma of the larynx
and adenocarcionoma of the prostate. J Dermatol. 29:516–521. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Rai RS, Deb P, Rai R, Gupta E and Panayach
JS: Synchronous primary triple neoplasia (renal cell carcinoma and
prostate cancer in combination with thyroid neoplasm). Report of an
unusual case. Minerva Urol Nefrol. 59:451–454. 2007.PubMed/NCBI
|
|
31
|
Jaudah AM, Kanaan HD and Hasan JF:
Multiple primary malignancies of thyroid, kidneys and prostate:
Synchronous and metachronous presentation in one patient. New Egypt
J Med. 39:33–36. 2008.
|
|
32
|
Yamashita M, Jinbu Y, Hiratsuka M,
Shinozaki Y, Itoh H and Kusama M: A case of quadruple primary
cancer including lower lip cancer. Asian J Oral Maxillofac Surg.
22:172–174. 2010. View Article : Google Scholar
|
|
33
|
Salem A, Abu-Hijlih R, Abdelrahman F,
Turfa R, Amarin R, Farah N, Sughayer M, Almousa A and Khader J:
Multiple primary malignancies: Analysis of 23 patients with at
least three tumors. J Gastrointest Cancer. 43:437–443. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Guven BC, Guzide Ayse GO and Ramazan S:
Triple synchronous primary cancers of thyroid, bladder and
prostate: A case report. Kuwait Med J. 46:62–64. 2014.
|
|
35
|
Mukaiyama Y, Suzuki M, Morikawa T, Mori Y,
Takeshima Y, Fujimura T, Fukuhara H, Nakagawa T, Nishimatsu H, Kume
H and Homma Y: Multiple primary malignant neoplasms of the glottis,
renal pelvis, urinary bladder, oral floor, prostate, and esophagus
in a Japanese male patient: A case report. World J Surg Oncol.
12:2942014. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Mohammed A, Al-Zahrani A, Mansour M,
Ghanem H, El Saify A and Hani EK: Triple primary carcinomas:
Prostatic adenocarcinoma, bladder urethral carcinoma and papillary
thyroid carcinoma: A case report. Am J Cancer Case Rep. 3:24–28.
2015.
|
|
37
|
Testori A, Cioffi U, De Simone M, Bini F,
Vaghi A, Lemos AA, Ciulla MM and Alloisio M: Multiple primary
synchronous malignant tumors. BMC Res Notes. 27:7302015. View Article : Google Scholar
|
|
38
|
Pastore AL, Palleschi G, Leto A, Silvestri
L, Porta N, Petrozza V and Carbone A: A novel combination of triple
metachronous malignancies of the kidney, oropharynx and prostate: A
case report. Oncol Lett. 10:917–920. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Adel M, Liao CT, Lee LY, Hsueh C, Lin CY,
Fan KH, Wang HM, Ng SH, Lin CH, Tsao CK, et al: Incidence and
outcomes of patients with oral cavity squamous cell carcinoma and
fourth primary tumors: A long-term follow-up study in a betel quid
chewing endemic area. Medicine (Baltimore). 95:e29502016.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Donin N, Filson C, Drakaki A, Tan HJ,
Castillo A, Kwan L, Litwin M and Chamie K: Risk of second primary
malignancies among cancer survivors in the United States, 1992
through 2008. Cancer. 122:3075–3086. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Babacan NA, Aksoy S, Cetin B, Ozdemir NY,
Benekli M, Uyeturk U, Ali Kaplan M, Kos T, Karaca H, Oksuzoglu B,
et al: Multiple primary malignant neoplasms: Multi-center results
from Turkey. J BUON. 17:770–775. 2012.PubMed/NCBI
|
|
42
|
Utada M, Ohno Y, Hori M and Soda M:
Incidence of multiple primary cancers and interval between first
and second primary cancers. Cancer Sci. 105:890–896. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Yamashita T, Araki K, Tomifuji M, Tanaka
Y, Harada E, Suzuki T, Miyamoto S and Shiotani A: Clinical features
and treatment outcomes of Japanese head and neck cancer patients
with a second primary cancer. Asia Pac J Clin Oncol. 13:172–178.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Hung CY, Ueng SH, Lin YC and Chou WC:
Metastatic carcinoma of the urinary bladder in a 67-year-old female
with underlying triple primary cancers. J Cancer Res Pract.
3:49–53. 2016. View Article : Google Scholar
|
|
45
|
Shiels MS, Gibson T, Sampson J, Albanes D,
Andreotti G, Beane Freeman L, Berrington de Gonzalez A, Caporaso N,
Curtis RE, Elena J, et al: Cigarette smoking prior to first cancer
and risk of second smoking-associated cancers among survivors of
bladder, kidney, head and neck, and stage I lung cancers. J Clin
Oncol. 32:3989–3995. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Tiwana MS, Hay J, Wu J, Wong F, Cheung W
and Olson RA: Incidence of second metachronous head and neck
cancers: Population-based outcomes over 25 years. Laryngoscope.
124:2287–2291. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Kundra V: Prostate cancer imaging. Semin
Roentgenol. 41:139–149. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Kimura T, Onozawa M, Miyazaki J, Matsuoka
T, Joraku A, Kawai K, Nishiyama H, Hinotsu S and Akaza H:
Prognostic impact of young age on stage IV prostate cancer treated
with primary androgen deprivation therapy. Int J Urol. 21:578–583.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Miller KD, Siegel RL, Lin CC, Mariotto AB,
Kramer JL, Rowland JH, Stein KD, Alteri R and Jemal A: Cancer
treatment and survivorship statistics, 2016. CA Cancer J Clin.
66:271–289. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2017. CA Cancer J Clin. 67:7–30. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Strobel K, Haerle SK, Stoeckli SJ, Schrank
M, Soyka JD, Veit-Haibach P and Hany TF: Head and neck squamous
cell carcinoma (HNSCC)-detection of synchronous primaries with
(18)F-FDG PET/CT. Eur J Nucl Med Mol Imaging. 36:919–927. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Kondo N, Tsukuda M and Nishimura G:
Diagnostic sensitivity of 18fluorodeoxyglucose positron
emission tomography for detecting synchronous multiple primary
cancers in head and neck cancer patients. Eur Arch
Otorhinolaryngol. 269:1503–1507. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Hiraoka A, Hirooka M, Ochi H, Koizumi Y,
Shimizu Y, Shiraishi A, Yamago H, Tanihira T, Miyata H, Ninomiya T,
et al: Importance of screening for synchronous malignant neoplasms
in patients with hepatocellular carcinoma: Impact of FDG PET/CT.
Liver Int. 33:1085–1091. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Minamimoto R, Senda M, Terauchi T,
Jinnouchi S, Inoue T, Iinuma T, Inoue T, Ito K, Iwata H, Uno K, et
al: Analysis of various malignant neoplasms detected by FDG-PET
cancer screening program: Based on a Japanese Nationwide Survey.
Ann Nucl Med. 25:45–54. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Murakami R, Uozumi H, Hirai T, Nishimura
R, Shiraishi S, Ota K, Murakami D, Tomiguchi S, Oya N, Katsuragawa
S and Yamashita Y: Impact of FDG-PET/CT imaging on nodal staging
for head-and-neck squamous cell carcinoma. Int J Radiat Oncol Biol
Phys. 68:377–382. 2007. View Article : Google Scholar : PubMed/NCBI
|