Introduction
Hepatocellular carcinoma (HCC) is the sixth most
common type of cancer and is the third leading cause of
cancer-associated mortality worldwide (1). According to the guidelines of the
American Association for the Study of Liver Diseases, surgical
resection, liver transplantation and radiofrequency ablation (RFA)
are three curative treatment options for HCC (2). However, as the majority of patients are
asymptomatic at early stages, only a small portion of patients are
amenable to curative treatments. Despite the advances in technology
and surgical treatment in recent years, the prognosis of patients
with HCC following curative surgical resection remains poor, with a
relatively high post-operative recurrence rate (3–5). The
molecular mechanisms underlying the recurrence of HCC are not
well-understood. However, previous studies have demonstrated that
inflammation serves an important role in the development,
progression and metastasis of HCC, with a variety of inflammatory
cells and mediators identified within the HCC microenvironment
(6–8).
Myeloid-derived innate immune cells, particularly macrophages and
neutrophils, serve important roles in the maintenance of the tumor
microenvironment (9).
The S100 protein family belongs to a group of
inflammatory molecules which are primarily composed of
calcium-binding proteins and have been identified to be involved in
a range of pathological processes including infections,
cardiovascular diseases and malignancies. There are ~25 members of
the S100 protein family have been identified to date, the majority
of which are located on chromosome 1q21 which is frequently
associated with genomic rearrangement, forming the genetic
background of tumor progression (10). An association between S100 proteins
and various types of cancer has been revealed in multiple studies
(10,11). Of the ~25 members, S100A8, S100A9 and
S100A12 belong to the calgranulin S100 protein subfamily.
Calgranulins are widely expressed on myeloid-derived immune cells,
particularly on neutrophils and monocytes/macrophages (12,13). It
has been reported that S100A8 and S100A9, which usually form
heterodimers with each other, are involved in the development of
liver malignancies (14–16). In contrast with S100A8 and S100A9,
S100A12 functions as a homodimer. Funk et al (17) demonstrated the association between
high expression levels of S100A12 on tumor cells with favorable
prognosis for oropharyngeal squamous cell carcinoma. However,
whether S100A12 is associated with the prognosis of HCC remains
unknown. The present study investigated the prognostic value of
S100A12 in HCC following curative surgical resections.
Materials and methods
Study populations
A total of 139 patients diagnosed with HCC who
underwent curative surgical resection in Zhongshan Hospital (Fudan
University, Shanghai, China) between December 2005 and June 2006
were enrolled in the present study. Patient inclusion required the
fulfilment of the following criteria: i) Received curative surgical
resection without gross residual tumor; ii) preoperative liver
functions classified as Child-Pugh A (18); and iii) histopathological examination
confirmed HCC diagnosis. Patients were routinely followed up every
2 months during the first postoperative year and every 3–4 months
thereafter (19). The median
follow-up time was 34.33 months in the present study. Tumor
specimens were prepared for the construction of tissue microarrays
according to a previously published protocol (20). Patient characteristics are presented
in Table I. The present study was
approved by the Zhongshan Hospital Research Ethics Committee and
written informed consent was obtained from all patients.
 | Table I.Basic clinicopathological parameters
of included patients. |
Table I.
Basic clinicopathological parameters
of included patients.
|
| Intratumoral S100A12
expression |
|
|---|
|
|
|
|
|---|
| Characteristics | Low | High | P-value |
|---|
| Age, years | 52.31±11.74 | 51.12±8.89 | 0.573 |
| Sex |
|
| 0.759 |
| Male | 98 | 21 |
|
|
Female | 16 | 4 |
|
| HBsAg |
|
| 0.526 |
|
Negative | 17 | 2 |
|
|
Positive | 97 | 23 |
|
| Concurrent
cirrhosis |
|
| 0.832 |
| No | 89 | 20 |
|
|
Yes | 25 | 5 |
|
| AFP, ng/ml |
|
| 0.726 |
|
≤20 | 36 | 7 |
|
|
>20 | 78 | 18 |
|
| Tumor size, cm | 5.60±3.64 | 5.68±4.21 | 0.924 |
| Tumor number |
|
| 0.765 |
|
Solitary | 95 | 22 |
|
|
Multiple | 19 | 3 |
|
| Tumor
encapsulation |
|
| 0.060 |
| No | 55 | 7 |
|
|
Yes | 59 | 18 |
|
| Vascular tumor
thrombus |
|
| 0.383 |
| No | 66 | 12 |
|
|
Yes | 48 | 13 |
|
| Tumor
differentiation |
|
| 0.010a |
|
I–II | 88 | 13 |
|
|
III–IV | 26 | 12 |
|
| TNM stage |
|
| 0.549 |
|
I–II | 97 | 20 |
|
|
III–IV | 17 | 5 |
|
Immunohistochemistry
Tumor tissues were fixed in 10% neutral buffered
formalin at room temperature for 12–24 h. Paraffin embedded tumor
tissues were sliced into sections with a thickness of 5 µm.
Paraffin-embedded tumor sections were incubated in 10%
H2O2 for 15 min to minimize the non-specific
staining due to endogenous peroxidase prior to being incubated in
10 mM citrate buffer (pH 6.0) for 30 min in a steam-cooker for
antigen retrieval. Non-specific background staining was blocked
with 10% goat serum (Shanghai Haoran Biological Technology Co.,
Ltd., Shanghai, China) for 30 min and subsequently primary mouse
anti-S100A12 (1:200; cat. no. LS-C335557; LifeSpan BioSciences,
Inc., Seattle, WA, USA), mouse anti-cluster of differentiation
(CD)15 (1:100; cat. no. ab754; Abcam, Cambridge, UK) and mouse
anti-CD68 (1:100; cat. no. ab49777; Abcam) antibodies were applied
for incubation overnight at 4°C, according to the manufacturer's
protocol. Subsequently, sections were incubated with ready-to-use
horseradish peroxidase (HRP)-conjugated anti-mouse IgG (HRP Polymer
Quanto; cat. no. TL-015-QHD; Lab Vision Corporation, Fremont, CA,
USA) at room temperature for 30 min followed by incubation with
mixture of DAB Quanto Substrate (cat. no. TL-015-QHD; Lab Vision
Corporation) and DAB Quanto Chromogen (cat. no. TL-015-QHD; Lab
Vision Corporation) at a ratio of 1,000:30 at room temperature for
1–5 min.
For immunofluorescence staining, frozen tumor
sections were blocked with 10% donkey serum (Jackson ImmunoResearch
Laboratories, Inc., West Grove, PA, USA) for at room temperature
for 30 min before incubation with primary rabbit anti-S100A12
antibody (1:100; cat. no. LS-C335557, LifeSpan BioSciences, Inc.)
together with mouse anti-CD11B (1:100, cat. no. ab34216, Abcam),
mouse anti-CD15 (1:100, cat. no. ab754, Abcam) or mouse anti-CD68
(1:100, cat. no. ab53444, Abcam) antibody overnight at 4°C.
Sections were washed three times in PBS followed by incubation with
Alexa Fluor 546-conjugated anti-rabbit IgG (1:250; cat. no. A10040;
Invitrogen; Thermo Fisher Scientific, Inc., Waltham, MA, USA) and
Alexa Fluor 488-conjugated anti-mouse IgG (1:250; cat. no. A21202;
Invitrogen; Thermo Fisher Scientific, Inc.) secondary antibodies
for 2 h at 37°C. Sections were then stained with 10 µg/ml DAPI for
10 min. Sections were washed in PBS and mounted with anti-fade
reagent (SouthernBiotech, Birmingham, AL, USA) and observed using a
confocal fluorescence microscope (1,260X objective magnification;
Olympus Corporation, Tokyo, Japan).
Tissue microarray analysis
The assessment of S100A12 expression was performed
by a computerized image system comprising a Leica charge-coupled
device camera DFC420 connected to a Leica DM IRE2 microscope (Leica
Microsystems, Ltd., Milton Keynes, UK). Images of three random
‘hotspots’ where S100A12 was most populous at magnification ×20,
were captured using Leica QWin Plus software (v.3; Leica
Microsystems, Ltd.), and the integrated optical total positive
staining area (TPSA) of these images was determined using Image-Pro
Plus software version 6 (Media Cybernetics, Inc. Rockville, MD,
USA). The camera parameter was constant throughout the whole
process. An optimal TPSA was selected as a threshold to define the
expression of S100A12 as low or high on the basis of the log-rank
test as described previously (21).
Three experienced researchers, who are experienced in the
pathological examination of HCC, reviewed the results
independently. Any discrepancies were resolved by discussion in
order to reach a consensus.
Statistical analysis
Quantitative variables of normal distribution are
presented as the mean ± standard deviation and compared using the
independent sample Student's t-test or one-way analysis of variance
test (Tukey post-hoc test) where applicable. Quantitative variables
of abnormal distribution are presented as the median and compared
with non-parametric tests (Mann Whitney-U test). Qualitative
variables are presented as relative frequencies and compared using
Pearson χ2 test or Fisher's exact test. The Kaplan-Meir
estimator survival analysis using a log-rank test was performed to
compare the overall survival (OS) and disease-free survival (DFS)
rates following curative surgical resection of HCC between
different groups. A multivariate Cox's proportional hazard model
was used to assess the association between S100A12 and patient
survival rate, together with other confounding factors including
tumor size, tumor number, presence of vascular tumor embolus,
tumor-node-metastasis (TNM) stage, tumor differentiation and
pre-operative serum γ-glutamyltranspeptidase (GGT). All statistical
analysis was performed using the commercially available software
SPSS (version 18.0; IBM Corp., Armonk, NY, USA). P<0.05 was
considered to indicate a statistically significant difference.
Results
Patient characteristics and S100A12
expression
A total of 139 patients were included in the present
study. Measured by the TPSA, intratumoral S100A12 expression was
low in 114 patients (TPSA, <1,600 µm2) and high in 25
patients (TPSA, >1,600 µm2). Basic
clinicopathological parameters, including age, sex, hepatitis B
surface antigen, concurrent cirrhosis, α-fetoprotein, tumor size,
tumor number, presence of vascular tumor thrombus, TNM stage and
tumor Edmonson-Steiner differentiation score (I–IV, with an,
increased score indicating a worse differentiation) (22), were collected (Table I).
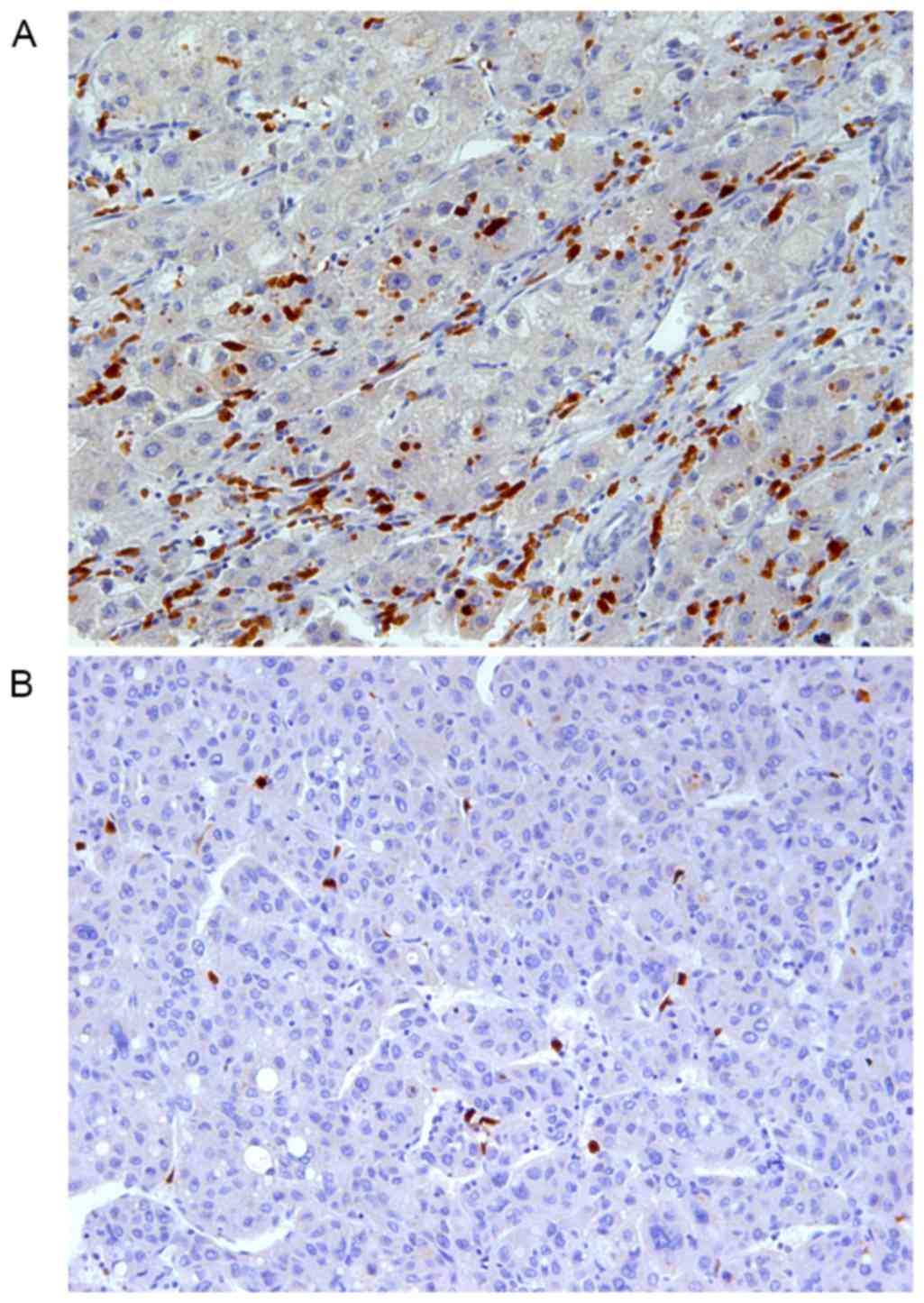
S100A12 was exclusively expressed in the cytoplasm
of stroma cells with relatively smaller size compared with the
tumor cells (Fig. 1). The expression
of S100A12 was significantly decreased (P<0.001) on intratumoral
stroma cells (median TPSA, 270 µm2) compared with
peritumoral stroma cells (median TPSA, 836 µm2).
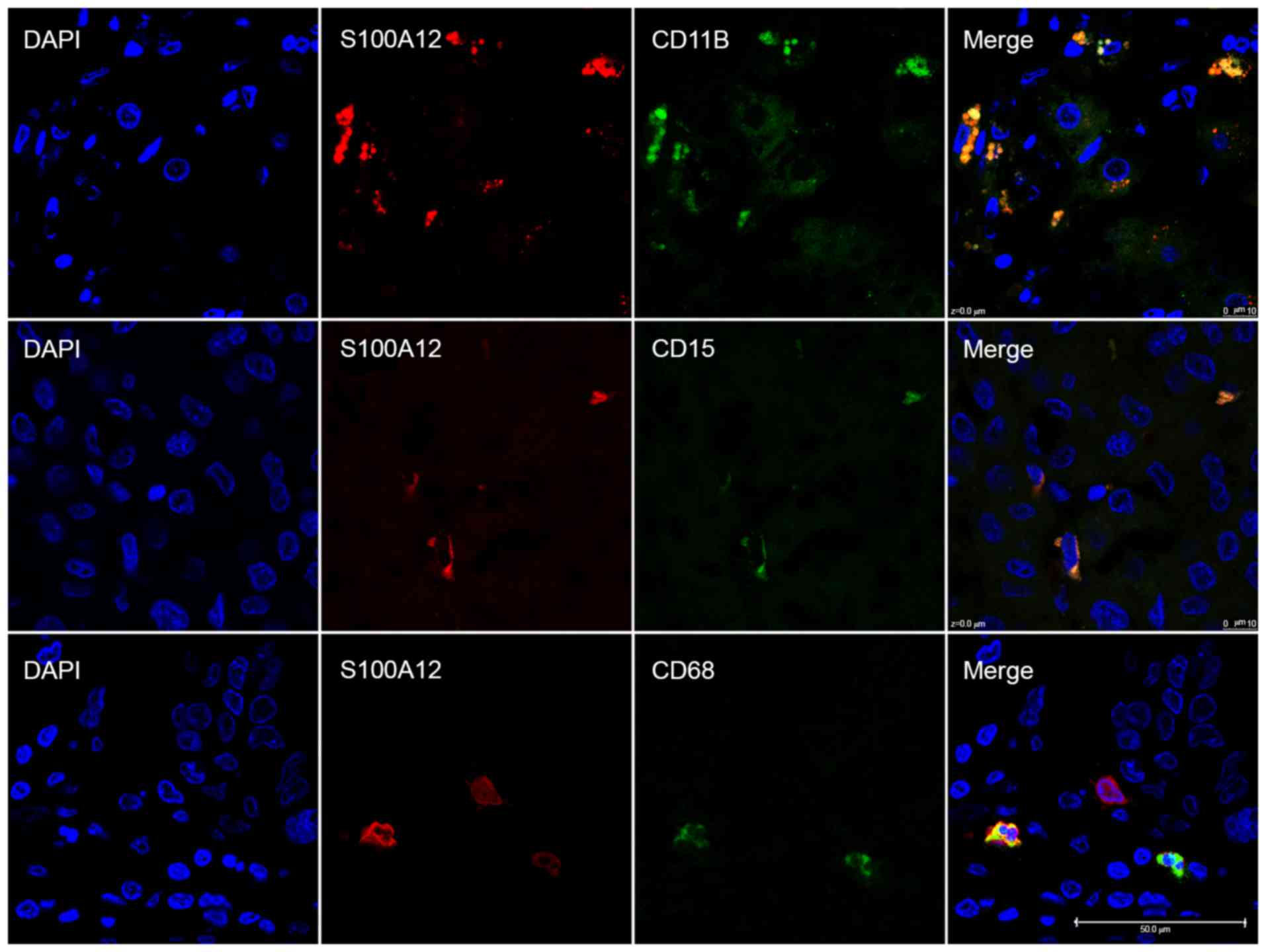
Immunofluorescence staining revealed that S100A12 was primarily
expressed on CD11B-positive myeloid-derived immune cells
particularly CD15-positive neutrophils and CD68-positive
macrophages, which serve important roles in the HCC tumor
microenvironment (Fig. 2). There was
no association between the TPSA of S100A12 and that of CD15
(P=0.603), CD68 (P=0.562) or the sum of the two (P=0.526) in 30 of
139 patients. A statistically significant association between
S100A12 expression and tumor differentiation was observed upon
evaluation with the Edmonson-Steiner score (P=0.010). Pre-operative
serum alanine aminotransferase (P=0.539), aspartate
aminotransferase (P=0.595), albumin (P=0.194), bilirubin (P=0.601),
prothrombin time (P=0.124) and GGT (P=0.401), which all reflect the
severity of liver damage or the activity of inflammation, were not
statistically different between high and low S100A12 expression.
There was no association between S100A12 expression and other
clinicopathological parameters. There were 67 patients at TNM stage
I and 50 at stage II in total. The expression of S100A12 between
TNM I and II was not significantly different with a P-value of
0.620.
Prognostic value of S100A12 expression
within tumor tissues
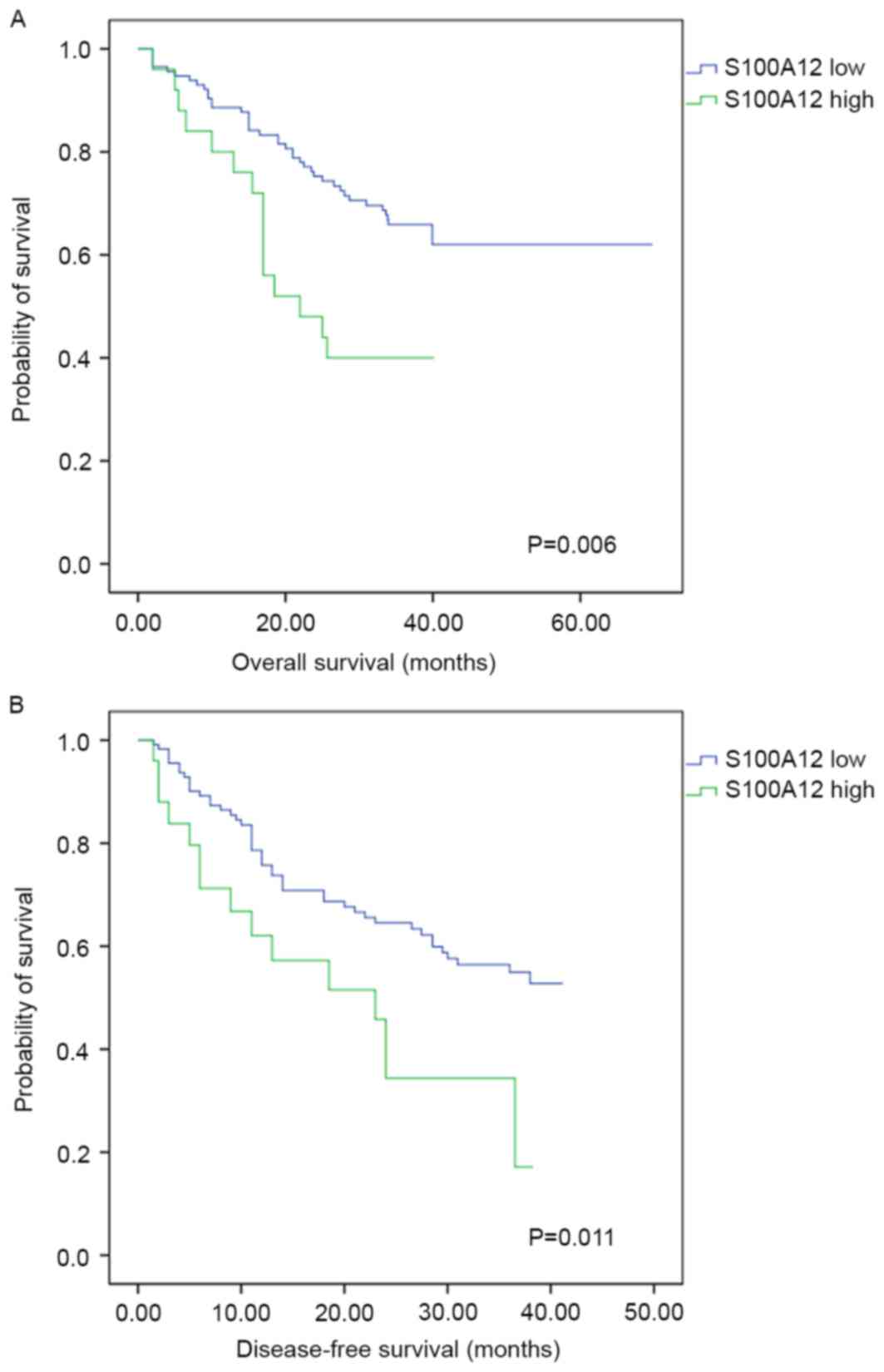
Measured by the TPSA, high expression of S100A12 on
intratumoral stroma cells was associated with a poorer OS rate
(χ2=7.519, P=0.006). The 1-, 2- and 3-year OS rates were
88.6, 75.3 and 65.8% for patients with low S100A12 expression,
compared with 80.0, 48.0 and 40.0% for patients with high S100A12
expression. The DFS rate of patients with low expression of S100A12
was significantly increased, with 1-, 2- and 3-year DFS rates of
78.6, 64.5 and 54.9%, compared with 62.0, 34.3 and 17.2% for
patients with high S100A12 expression (χ2=6.471;
P=0.011; Fig. 3). There were no
statistically significant associations between peritumoral S100A12
expression and the OS (P=0.709) or DFS (P=0.759) rate following
surgical resection of HCC.
With the exception of S100A12, univariate analysis
revealed that tumor size (P<0.001), tumor number (P=0.015),
presence of vascular tumor thrombus (P=0.004), tumor
differentiation score (P=0.006), TNM stage (P<0.001) and
pre-operative serum GGT (P<0.001) were associated with the OS
rate of patients with HCC. Tumor size (P<0.001), presence of
vascular tumor thrombus (P=0.035), TNM stage (P=0.003) and
pre-operative serum GGT (P=0.017) were also associated with the DFS
of patients with HCC. Multivariate Cox's proportional hazard model
analysis demonstrated that high expression of S100A12 was an
independent prognostic factors for the OS (P=0.001) and DFS
(P=0.007) rates of patients with HCC, as presented in Table II.
 | Table II.Multivariate analysis of factors
associated with overall and disease-free survival rates of
hepatocellular carcinoma following curative surgical resection. |
Table II.
Multivariate analysis of factors
associated with overall and disease-free survival rates of
hepatocellular carcinoma following curative surgical resection.
|
| Overall
survival | Disease-free
survival |
|---|
|
|
|
|
|---|
| Characteristic | HR (95% CI) | P-value | HR (95% CI) | P-value |
|---|
| Tumor size, cm |
| >5
vs. ≤5 | 2.81
(1.32–5.98) | 0.007a | 1.96
(1.03–3.74) | 0.040a |
| Tumor number |
|
Solitary vs. multiple | 1.15
(0.42–3.13) | 0.791 | NA | NA |
| Vascular tumor
thrombus |
| Yes vs.
no | 0.84
(0.42–1.68) | 0.626 | 1.12
(0.62–2.05) | 0.704 |
| Tumor
differentiation |
| III–IV
vs. I–II | 2.00
(0.97–4.11) | 0.059 | NA | NA |
| TNM stage |
| III–IV
vs. I–II | 2.36
(0.79–7.12) | 0.126 | 1.67
(0.78–3.56) | 0.188 |
| GGT, U/l |
| >91
vs. ≤91 | 2.60
(1.40–4.82) | 0.002a | 1.70
(0.95–3.05) | 0.075 |
| S100A12
expression |
| Low vs.
high | 3.17
(1.53–6.57) | 0.002a | 2.53
(1.29–4.96) | 0.007a |
Discussion
There are a limited number of studies on S00A12 and
its association with cancer incidence. One reason for the limited
knowledge about S100A12 may be that S100A12 is constitutionally
expressed in humans without an ortholog in rodents. With
experimental studies in the field of cancer research now primarily
based on model organisms, particularly rodents, this may be a
limiting factor.
Consistent with the literature, the results of the
present study demonstrated that S100A12 was primarily expressed on
myeloid-derived immune cells, including neutrophils and
monocytes/macrophages (12,13). The immunohistochemistry results of the
present study revealed that S100A12 was not expressed on tumor
cells or vascular endothelial cells. Immunofluorescence staining
demonstrated that S100A12 was predominantly expressed on
CD11B-positive myeloid-derived immune cells, particularly on
CD15-positive neutrophils and CD68-positive macrophages. The
results of the present study revealed that intratumoral S100A12
served as an independent prognostic factor for HCC following
curative surgical resection. High expression of S100A12 on
intratumoral stroma cells indicated poor OS and DFS rates following
curative surgical resection.
Tumor-associated macrophages and neutrophils have
been intensively studied (9) and an
association with HCC has been demonstrated. However, a previous
study revealed that intratumoral macrophage infiltration was not
associated with the OS or DFS rates of HCC following curative
surgical resection (20). Kuang et
al (23) also demonstrated that
intratumoral neutrophils were not associated with the prognosis of
HCC. There was no association between S100A12 with CD15 (P=0.603),
CD68 (P=0.562) or the two together (P=0.526). It may be that
certain types of macrophages or neutrophils that infiltrate tumor
tissues that serve a critical role in the progression of HCC. Erbel
et al (24) identified a new
type of atherosclerotic plaque-associated MMP7-positive,
S100A8-positive and CD68-positive macrophage termed M4 macrophage,
which is distinct from the typical M1 and M2 macrophage type. As an
analog of S100A12, S100A8 expression was interleukin 10- and
prostaglandin E2-dependent, which suggested
anti-inflammatory properties of S100A12-positive macrophages
(25). In addition, neutrophils may
be involved in the progression of HCC through an S100A12 signaling
pathway, an area where further investigation is required.
Neutrophils expressing S100A12 may be associated with tumor
progression and may account for the post-operative recurrence of
HCC. As members of the calgranulins, S100A8 and S100A9 may possess
similar functions to those of S100A12. A previous study revealed
that S100A8 and S100A9 expression was induced by nuclear factor κB,
promoting HCC progression by activating the reactive oxygen species
signaling pathway (16).
Additionally, S100A9 alone increased the viability and invasiveness
of human HCC cells by activating the mitogen-activated protein
kinase signaling pathway (15).
In the present study, tumor size and pre-operative
serum GGT were also identified to be independent prognostic factors
of post-operative OS rates, and tumor size was identified to be an
independent prognostic factor of post-operative DFS rates. These
results were consistent with those of previous studies; tumor size
was an accepted prognostic factor of HCC following surgical
resection (26) and GGT was reported
to be associated with poor prognosis following surgical resection,
RFA and transcatheter arterial chemoembolization (27–29). Fu
et al (27) revealed that high
pre-operative serum GGT was associated with poor OS and DFS rates
following surgical resection of HCC, which supports the results of
the present study. However, the underlying molecular mechanism
remains elusive (27).
The results of the present study revealed an
association between S100A12 and poor tumor differentiation, which
was in accordance with previous studies (10). Similar results were reported for
S100A9 and its association with tumor differentiation in HCC
(30). It is known that crosstalk
between tumor cells and surrounding immune cells contributes to the
progression, invasion and metastasis of tumor cells (9). S100A12 expression in tumor-infiltratory
myeloid-derived immune cells may be induced by tumor cells,
attracting various inflammatory cells to migrate to the tumor site
and exert pro- and anti-inflammation activities by secreting
cytokines of diverse functions. Transforming growth factor β1
(TGF-β1) and other inflammatory mediators have been revealed to be
involved in the process of epithelial-mesenchymal transition (EMT),
which is associated with the dedifferentiation of cancer cells
(31–33). Another member of the S100 protein
family, S100A4, was reported to upregulate SNAI1, which was
downstream of TGF-β1, serving a role in the EMT of HCC cells
(34). As with S100A4, a similar
process may explain the association between S100A12 and poor tumor
differentiation during the course of HCC progression.
There were a number of limitations to the present
study. First, S100A12 is unique to humans and is expressed on
multiple myeloid-derived immune cells including neutrophils and
monocytes/macrophages, which impeded further knowledge of the
underlying molecular mechanisms by which S100A12 influences HCC
progression and recurrence. Secondly, as the expression of S100A12
within HCC tissues was low in general, there was only a small
portion of patients with high intratumoral S100A12 expression. The
sample size of the high-S100A12 group was relatively small which
could limit the reliability of results. Further studies including
incorporating more eligible patients are required.
The present study revealed that S100A12 may provide
a potential target for the immune therapy of HCC. S100A12 may be a
potential target for antitumor treatment. Also, the decrease in
S100A12 expression may be a marker of clinical effectiveness of
immune therapy for HCC.
Acknowledgements
The authors would like to Mrs Ke Qiao from Key
Laboratory of Medical Molecular virology, Ministry of Education and
Public Health, School of Basic Medical Sciences, Fudan University
(Shanghai, China) for her technical expertise in microscopic
imaging techniques.
Funding
The collection, analysis and interpretation of data
and manuscript writing were supported by grants from the National
Natural Science Foundation of China (grant nos. 81372655 and
81472224) and the National Key Basic Research Program [(973
project; grant no. 2015CB554005) from the Ministry of Science and
Technology of China.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
HS and HC designed the study. HC contributed to the
writing of the manuscript. HC and BY analyzed the
clinicopathological data and performed the survival analysis. HC
and BY performed the immunohistochemistry staining of the tissue
microarray. HC and XZ performed the immunofluorescence staining of
HCC frozen sections. YZ, ZC and CW collected the
clinicopathological data and follow-up information of patients with
HCC. All authors read and approved the final manuscript.
Ethics approval and consent to
participate
The present study was approved by the Zhongshan
Hospital Research Ethics Committee. Written informed consent for
the use of their tissue and clinicopathologic data was obtained
from all patients.
Patient consent for publication
There is no patient consent for publication.
However, all identifying information has been removed in the
present study.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Global Burden of Disease Cancer
Collaboration, ; Fitzmaurice C, Dicker D, Pain A, Hamavid H,
Moradi-Lakeh M, MacIntyre MF, Allen C, Hansen G, Woodbrook R, et
al: The global burden of cancer 2013. JAMA Oncol. 1:505–527. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Bruix J and Sherman M; American
Association for the Study of Liver Diseases, : Management of
hepatocellular carcinoma: An update. Hepatology. 53:1020–1022.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Roayaie S, Obeidat K, Sposito C, Mariani
L, Bhoori S, Pellegrinelli A, Labow D, Llovet JM, Schwartz M and
Mazzaferro V: Resection of hepatocellular cancer </=2 cm:
Results from two Western centers. Hepatology. 57:1426–1435. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Shrager B, Jibara G, Schwartz M and
Roayaie S: Resection of hepatocellular carcinoma without cirrhosis.
Ann Surg. 255:1135–1143. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Hasegawa K, Kokudo N, Imamura H, Matsuyama
Y, Aoki T, Minagawa M, Sano K, Sugawara Y, Takayama T and Makuuchi
M: Prognostic impact of anatomic resection for hepatocellular
carcinoma. Ann Surg. 242:252–259. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Alison MR, Nicholson LJ and Lin WR:
Chronic inflammation and hepatocellular carcinoma. Recent Results
Cancer Res. 185:135–148. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Hu B, Yang XR, Xu Y, Sun YF, Sun C, Guo W,
Zhang X, Wang WM, Qiu SJ, Zhou J and Fan J: Systemic
immune-inflammation index predicts prognosis of patients after
curative resection for hepatocellular carcinoma. Clin Cancer Res.
20:6212–6222. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Huang J, Xu L, Luo Y, He F, Zhang Y and
Chen M: The inflammation-based scores to predict prognosis of
patients with hepatocellular carcinoma after hepatectomy. Med
Oncol. 31:8832014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Galdiero MR, Bonavita E, Barajon I,
Garlanda C, Mantovani A and Jaillon S: Tumor associated macrophages
and neutrophils in cancer. Immunobiology. 218:1402–1410. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Chen H, Xu C, Jin Q and Liu Z: S100
protein family in human cancer. Am J Cancer Res. 4:89–115.
2014.PubMed/NCBI
|
|
11
|
Salama I, Malone PS, Mihaimeed F and Jones
JL: A review of the S100 proteins in cancer. Eur J Surg Oncol.
34:357–364. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Goyette J and Geczy CL:
Inflammation-associated S100 proteins: New mechanisms that regulate
function. Amino Acids. 41:821–842. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Gross SR, Sin CG, Barraclough R and
Rudland PS: Joining S100 proteins and migration: for better or for
worse, in sickness and in health. Cell Mol Life Sci. 71:1551–1579.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Wu R, Duan L, Cui F, Cao J, Xiang Y, Tang
Y and Zhou L: S100A9 promotes human hepatocellular carcinoma cell
growth and invasion through RAGE-mediated ERK1/2 and p38 MAPK
pathways. Exp Cell Res. 334:228–238. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Wu R, Duan L, Ye L, Wang H, Yang X, Zhang
Y, Chen X, Zhang Y, Weng Y, Luo J, et al: S100A9 promotes the
proliferation and invasion of HepG2 hepatocellular carcinoma cells
via the activation of the MAPK signaling pathway. Int J Oncol.
42:1001–1010. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Nemeth J, Stein I, Haag D, Riehl A,
Longerich T, Horwitz E, Breuhahn K, Gebhardt C, Schirmacher P, Hahn
M, et al: S100A8 and S100A9 are novel nuclear factor kappa B target
genes during malignant progression of murine and human liver
carcinogenesis. Hepatology. 50:1251–1262. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Funk S, Mark R, Bayo P, Flechtenmacher C,
Grabe N, Angel P, Plinkert PK and Hess J: High S100A8 and S100A12
protein expression is a favorable prognostic factor for survival of
oropharyngeal squamous cell carcinoma. Int J Cancer. 136:2037–2046.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
European Association for the Study of the
Liver, . Electronic address: simpleeasloffice@easloffice.eu;
European Association for the Study of the Liver: EASL Clinical
Practice Guidelines: Management of hepatocellular carcinoma. J
Hepatol. 69:182–236. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Sun HC, Zhang W, Qin LX, Zhang BH, Ye QH,
Wang L, Ren N, Zhuang PY, Zhu XD, Fan J and Tang ZY: Positive serum
hepatitis B e antigen is associated with higher risk of early
recurrence and poorer survival in patients after curative resection
of hepatitis B-related hepatocellular carcinoma. J Hepatol.
47:684–690. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zhu XD, Zhang JB, Zhuang PY, Zhu HG, Zhang
W, Xiong YQ, Wu WZ, Wang L, Tang ZY and Sun HC: High expression of
macrophage colony-stimulating factor in peritumoral liver tissue is
associated with poor survival after curative resection of
hepatocellular carcinoma. J Clin Oncol. 26:2707–2716. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Budczies J, Klauschen F, Sinn BV, Győrffy
B, Schmitt WD, Darb-Esfahani S and Denkert C: Cutoff Finder: A
comprehensive and straightforward Web application enabling rapid
biomarker cutoff optimization. PLoS One. 7:e518622012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Liu GJ, Xu HX, Lu MD, Xie XY, Xu ZF, Zheng
YL and Liang JY: Correlation between enhancement pattern of
hepatocellular carcinoma on real-time contrast-enhanced ultrasound
and tumour cellular differentiation on histopathology. Br J Radiol.
80:321–330. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Kuang DM, Zhao Q, Wu Y, Peng C, Wang J, Xu
Z, Yin XY and Zheng L: Peritumoral neutrophils link inflammatory
response to disease progression by fostering angiogenesis in
hepatocellular carcinoma. J Hepatol. 54:948–955. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Erbel C, Tyka M, Helmes CM, Akhavanpoor M,
Rupp G, Domschke G, Linden F, Wolf A, Doesch A, Lasitschka F, et
al: CXCL4-induced plaque macrophages can be specifically identified
by co-expression of MMP7+S100A8+ in vitro and in vivo. Innate Immu.
21:255–265. 2015. View Article : Google Scholar
|
|
25
|
Hsu K, Chung YM, Endoh Y and Geczy CL:
TLR9 ligands induce S100A8 in macrophages via a STAT3-dependent
pathway which requires IL-10 and PGE2. PLoS One. 9:e1036292014.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Schlachterman A, Craft WW Jr, Hilgenfeldt
E, Mitra A and Cabrera R: Current and future treatments for
hepatocellular carcinoma. World J Gastroenterol. 21:8478–8491.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Fu S, Guo Z, Li S, Kuang M, Hu W, Hua Y,
He X and Peng B: Prognostic value of preoperative serum
gamma-glutamyltranspeptidase in patients with hepatocellular
carcinoma after hepatectomy. Tumour Biol. 37:3433–3440. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Ma H, Zhang L, Tang B, Wang Y, Chen R,
Zhang B, Chen Y, Ge N, Wang Y, Gan Y, et al:
γ-Glutamyltranspeptidase is a prognostic marker of survival and
recurrence in radiofrequency-ablation treatment of hepatocellular
carcinoma. Ann Surg Oncol. 21:3084–3089. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Zhang JB, Chen Y, Zhang B, Xie X, Zhang L,
Ge N, Ren Z and Ye SL: Prognostic significance of serum
gamma-glutamyl transferase in patients with intermediate
hepatocellular carcinoma treated with transcatheter arterial
chemoembolization. Eur J Gastroenterol Hepatol. 23:787–793. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Arai K, Yamada T and Nozawa R:
Immunohistochemical investigation of migration inhibitory
factor-related protein (MRP)-14 expression in hepatocellular
carcinoma. Med Oncol. 17:183–188. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Pang MF, Georgoudaki AM, Lambut L,
Johansson J, Tabor V, Hagikura K, Jin Y, Jansson M, Alexander JS,
Nelson CM, et al: TGF-β1-induced EMT promotes targeted migration of
breast cancer cells through the lymphatic system by the activation
of CCR7/CCL21-mediated chemotaxis. Oncogene. 35:748–760. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Ricciardi M, Zanotto M, Malpeli G, Bassi
G, Perbellini O, Chilosi M, Bifari F and Krampera M:
Epithelial-to-mesenchymal transition (EMT) induced by inflammatory
priming elicits mesenchymal stromal cell-like immune-modulatory
properties in cancer cells. Br J Cancer. 112:1067–1075. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
van Zijl F, Zulehner G, Petz M, Schneller
D, Kornauth C, Hau M, Machat G, Grubinger M, Huber H and Mikulits
W: Epithelial-mesenchymal transition in hepatocellular carcinoma.
Future Oncol. 5:1169–1179. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Zheng X, Gai X, Wu Z, Liu Q and Yao Y:
Metastasin leads to poor prognosis of hepatocellular carcinoma
through partly inducing EMT. Oncol Rep. 29:1811–1818. 2013.
View Article : Google Scholar : PubMed/NCBI
|