Introduction
The most common types of malignant tumors in women
are breast, uterine, cervical, and ovarian cancers. Cervical cancer
is one of the most common types of cancers and is associated with
high rates of malignancy-related death in women worldwide. Cervical
cancer-related morbidity and mortality rates have decreased over
the last 30 years in many countries due to the widespread use of
Pap smear screening tests; however, patients with advanced stage
cervical cancer still have a poor prognosis (1,2). According
to the GLOBOCAN series reported by the International Agency for
Research on Cancer, cancers in women have a relatively poor
prognosis, with a mortality-to-incidence ratio of 32.5% (3). In patients with cervical cancer, tumor
size, lymph node metastasis, and lymph-blood vessel invasion are
independent prognostic factors for survival. However, due to the
heterogeneity of the patient population, these factors may not
predict prognosis accurately (4,5).
Therefore, the identification of novel prognostic biomarkers is
urgently needed to improve the prognosis of women with cancer.
Approximately 98% of transcripts in the human genome
represent RNA that does not encode proteins (6). Although these noncoding RNAs (ncRNAs)
have previously been considered transcriptional noise, there is now
evidence that they play important roles in most cellular processes,
including cell proliferation, differentiation, apoptosis,
metabolism, and immunity. In addition, ncRNAs affect cancer cell
phenotypes and gene regulation (7,8). In recent
years, long ncRNAs (lncRNAs) have also been shown to affected DNA
binding and the expression of various genes, including chromatin
modification complex protein-related activities. Additionally,
lncRNAs regulate gene expression in response to external stimuli or
DNA damage. However, the biological functions and molecular
mechanisms of lncRNAs in human diseases and cancers remain largely
unknown (9–11).
Steroid receptor activator (SRA), an lncRNA
located on chromosome 5q31.3, has been shown to activate human
hormone receptors that are strongly associated with gynecologic
cancers, such as ovarian and breast cancers (12,13).
SRA modulates the functions of a variety of transcription
factor modulators and can act as a separate scaffold by enhancing
the transcriptional activity of the steroid receptor in the
reporter gene. SRA is involved in normal biological
processes, such as cell death, lipogenesis, steroidogenesis, muscle
formation, and insulin signaling, and has been shown to have roles
in breast cancer, prostate cancer, abnormal cardiac development,
and reduced fertility. Moreover, SRA functionally interacts
with proteins involved in cleavage and a number of nuclear
receptors, such as retinoic acid receptors (14). Although SRA is known to be
related to the progression of malignant tumors, its role in
cervical cancer has not been elucidated. Previous studies have
shown that SRA expression in cervical cancer cell lines is
associated with the progression of malignant tumors (15). However, the clinical relevance of
SRA expression is still unclear.
Accordingly, in this study, we investigated the
expression of SRA in cervical cancer cell lines and analyzed
the relationships among SRA expression, clinicopathological
findings, and disease prognosis. Functional analysis was also
performed to investigate the effects of SRA on cancer cell
invasion and migration in vitro. Finally, we investigated
whether SRA was involved in the epithelial-mesenchymal
transition (EMT), as a major mechanism leading to metastasis, in
cervical cell lines.
Patients and methods
Patients and tissue samples
In total, 100 women who underwent surgery between
2012 and 2017 at Yonsei Severance Hospital, Yonsei University
(Seoul, Korea) were included in this study. Specimens from patients
with newly diagnosed invasive stage IA to IVB cervical cancer
(International Federation of Gynecology and Obstetrics) who had not
received prior treatment were evaluated. Additionally, 22 normal
cervical tissues from patients undergoing simple hysterectomy
because of uterine leiomyomata were obtained as controls. This
study was approved by the Ethics Committee of Yonsei Severance
Hospital, and informed consent was obtained from all patients. All
specimens were immediately frozen in liquid nitrogen and stored at
−80°C until RNA extraction.
Cell lines and cell culture
The human cervical squamous carcinoma SiHa cells was
obtained from the Korean Cell Line Bank (Seoul, Korea) and provided
by the Korea Gynecologic Cancer Bank through the Bio and Medical
Technology Development Program of the Minister of Science,
Information and Communication Technology and Future Planning,
Korea. A total of 293 cells were purchased from American Type
Culture Collection (Manassas, VA, USA). SiHa and 293 cells were
cultured in Dulbecco's modified Eagle's medium. All culture media
were supplemented with 10% (v/v) fetal bovine serum and 1%
penicillin/streptomycin, and cell lines were maintained at 37°C in
a humidified atmosphere of 5% CO2 and 95% air. Culture
medium was replaced with fresh medium every 2–3 days, and cells
that had been passaged less than 20 times were used in the
experiments.
Quantitative real-time PCR
(qRT-PCR)
Total RNA was extracted using TRIzol reagent
(Invitrogen; Thermo Fisher Scientific, Inc., Waltham, MA, USA)
according to the manufacturer's instructions. One microgram of
total RNA was reverse transcribed into first-strand cDNA using a
reverse transcription reagent kit (Bioline, London, UK). The cDNA
template was amplified by qRT-PCR using SensiFAST SYBR Hi-ROX Mix
(Bioline). qRT-PCR was performed on an ABI StepOnePlus Real-Time
PCR System (Applied Biosystems, Foster City, CA, USA). All
quantifications were performed with U6 as the internal
standard. Relative gene expression was analyzed using the
2−ΔΔCT method, and the results were expressed as extent
of change with respect to control values. qRT-PCR experiments were
replicated at least three times. Primers used for PCR are shown in
Table I.
 | Table I.Primer sequences used in this
study. |
Table I.
Primer sequences used in this
study.
|
| Primer
sequence | Product |
|---|
|
|
|
|
|---|
| Gene | Forward
(5′-3′) | Reverse
(5′-3′) | Size (base
pair) |
|---|
| SRA |
CTCCCTTCTTACCACCACCA |
TGCAGATACACAGGGAGCAG | 217 |
| U6 |
CTCGCTTCGGCAGCACA |
AACGCTTCAGGAATTTGCGT | 92 |
| E-cadherin |
ATTCTGATTCTGCTGCTCTTG |
AGTAGTCATAGTCCTGGTCCT | 421 |
| N-cadherin |
CCCAAGACAAAGAGACCCAG |
GCCACTGTGCTTACTGAATTG | 140 |
| Snail |
GAGGCGGTGGCAGACTAG |
GACACATCGGTCAGACCAG | 178 |
| Wnt |
TGTGAGGTGAAGACCTGCTG |
AAAGTTGGGGGAGTTCTCGT | 207 |
| Vimentin |
TGGATTCACTCCCTCTGGTT |
GGTCATCGTGATGCTGAGAA | 111 |
| Twist |
CGGGAGTCCGCAGTCTTA |
TGAATCTTGCTCAGCTTGTC | 150 |
Plasmid constructs and generation of
stable cell lines
Full-length human SRA cDNA was amplified by
PCR and inserted into the pLenti6/V5-D-TOPO vector using the
ViraPower Lentiviral Expression System (Invitrogen; Thermo Fisher
Scientific, Inc.) according to the manufacturer's protocol. The
plasmid was transfected into 293FT cells for packaging, and the
resulting lentivirus was used to infect the desired cell lines. The
selection of SRA stably transfected cells was performed in
medium containing blasticidin (Invitrogen; Thermo Fisher
Scientific, Inc.).
Cell proliferation assay
Cell proliferation was evaluated using Cell Counting
kit-8 (CCK-8) assays (Dojindo Laboratories, Kumamoto, Japan). Cells
were seeded into 6-well flat-bottomed plates (1×105
cells/well) in 2 ml complete medium. The cells were incubated
overnight to allow for cell attachment and recovery and were
subsequently subjected to SRA1 overexpression for 24, 48,
72, or 96 h. Next, 10 µl CCK-8 solution was added to each well, and
cells were incubated for 1 h. The absorbance was measured at 450 nm
using an auto-microplate reader to calculate the number of viable
cells in each well. The cell survival rate was expressed as the
absorbance relative to that of the SiHa, veoctor cells. Three
independent experiments were performed in triplicate.
Matrigel invasion assay
Matrigel invasion assays were performed using BD
Biocoat Matrigel Invasion Chambers (pore size: 8 µm, 24-well; BD
Biosciences, Bedford, MA, USA), according to the manufacturer's
protocol. Briefly, 1×105 cells were plated in the upper
chambers in serum-free medium, and complete medium was added to the
bottom chamber. The chambers were then incubated for 48 h at 37°C
in an atmosphere containing 5% CO2. Noninvading cells
were removed from the upper chambers using cotton-tipped swabs.
Cells that had invaded through the pores into the lower side of the
filter were stained (Diff Quik; Sysmes, Kobe, Japan), and these
cells were then counted using a hemocytometer. Invasion cell was
measured using ImageJ software (NIH, Bethesda, MD, USA) and the
percentage of the invasion cell was calculated. Results were
standardized to SiHa cells. The number of cells that invaded the
membrane was counted in 10 fields under the ×20 objective lens.
Original magnification, ×200. The assay was replicated at least
three times.
Wound healing migration assay
Cell migration was assessed using wound healing
assays. Briefly, 1×106 cells were seeded into 6-well
culture plates with serum-containing medium and allowed to grow to
90% confluence in complete medium. The serum-containing medium was
removed, and cells were serum starved for 24 h. When the cell
density reached ~100% confluence, an artificial homogenous wound
was created by scratching the monolayer with a sterile 200-µl
pipette tip. After scratching, the cells were washed with
serum-free medium. Images of cells migrating into the wound were
captured at 0, 24, and 48 h using a microscope. Scratch width was
measured using NIH ImageJ software, and the percentage of the
scratch area closed was calculated as (width at 0 h-width at 48
h)/width at 0 h. Results were standardized to SiHa cells. The
migration cells was counted in 10 fields under the ×20 objective
lens. Original magnification, ×200. The assay was performed in
triplicate.
Western blot analysis
Proteins were extracted with RIPA buffer (Thermo
Fisher Scientific, Inc.). Protein concentrations were measured
using a Pierce BCA Protein assay kit (Thermo Fisher Scientific,
Inc.). After boiling with 5X sample buffer, proteins were resolved
on 10% sodium dodecyl sulfate-polyacrylamide gels and transferred
electrophoretically to polyvinylidene difluoride membranes
(Millipore, Billerica, MA, USA). Membranes were blocked with 5%
nonfat dried milk in 1X Tris-buffered saline containing 0.1%
Tween-20 (pH 7.6) at room temperature for 1 h and were then
incubated with primary antibodies at 4°C overnight under constant
agitation. The primary antibodies included rabbit anti-human
E-cadherin (1:1,000 dilution), rabbit anti-human β-catenin (1:1,000
dilution), and mouse anti-human Snail (1:1,000 dilution; all Cell
Signaling Technology, Danvers, MA, USA). Proteins were visualized
using an enhanced chemiluminescence system (Amersham, Little
Chalfont, UK), and band intensities were quantified using a
Luminescent Image Analyzer (LAS-4000 mini; Fujifilm, Uppsala,
Sweden).
Statistical analysis
The results of the statistical analyses (SPSS,
version 24.0; IBM, Chicago, IL, USA) were expressed as means ±
standard deviations (SDs). Pearson's χ2 tests, Student's
t-tests, and Fisher's exact tests were used to evaluate the
associations between SRA expression and clinicopathological
characteristics. To evaluate the performance of the models with
respect to their discrimination ability, statistics were used
chi-square values from the logrank test from the receiver operating
characteristic (ROC) analysis. The median value (1.64) was set as
the cut-off value. The groups were classification into high and low
SRA expression groups at values above and below the cut-off value
(1.64), respectively. The Kaplan-Meier method was used to analyze
overall survival times. The log-rank test was used to estimate
between group differences. Stepwise Cox regression model analysis
was used for multivariate survival analysis of the parameters that
were significant in the univariate analysis. The statistical tests
were two-sided; differences with P<0.05 was considered to
indicate a statistically significant difference.
Results
SRA levels were elevated in patients
with cervical cancer having poor prognoses
To determine whether SRA expression in
tissues was linked to the clinicopathological features of cervical
cancer, we evaluated the expression of SRA in cervical
cancer tissues (n=100) and corresponding normal tissues (n=22).
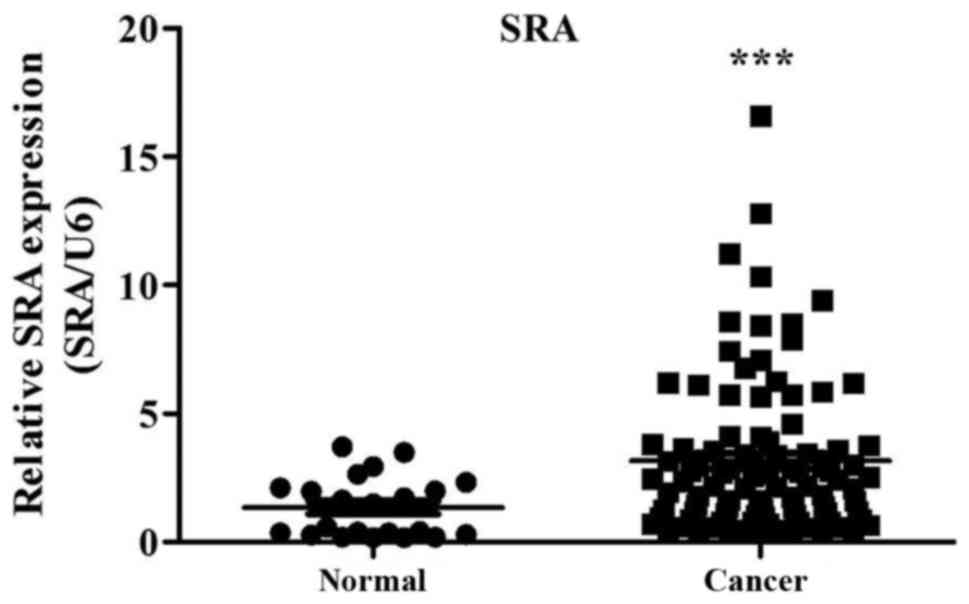
SRA expression in cervical cancer tissues was more than
3.15-fold that of noncancerous tissues (P=0.00007; Fig. 1). Additionally, we examined the
relationships between SRA expression and clinicopathological
information in 100 patients with cervical cancer (Table II). The mean follow-up period was 60
months. Additionally, patients with high SRA expression
exhibited higher rates of lymphatic invasion and invasive cell type
relative to patients with low SRA expression; this
relationship was statistically significant.
 | Table II.Clinicopathological features and
SRA expression in patients with cervical cancer. |
Table II.
Clinicopathological features and
SRA expression in patients with cervical cancer.
|
|
| SRA
expression |
|
|---|
|
|
|
|
|
|---|
| Variables | n (%) | Low | High |
P-valuea |
|---|
| Age (years, mean ±
SD) | 100 | 49.425±1.84 | 51.95±1.61 | 0.339 |
| Stage |
|
|
| 0.094 |
| I,
II | 64 | 22 | 42 |
|
| III,
IV | 36 | 18 | 18 |
|
| Lymph node
metastasis |
|
|
| 0.414 |
|
Yes | 35 | 15 | 20 |
|
| No | 65 | 25 | 40 |
|
| Lymphatic
invasion |
|
|
| 0.033 |
|
Yes | 37 | 10 | 27 |
|
| No | 63 | 30 | 33 |
|
| Tumor size
(cm) |
|
|
| 0.478 |
|
0-5.9 | 45 | 19 | 29 |
|
| ≥6 | 53 | 21 | 32 |
|
| Recurrence |
|
|
| 0.515 |
|
Yes | 29 | 12 | 17 |
|
| No | 71 | 28 | 43 |
|
| Cell type |
|
|
| 0.025 |
|
Squamous cell carcinoma | 63 | 20 | 43 |
|
|
Adenocarcinoma | 17 | 9 | 8 |
|
|
Mixed | 8 | 3 | 5 |
|
|
Other | 10 | 7 | 3 |
|
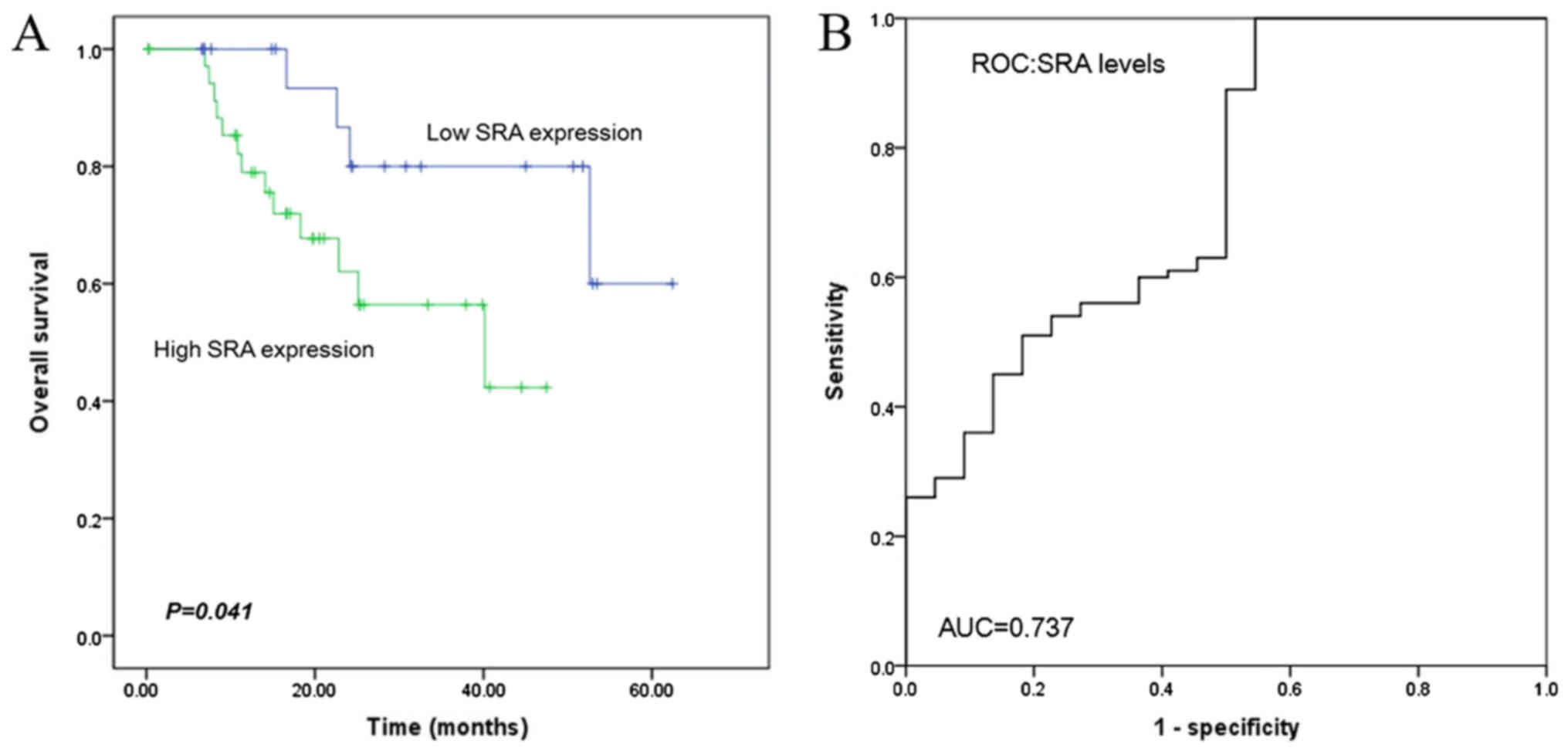
The median 5-year survival duration was
significantly higher in the low SRA expression group than in
the high SRA expression group (19.7 and 25.1 months,
respectively; log-rank test: P=0.041; Fig. 2A). The risk model in SRA data had an
area under curve (AUC) of 0.737 in predicting (P=0.001; Fig. 2B). Moreover, Cox univariate
proportional hazards analysis showed that stage [hazard ratio (HR)
=2.809; P=0.004], tumor size (HR=2.260; P=0.011), and recurrence
(HR=2.479; P=0.05) were independent prognostic factors for overall
survival (Table II). Cox
multivariate proportional hazards analysis showed that SRA
expression (HR=3.714; P=0.031), stage (HR=2.809; P=0.004), tumor
size (HR=2.143; P=0.047), and lymphatic invasion (HR=3.44, P=0.03)
were independent prognostic factors for overall survival (Table III). Both univariate and
multivariate proportional hazards analyses showed that stage and
tumor size were independent prognostic factors of overall
survival.
 | Table III.Univariate and multivariate analyses
of parameters associated with overall survival in 100 patients with
cervical cancer. |
Table III.
Univariate and multivariate analyses
of parameters associated with overall survival in 100 patients with
cervical cancer.
|
| Univariate
analysis | Multivariate
analysis |
|---|
|
|
|
|
|---|
| Factor | Hazard ratio (95%
CI) | P-value | Hazard ratio (95%
CI) | P-value |
|---|
| SRA
expression | 2.096
(0.747–5.729) | 0.162 | 3.714
(1.128–12.230) | 0.031 |
| Age (years) | 1.014
(0.976–1.052) | 0.483 | 1.023
(0.983–1.064) | 0.27 |
| Stage | 2.265
(1.413–3.631) | 0.001 | 2.809
(1.388–5.686) | 0.004 |
| Tumor size | 2.260
(1.208–4.231) | 0.011 | 2.143
(1.011–4.542) | 0.047 |
| Lymph node
metastasis | 2.252
(0.907–5.588) | 0.08 | 0.751
(0.240–2.350) | 0.623 |
| Lymphovascular
invasion | 1.407
(0.564–3.507) | 0.464 | 3.44
(1.13–10.468) | 0.03 |
| Recurrence | 2.479
(1.001–6.136) | 0.05 | 2.68
(0.734–9.782) | 0.136 |
| Histology | 1.185
(0.765–1.837) | 0.447 | 1.434
(0.883–2.330) | 0.145 |
Overexpression of SRA increased cell
proliferation in cervical cancer cells
We previously examined SRA expression levels
in several cervical cancer cell lines by qRT-PCR (15). Here, lentiviral-mediated
overexpression of SRA was performed to determine the
functional role of this lncRNA in SiHa cells. SRA-expressing
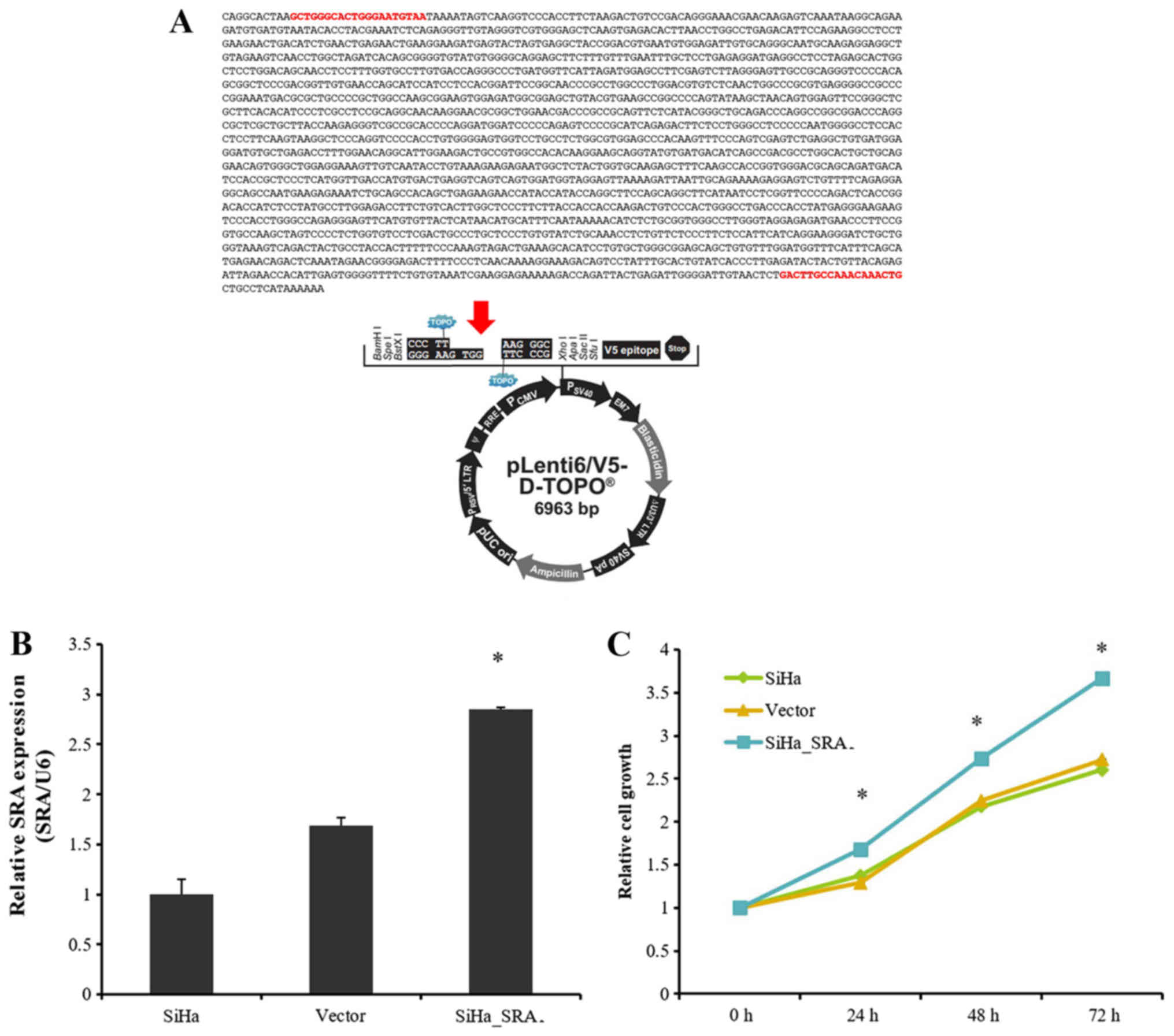
plasmid was prepared using as template a PCR product that contained
the SRA sequences (Fig. 3A). RT-PCR
analysis showed that SRA was successfully overexpressed in
SiHa cells compared with that in control cells (P=0.01; Fig. 3B). We next examined the impact of
SRA overexpression on cell proliferation. The results of
CCK-8 assays showed that overexpression SRA in SiHa cells
increased cell proliferation (Fig.
3C), suggesting that SRA was involved in the
proliferation of cervical cancer cells.
SRA overexpression affected cervical
cancer cell migration and invasion
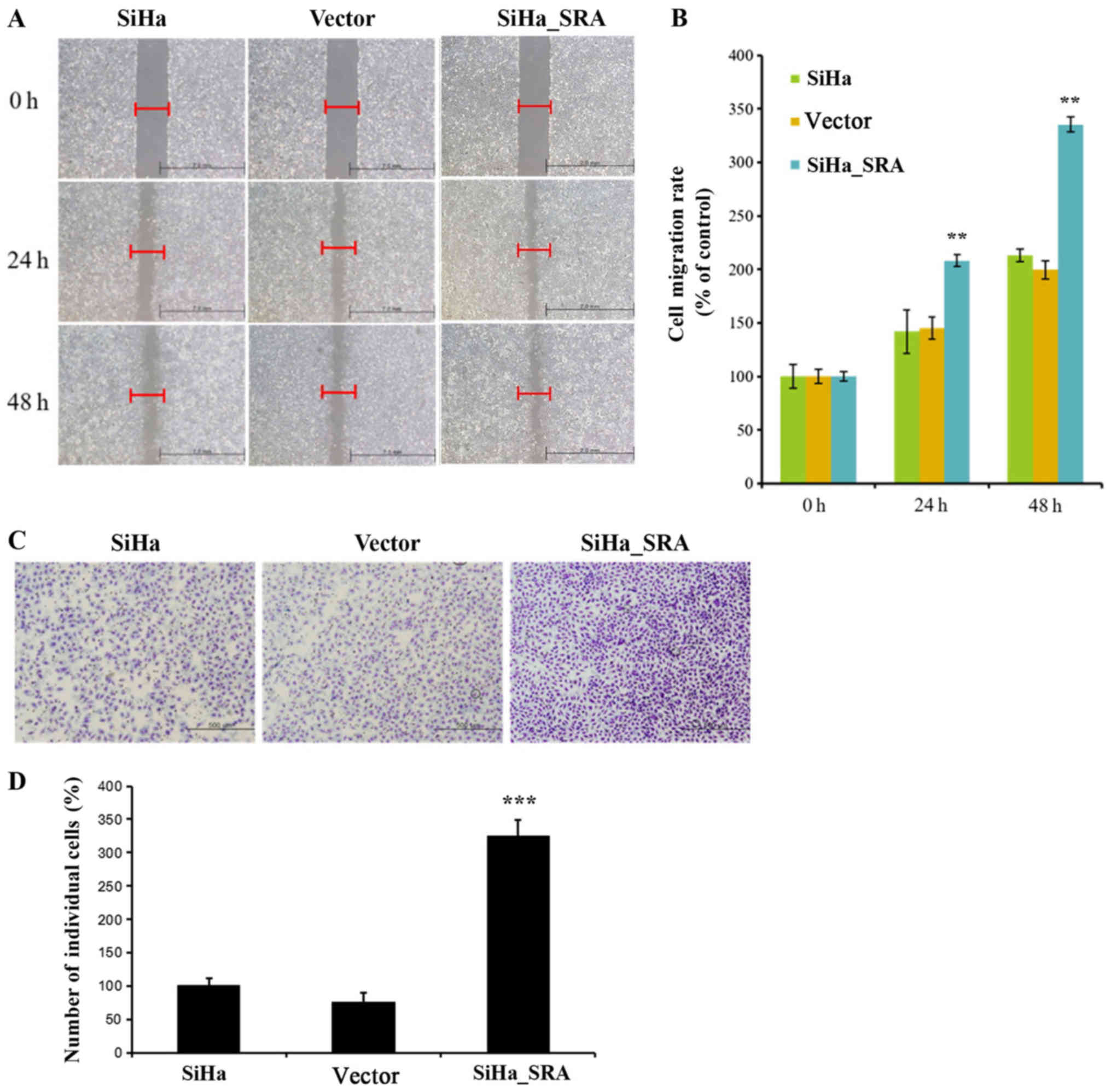
Next, the effects of SRA on the invasive and
migratory behaviors of cells were assessed by Matrigel invasion and
wound healing assays. Overexpression of SRA resulted in increased
migration of SiHa cells relative to empty vector-expressing
controls (P=0.001; Fig. 4A). There
was a significant difference between the scratch width percentages
of each cell line 24 and 48 h after in the SiHa, empty vector and
SRA overexpression cells (P=0.001; Fig.
4B). Furthermore, SRA overexpression in SiHa cells
significantly increased invasion relative to that in empty
vector-expressing cells (P=0.001; Fig.
4C). The invasion relative percentages of each cell line 48 h
after in SiHa, empty vector and SRA overexpression groups was
significantly different (P=0.001; Fig.
4D). The SiHa cells percentages were 100±4.58. The empty cell
line percentages were 72±13.52. The SRA overexpression cell line
percentages were 173±5.38. Altogether, these results indicated that
SRA promoted SiHa cell invasion and migration in vitro.
Overexpression SRA increased the
expression of EMT-associated genes in cervical cancer cells
Because the EMT is important in cell migration and
invasion, the present study examined whether SRA was required for
EMT using RT-qPCR and western blotting. The overexpression of SRA
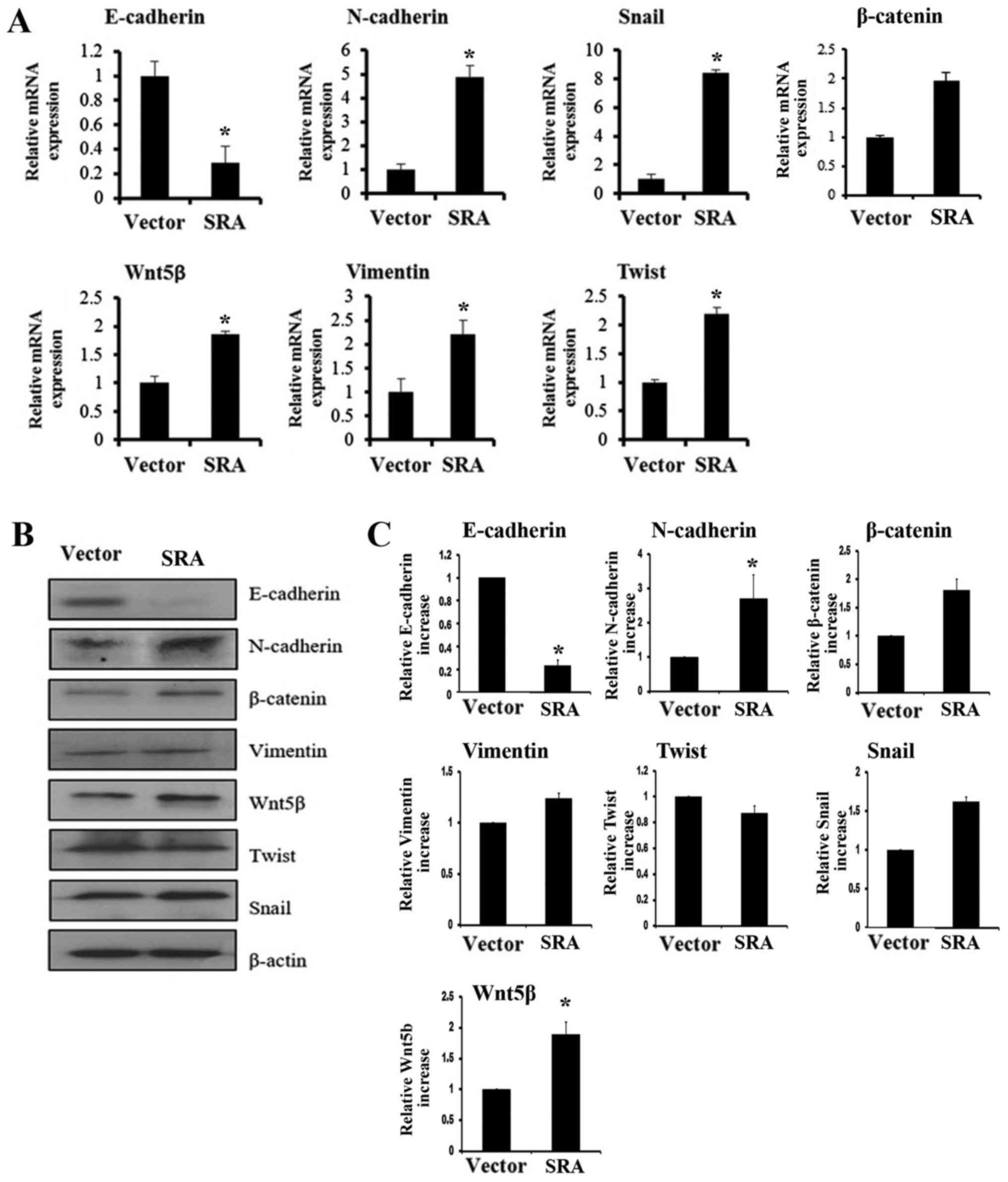
decreased E-cadherin expression and increased N-cadherin, Snail,
Wnt5β, Vimentin and β-catenin expression (Fig. 5A). Levels of protein in cells with
overexpressed SRA had decreased E-cadherin expression and increased
N-cadherin, β-catenin, Vimentin, Wnt5β, Twist and Snail expression
(Fig. 5B). The western blot density
percentages of each cell line 48 h after in the SiHa, empty vector
and SRA overexpression cells are presented in Fig. 5C. In addition, the expression of
Snail, a transcription factor that mediates the EMT, was
upregulated in SRA-overexpressing SiHa cells compared with that in
cells transfected with the empty vector (Fig. 5A-C). These data suggested that
upregulation of EMT-associated genes could partly explain the
involvement of SRA in cervical cancer cell migration and
invasion.
Discussion
A deeper understanding of the molecular mechanisms
underlying cervical cancer progression and metastasis is essential
for the development of more effective therapeutic treatments and
for identifying new diagnostic markers for cervical cancer. lncRNAs
are transcripts measuring 200 nucleotides or more that do not
encode proteins. Although the functional roles of small regulatory
ncRNAs, such as microRNAs, have been well established in human
cancers, little is known about the regulatory roles of lncRNAs and
their relevance to human disease. Many lncRNAs are capped, spliced,
and polyadenylated with protein-coding counterparts (16). lncRNAs have been shown to exhibit
tissue-specific expression patterns and have been functionally
characterized; the biosynthesis of these RNAs is important for a
variety of physiological processes, and abnormal expression of
lncRNAs may affect cancer development and progression (17). Moreover, lncRNAs are emerging as key
players in the complex mechanisms underlying malignant processes,
including tumorigenesis, drug resistance, and metastasis (18–20).
Cervical cancer remains one of the leading causes of
cancer-related death in women worldwide (21). Despite the development of advanced
therapeutic strategies, the prognosis in patients with cervical
cancer varies significantly and is hard to predict. Treatment
outcomes still depend primarily on early detection and diagnosis.
Recent studies have demonstrated that some abnormal molecular
biology changes may play central roles in the development of
cervical cancer (22). However, there
are few reports of the biology and function of SRA in
cervical cancer cells. Accordingly, in this study, investigated the
molecular function and clinical significance of SRA
expression in cervical cancer cell lines. We found that SRA
expression was higher in cervical cancer tissues than in comparable
noncancerous tissues. Moreover, SRA overexpression altered
cell growth, migration, and invasion in cervical cancer cells. The
metastatic effects of SRA appeared to be mediated, at least
in part, by the regulation of genes involved in cell migration,
invasion, and the EMT.
SRA is an lncRNA that acts as a putative
coactivator for steroid receptor-mediated transcription. Its
overexpression and consequent deregulated hormone signaling are
associated with breast, uterine, ovarian, and prostate cancers
(23). Therefore, we hypothesized
that expression of SRA may affect prognosis in patients with
cervical cancer. We discovered that SRA overexpression
increased cervical cancer cell proliferation, migration, and
invasion. Thus, SRA may be oncogenic in cervical cancer and
promote aggressive and metastatic characteristics. The EMT involves
alterations in cell phenotype, and several transcription factors
have been implicated in the regulation of EMT-related gene
expression. Although several studies have focused on
transcriptional regulators in the pathological EMT, few studies
have evaluated the roles of transcription factors in cervical
cancer (24).
In this study, high expression of SRA in
cervical cancer cells induced cell migration and invasion through
upregulation of EMT-related genes.
Loss of E-cadherin is thought to be an important
event of EMT, but N-cadherin causes a decrease in intercellular
junctions between two adjacent endotheliums, which causes cancer
cells to slip (25). In addition,
β-catenin is more mobile and weakens the slowly related mesenchymal
phenotype. The enhancement of the expression of transcription
factors such as Snail and Twist is associated with the loss of
intercellular adhesion (26),
vimentin constitutes the main component of the cytoskeleton of
mesenchymal cells, it's up-regulation is induced by EMT (1,27). Recent
study have been demonstrated that HOTAIR regulated the
expression of vascular endothelial growth factor, matrix
metalloproteinase-9 and EMT-related genes, which are important for
cell motility and metastasis (19).
We hypothesized that SRA may act an important
regulator of several signaling mechanisms associated with the EMT.
Our results suggested that SRA may contribute to the growth,
invasion, and recurrence of cervical cancer through induction of
the EMT. The recurrence rate of radical cervical cancer after
radical surgery is 15–30%, and the prognosis of patients with
recurrence is poor (28). A reliable
predictor of recurrence and progression is needed to improve the
prognosis of patients with cervical cancer. Cell type in cervical
cancer is related to patient survival, and squamous cell carcinoma
has been found to be most closely related to survival (29,30). Here,
we demonstrated that high SRA expression was related to low
overall survival rates in patients with squamous cell carcinoma.
Multi-scale modelling Pelvic lymph node metastasis is one of the
most important postoperative risk factors for relapse or failure to
survive. Therefore, patients with cervical cancer with metastasis
to pelvic lymph nodes require adjuvant therapy, such as
postoperative radiation therapy (31). We also found that high SRA
expression was related to advanced stage and metastasis. Thus,
assessment of SRA expression in patients with cervical
cancer can inform treatment decisions by predicting the risk of
progression or recurrence.
In summary, we found that patients with cervical
cancer had elevated SRA levels. Moreover, SRA
overexpression was positively correlated with clinicopathological
parameters in cervical cancer, and SRA was found to have a
role in promoting cell growth and invasion through modulation of
the EMT. These results suggested that SRA may have
applications in determination of the clinicopathological stage
and/or prognosis of patients with cervical cancer. Accordingly,
SRA may be a promising therapeutic target in cervical
cancer.
Acknowledgements
Not applicable.
Funding
The present study was supported by the Korea Health
Technology R&D Project through the Korea Health Industry
Development Institute, funded by the Ministry of Health and
Welfare, Republic of Korea (grant no. HI17C0321) and by the Basic
Science Research Program through the National Research Foundation
of Korea funded by the Ministry of Education, Science and
Technology (grant nos. NRF-2015R1A2A2A01008162,
NRF-2017R1D1A3B03032983 and NRF-2015 R1C1A2A01053516).
Availability of data and materials
The datasets used/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
HJK, SHL and YTK designed and coordinated the study
and LKK wrote the paper. LKK, KJE and SAP performed the research
and analyzed the data.
Ethics approval and consent to
participate
The present study was approved by the Ethics
Committee of Yonsei Severance Hospital, and written informed
consent was obtained from all patients.
Patient consent for publication
Written informed consent was obtained from all
patients.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
lncRNA
|
long noncoding RNA
|
|
SRA
|
steroid receptor activator
|
|
EMT
|
epithelial-to-mesenchymal
transition
|
References
|
1
|
Ferlay J, Soerjomataram I, Dikshit R, Eser
S, Mathers C, Rebelo M, Parkin DM, Forman D and Bray F: Cancer
incidence and mortality worldwide: Sources, methods and major
patterns in GLOBOCAN 2012. Int J Cancer. 136:E359–E386. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Yang W, Hong L, Xu X, Wang Q, Huang J and
Jiang L: LncRNA GAS5 suppresses the tumorigenesis of cervical
cancer by downregulating miR-196a and miR-205. Tumour Biol.
39:10104283177113152017. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Dryden-Peterson S, Bvochora-Nsingo M,
Suneja G, Efstathiou JA, Grover S, Chiyapo S, Ramogola-Masire D,
Kebabonye-Pusoentsi M, Clayman R, Mapes AC, et al: HIV infection
and survival among women with cervical cancer. J Clin Oncol.
34:3749–3757. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Chuang LT, Feldman S, Nakisige C, Temin S
and Berek JS: Management and care of women with invasive cervical
cancer. J Clin Oncol. 34:3354–3355. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Perez DS, Hoage TR, Pritchett JR,
Ducharme-Smith AL, Halling ML, Ganapathiraju SC, Streng PS and
Smith DI: Long, abundantly expressed non-coding transcripts are
altered in cancer. Hum Mol Genet. 17:642–655. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Mattick JS: The functional genomics of
noncoding RNA. Science. 309:1527–1528. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Guttman M, Donaghey J, Carey BW, Garber M,
Grenier JK, Munson G, Young G, Lucas AB, Ach R, Bruhn L, et al:
lincRNAs act in the circuitry controlling pluripotency and
differentiation. Nature. 477:295–300. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Rinn JL, Kertesz M, Wang JK, Squazzo SL,
Xu X, Brugmann SA, Goodnough LH, Helms JA, Farnham PJ, Segal E and
Chang HY: Functional demarcation of active and silent chromatin
domains in human HOX loci by noncoding RNAs. Cell. 129:1311–1323.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ponting CP, Oliver PL and Reik W:
Evolution and functions of long noncoding RNAs. Cell. 136:629–641.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Hung T, Wang Y, Lin MF, Koegel AK, Kotake
Y, Grant GD, Horlings HM, Shah N, Umbricht C, Wang P, et al:
Extensive and coordinated transcription of noncoding RNAs within
cell-cycle promoters. Nat Genet. 43:621–629. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Lanz RB, McKenna NJ, Onate SA, Albrecht U,
Wong J, Tsai SY, Tsai MJ and O'Malley BW: A steroid receptor
coactivator, SRA, functions as an RNA and is present in an SRC-1
complex. Cell. 97:17–27. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Ling H, Vincent K, Pichler M, Fodde R,
Berindan-Neagoe I, Slack FJ and Calin GA: Junk DNA and the long
non-coding RNA twist in cancer genetics. Oncogene. 34:5003–5011.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Leygue E: Steroid receptor RNA activator
(SRA1): Unusual bifaceted gene products with suspected relevance to
breast cancer. Nucl Recept Signal. 5:e0062007.PubMed/NCBI
|
|
14
|
Colley SM and Leedman PJ: Steroid receptor
RNA activator-A nuclear receptor coregulator with multiple
partners: Insights and challenges. Biochimie. 93:1966–1972. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Eoh KJ, Paek J, Kim SW, Kim HJ, Lee HY,
Lee SK and Kim YT: Long non-coding RNA, steroid receptor RNA
activator (SRA), induces tumor proliferation and invasion through
the NOTCH pathway in cervical cancer cell lines. Oncol Rep.
38:3481–3488. 2017.PubMed/NCBI
|
|
16
|
Carninci P, Kasukawa T, Katayama S, Gough
J, Frith MC, Maeda N, Oyama R, Ravasi T, Lenhard B, Wells C, et al:
The transcriptional landscape of the mammalian genome. Science.
309:1559–1563. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Hall PA and Russell SH: New perspectives
on neoplasia and the RNA world. Hematol Oncol. 23:49–53. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Mercer TR, Dinger ME and Mattick JS: Long
non-coding RNAs: Insights into functions. Nat Rev Genet.
10:155–159. 2009. View
Article : Google Scholar : PubMed/NCBI
|
|
19
|
Kim HJ, Lee DW, Yim GW, Nam EJ, Kim S, Kim
SW and Kim YT: Long non-coding RNA HOTAIR is associated with human
cervical cancer progression. Int J Oncol. 46:521–530. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Gupta RA, Shah N, Wang KC, Kim J, Horlings
HM, Wong DJ, Tsai MC, Hung T, Argani P, Rinn JL, et al: Long
non-coding RNA HOTAIR reprograms chromatin state to promote cancer
metastasis. Nature. 464:1071–1076. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Schiffman M, Castle PE, Jeronimo J,
Rodriguez AC and Wacholder S: Human papillomavirus and cervical
cancer. Lancet. 370:890–907. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Lui WO, Pourmand N, Patterson BK and Fire
A: Patterns of known and novel small RNAs in human cervical cancer.
Cancer Res. 67:6031–6043. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Fatima R, Akhade VS, Pal D and Rao SM:
Long noncoding RNAs in development and cancer: Potential biomarkers
and therapeutic targets. Mol Cell Ther. 3:52015. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Lee MY and Shen MR: Epithelial-mesenchymal
transition in cervical carcinoma. Am J Transl Res. 4:1–13.
2012.PubMed/NCBI
|
|
25
|
Ramis-Conde I, Chaplain MA, Anderson AR
and Drasdo D: Multi-scale modelling of cancer cell intravasation:
The role of cadherins in metastasis. Phys Biol. 6:0160082009.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Calaf GM, Balajee AS, Montalvo-Villagra
MT, Leon M, Daniela NM, Alvarez RG, Roy D, Narayan G and
Abarca-Quinones J: Vimentin and Notch as biomarkers for breast
cancer progression. Oncol Lett. 7:721–727. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Martin TA, Goyal A, Watkins G and Jiang
WG: Expression of the transcription factors snail, slug, and twist
and their clinical significance in human breast cancer. Ann Surg
Oncol. 12:488–496. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Delgado G, Bundy B, Zaino R, Sevin BU,
Creasman WT and Major F: Prospective surgical-pathological study of
disease-free interval in patients with stage IB squamous cell
carcinoma of the cervix: A Gynecologic Oncology Group study.
Gynecol Oncol. 38:352–357. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Wentz WB and Reagan JW: Survival in
cervical cancer with respect to cell type. Cancer. 12:384–388.
1959. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Biewenga P, van der Velden J, Mol BW,
Stalpers LJ, Schilthuis MS, van der Steeg JW, Burger MP and Buist
MR: Prognostic model for survival in patients with early stage
cervical cancer. Cancer. 117:768–776. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Kodama J, Seki N, Masahiro S, Kusumoto T,
Nakamura K, Hongo A and Hiramatsu Y: Prognostic factors in stage
IB-IIB cervical adenocarcinoma patients treated with radical
hysterectomy and pelvic lymphadenectomy. J Surg Oncol. 101:413–417.
2010.PubMed/NCBI
|