Introduction
Nodular goiter (NG), thyroid adenoma (TA) and
thyroid cancer (TC) are common thyroid diseases, and their
incidence rates are rapidly increasing worldwide (1–3), and in
China (4).
NG is a benign thyroid disease and, like
hyperplasia, stems from recurrent attacks of simple goiter. It is
diagnosed following thyroid ultrasonography and fine-needle
aspiration (FNA); if the results suggest malignancy or malignant
behavior, the patient is referred for a thyroidectomy (5).
TA is the most prevalent benign thyroid tumor; it
primarily originates from thyroid follicular cells, and has a
favorable prognosis following surgical resection (6).
TC is the most common malignant tumor among all
endocrine system and head and neck neoplasms, and has a low but
rapidly increasing incidence rate worldwide (7). According to the Surveillance,
Epidemiology, and End Results (SEER) database (http://seer.cancer.gov/statfacts/html/ld/thyro.html),
the number of new cases of TC has increased from 4.8 (per 100,000)
in 1975 to 6.2 (per 100,000) in 1995, and 15.1 (per 100,000) in
2013. In China, it was estimated that the new cases and mortalities
increased to 90,000 and 6,800, respectively, in 2015, according to
the National Central Cancer Registry (NCCR) data of the average
incidence rates from 2009–2011 in 72 population-based cancer
registries (4). Differentiated TC
originates from follicular thyroid cells, and includes papillary TC
(PTC) and follicular TC, which account for 80–85 and 10–15% of TC
cases, respectively (8).
Despite being the most commonly used diagnostic
tool, the ultrasonography-guided FNA biopsy is not useful to
distinguish between benign nodules, follicular adenoma and
follicular carcinoma in cytology (3,9–11). Therefore, it would be beneficial in
clinical practice to identify biomarkers that may distinguish NG,
TA and TC in a convenient and noninvasive manner.
Fatty acids are important nutrients and bioactive
molecules that are involved in energy storage, signal pathways and
key biochemical activities (12,13). There
are a number of studies demonstrating that fatty acids, in
particular ω-3 and ω-6 fatty acids, are closely associated with the
risk of certain diseases, including cancer, diabetes, and
cardiovascular diseases (14,15). The thyroid is an important metabolic
organ, which synthesizes thyroid hormones and controls energetic
metabolism, that is closely associated with fatty acids (16,17).
Schneider and Chen (3) hypothesized
that an increasing body mass index (BMI), which is closely
associated with fatty acids, is a possible explanation for the
increasing incidence of TC. Furthermore, additional studies have
reported differences in fatty acids in the serum (18–20), urine
(21), and thyroid tissue samples
(22,23) between TC patients and healthy
controls.
Although there is a possibility that benign
diseases, including NG and TA, may become malignant, it is
unconfirmed whether these diseases are the precursors of TC and
whether they share any common etiology, including body fat
percentage and fat intake. Therefore studying the association
between fatty acids and NG, TA and TC simultaneously may provide
insights that would be beneficial in clinical practice.
Consequently, the present study utilized a gas chromatography-flame
ionization detector (GC-FID) method to measure the percentages of
polyunsaturated fatty acids (PUFAs) in 122 plasma samples from
patients with thyroid diseases.
Materials and methods
Participants
A total of 122 patients with thyroid diseases were
recruited at 2 time points from Wenzhou Medical School Subsidiary
Hospital (Wenzhou, China), including 97 patients with thyroid
carcinoma (female, n=77), 11 patients with NG (female, n=7), and 14
patients with TA (female, n=9). All blood samples were collected
following overnight fasting. Following centrifugation at 3,000 × g
at room temperature for 15 min, plasma samples were removed and
stored at −80°C until measurement. Clinical parameters, including
age, sex, weight, height, fasting blood glucose, systolic/diastolic
blood pressure, thyroid hormone (Thy), triiodothyronine (T3),
thyroid-stimulating hormone (TSH), free tetraiodothyronine (FT4),
free triiodothyronine (FT3), thyroglobulin antibody (TGA),
anti-thyroperoxidase antibody (TPOA), thyroglobulin (HTG), and
parathormone (PTH), were obtained from the hospital researchers.
The study was approved by Institutional Review Board of the First
Affiliated Hospital of Wenzhou Medical University and informed
consent was obtained from all individual participants included in
the study.
Chemicals and reagents
The fatty acid methyl esters (FAMEs; including 38
FAMEs) internal standard (IS) C21:0 (purity >99%), used as the
calibration standard solution, was purchased from Nu-Chek Prep,
Inc. (Elysian, MN, USA). High-performance liquid chromatography
(HPLC)-grade methanol, dichloromethane, n-hexane, deionized
H2O, and iso-octane were purchased from Honeywell
(Morris Plains, NJ, USA). NaCl (purity >99.5%) was purchased
from Jiangsu Hengrui Medicine Co., Ltd. (Lianyungang, China).
Na2SO4 (purity >99%) and
H2SO4 (purity >95%) were purchased from
Sinopharm Chemical Reagent Co., Ltd. (Shanghai, China).
Profile of fatty acids in plasma
Fatty acids were extracted from the plasma sample
using methanol/dichloromethane (V/V=1:1), then dried with nitrogen,
trans-methylated with methanol/concentrated sulfuric acid
(V/V=25:1) and bathed in 80°C water for 1 h. FAMEs were extracted
by n-hexane, then dried by nitrogen, and finally dissolved in
iso-octane for GC-FID (Agilent Technologies, Inc., Santa Clara, CA,
USA) equipped with a 100-m HP-88 NEFA phase column (100 m × 0.25 mm
× 0.2 µm; Agilent Technologies, Inc.).
Four ω-3 and seven ω-6 fatty acids evaluated in the
present study. The percentage of each individual fatty acid was
calculated according to a response value of the standards using
C21:0 as the IS. The standards were used to adjust the measurement
deviation, and the IS was used to adjust the extraction procedure
deviation. A uniform quality control (QC) sample was inserted into
every 12 samples. The coefficient of variation (CV) of QC was
<12% for all 11 fatty acids.
Statistics analysis
The clinical parameters were compared among three
groups by non-parametric Kruskal-Wallis test for continuous
variables, as the data did not follow a normal distribution. A
χ2 test was used for categorical variables. The
percentages or ratios of fatty acids were compared between TC, NG
and TA by the non-parametric Kruskal-Wallis test. Each group
comparison was performed by the rank-based ANOVA among three
groups. All tests were two-sided. P<0.05 was considered to
indicate a statistically significant difference. Statistical
analyses were performed using SAS 9.3 (SAS Institute, Inc., Cary,
NC, USA).
Results
Baseline clinical characteristics
The baseline clinical characteristics are summarized
shown in Table I. In thyroid-related
hormones, only the TSH was significantly different among the three
groups; and the level in the TC group was the highest. However, the
other hormones evaluated, including Thy, T3, FT4, FT3, TGA, TPOA,
and HTG, did not show any statistically significant differences
(Table I). No significant difference
among the three groups was observed for PTH, and the majority of
thyroid-related hormone indexes in the experimental population were
in the normal range according to ‘China Thyroid Disease Diagnosis
and Treatment Guidelines’ (24),
except TGA and TPOA. In total, 33 out of 95 patients exhibited
higher TGA, and 24 out of 93 patients exhibited higher TPOA. Out of
110 patients, 40 were in the normal range according to ‘China
Thyroid Disease Diagnosis and Treatment Guidelines’ (24) for all 9 thyroid-related hormone
parameters (data not shown).
 | Table I.Baseline characteristics and
thyroid-related hormone levels in the TC, TA and NG groups. |
Table I.
Baseline characteristics and
thyroid-related hormone levels in the TC, TA and NG groups.
|
| Value |
|
|---|
|
|
|
|
|---|
| Characteristic | TC | TA | NG | P-value |
|---|
| Age, years | 46 (38–55) | 43 (38–55) | 56 (41–62) | 0.25 |
| Sex, n |
|
|
|
|
|
Female | 77 | 9 | 8 | 0.55 |
|
Male | 20 | 5 | 3 |
|
| Height, cm
(range) | 160 (158–165) | 160 (157–170) | 158 (151–168) | 0.34 |
| Weight, kg
(range) | 60 (53.5–67) | 63 (49.5–79) | 57 (55–63) | 0.69 |
| BMI,
kg/m2 (range) | 23 (21–26) | 24 (20–28) | 23 (20–25) | 0.91 |
| Glu, mmol/l
(range) | 5.6 (4.9–6.5) | 5.7 (5.5–6.1) | 5.95 (5.2–7.3) | 0.44 |
| SBP, mmHg
(range) | 124 (117–140) | 125.5
(114–134) | 129 (118–135) | 0.91 |
| DBP, mmHg
(range) | 80 (74–87) | 80.5 (70–90) | 83 (79–88) | 0.77 |
| HT, % | 32/97 (34) | 2/11 (18) | 2/10 (20) | 0.56 |
| Thy, nmol/l
(range) | 106 (95–117) | 90 (85–111) | 108 (92–130) | 0.18 |
| T3, nmol/l
(range) | 1.6 (1.4–1.8) | 1.6 (1.3–1.7) | 1.75 (1.4–1.8) | 0.49 |
| TSH, mIU/l
(range) | 1.4
(0.88–1.91) | 1.13
(0.62–1.46) | 0.67
(0.48–1.6) | 0.03 |
| FT4, pmol/l
(range) | 11 (9–12) | 11 (10–12) | 12 (9.5–13) | 0.61 |
| FT3, pmol/l
(range) | 4.4 (4–4.8) | 4.1 (3.8–4.4) | 4.4 (4.1–4.71) | 0.16 |
| TGA, IU/ml
(range) | 0.9 (0.9–12.8) | 0.9 (0.9–0.9) | 0.9 (0.9–0.9) | 0.29 |
| TPOA, IU/ml
(range) | 106 (95–117) | 90 (85–110) | 108 (92–130) | 0.37 |
| PTH, pg/ml
(range) | 37 (28–43) | 31 (27–33) | 42 (32–47) | 0.20 |
PUFAs among three groups
The plasma levels of 22:6n-3, ω-3 PUFAs, 22:4n-6,
ω-6/ω-3 fatty acids, and total PUFAs were significantly different
among the three groups (Fig. 1A-D).
Total PUFA level (Fig. 1E) was
significantly increased in the TA group compared with the TC and NG
groups. The levels of 22:6n-3 (Fig.
1A) and total ω-3 PUFAs (Fig. 1B)
were significantly increased in the TA group compared with the TC
and NG groups. However, 20:5n-3 and 22:5n-3 did not demonstrate a
statistically significant difference among the three groups. In
addition, among ω-6 fatty acids, 22:4n-6 (Fig. 1H) was significantly increased in the
NG group compared with the TC and TA groups. A number of other ω-6
fatty acids, including 18:3n-6 (Fig.
1I), 20:2n-6 (Fig. 1J), 20:3n-6
(Fig. 1K), 20:4n-6 (Fig. 1L), and 22:5n-6 (Fig. 1M), demonstrated a similar pattern. The
ω-6/ω-3 (Fig. 1D) ratio was
significantly decreased in the TA group compared with the TC
group.
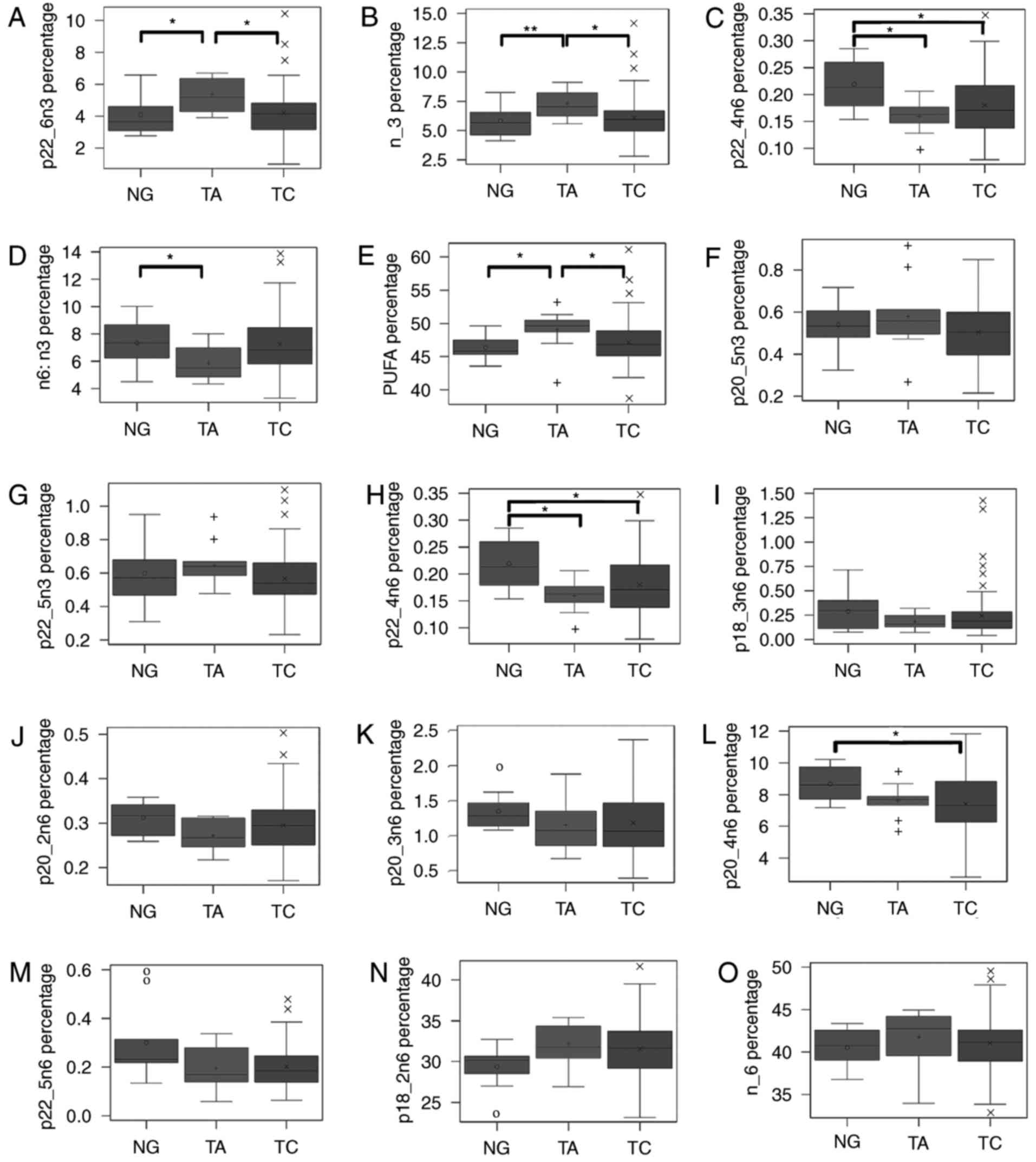 | Figure 1.Percentage and the ratio of fatty
acids and different kinds of fatty acids are compared among three
diseases. (A) Demonstrates the percentage of total PUFA; (B)
exhibits the ratio of total ω-6 to total ω-3; (C-J) were the
percentage of total ω-6, 18:2n-6, 18:3n-6, 20:2n-6, 20:3n-6,
20:4n-6, 22:4n-6, and 22:5n-6; (K-O) were the percentage of total
ω-3, 18:3n-3, 20:5n-3, 22:5n-3, and 22:6n-3. Fatty acids are
compared by Kruskal-Wallis test among three groups, and two groups
are compared using an analysis of based on rank adjusted by
Bonferroni Correction. *P<0.05. 0, +, and × denote median values
of NG, TA and TC, respectively, inside the interquartile range, and
outliers outside the interquartile range. |
PUFAs among three groups in
females
In females (Table
II), the present study did not identify a statistically
significant difference in any ω-3 fatty acids among the three
groups examined. While the ω-6 fatty acids 20:4n-6 and 22:4n-6 were
significantly different among three groups in females. In females,
the levels of 18:3n-6, 20:2n-6, 20:3n-6 and 22:5n-6 in the NG group
were higher than those of the other groups, and their pattern that
was similar to that of the whole study population (Fig. 1).
 | Table II.The percentage and the ratio of fatty
acids and different kinds of fatty acids in female are compared
among three diseases. |
Table II.
The percentage and the ratio of fatty
acids and different kinds of fatty acids in female are compared
among three diseases.
|
| Fatty acid level,
median (Q1, Q3) |
|
|---|
|
|
|
|
|---|
| Fatty acid | TC (n=78) | TA (n=9) | NG (n=8) | P-value |
|---|
| PUFA | 47 (45,49) | 50 (49,51) | 46 (45,48) | 0.01 |
| n-3 | 6.2 (5.2,6.9) | 7.4 (6.5,8.1) | 6.0 (4.6,7.9) | 0.08 |
|
18:3n-3 | 0.71
(0.59,1.0) | 0.53
(0.49,0.65) | 0.61
(0.49,0.84) | 0.13 |
|
20:5n-3 | 0.49
(0.4,0.61) | 0.57
(0.52,0.61) | 0.51
(0.45,0.55) | 0.24 |
|
22:5n-3 | 0.54
(0.48,0.67) | 0.6
(0.53,0.66) | 0.64
(0.45,0.86) | 0.66 |
|
22:6n-3 | 4.3 (3.3,5.1) | 5.7 (4.5,6.3) | 3.7 (3.0,6.0) | 0.08 |
| n-6 | 41 (39,43) | 43 (42,44) | 41 (37,42) | 0.15 |
|
18:2n-6 | 32 (29,34) | 33 (31,35) | 30 (27,31) | 0.05 |
|
18:3n-6 | 0.2
(0.12,0.29) | 0.16
(0.13,0.23) | 0.28
(0.11,0.4) | 0.70 |
|
20:2n-6 | 0.29
(0.26,0.33) | 0.27
(0.25,0.31) | 0.32
(0.3,0.34) | 0.18 |
|
20:3n-6 | 1.1 (0.87,1.5) | 1.2 (0.91,1.4) | 1.2 (1.1,1.6) | 0.38 |
|
20:4n-6 | 7.3 (6.3,8.8) | 7.8 (7.6,8.3) | 9.1 (8.1,9.9) | 0.04 |
|
22:4n-6 | 0.17
(0.14,0.21) | 0.16
(0.14,0.18) | 0.22
(0.18,0.28) | 0.02 |
|
22:5n-6 | 0.18
(0.13,0.25) | 0.2
(0.14,0.31) | 0.24
(0.22,0.56) | 0.06 |
| n-6:n-3 | 6.7 (5.7,8.1) | 5.8 (5.2,6.6) | 7.2 (4.6,9.3) | 0.30 |
PUFAs among three groups with normal
level of thyroid-related hormones
The percentage of fatty acids in patients with a
normal level of thyroid-related hormones were compared by
Kruskal-Wallis test among three groups (Table III). The results identified that
22:6n-3, total ω-3 fatty acids, 22:4n-6, and total PUFA levels were
significantly different among three groups, which were consistent
with the whole study population.
 | Table III.Percentage and ratio of fatty acids
compared among three groups of patients with five types of normal
level thyroid-related hormone. |
Table III.
Percentage and ratio of fatty acids
compared among three groups of patients with five types of normal
level thyroid-related hormone.
|
| Fatty acid level,
median (Q1, Q3) |
|
|---|
|
|
|
|
|---|
| Fatty acid | TC (N=56) | TA (N=8) | NG (N=6) | P-value |
|---|
| PUFA | 46 (44,49) | 50 (49,51) | 46 (45,48) | 0.03 |
| n-3 | 5.9 (4.9,6.7) | 7.4 (6.8,8.0) | 5.0 (4.5,6.0) | 0.02 |
|
18:3n-3 | 0.68
(0.58,1.1) | 0.53
(0.51,0.7) | 0.52
(0.43,0.63) | 0.12 |
|
20:5n-3 | 0.51
(0.4,0.61) | 0.57
(0.54,0.61) | 0.53
(0.51,0.55) | 0.24 |
|
22:5n-3 | 0.54
(0.43,0.68) | 0.6
(0.59,0.66) | 0.57
(0.47,0.68) | 0.65 |
|
22:6n-3 | 4.2 (3.1,4.8) | 5.7 (5.0,6.3) | 3.4 (3.0,3.7) | 0.04 |
| n-6 | 40 (38,43) | 43 (42,43) | 41 (39,43) | 0.21 |
|
18:2n-6 | 31 (28,33) | 33 (31,34) | 30 (29,31) | 0.20 |
|
18:3n-6 | 0.2
(0.12,0.29) | 0.15
(0.13,0.22) | 0.12
(0.11,0.32) | 0.64 |
|
20:2n-6 | 0.3
(0.25,0.33) | 0.27
(0.25,0.31) | 0.33
(0.27,0.35) | 0.24 |
|
20:3n-6 | 1.1 (0.87,1.6) | 1 (0.86,1.3) | 1.3 (1.2,1.4) | 0.50 |
|
20:4n-6 | 7.2 (6.3,8.3) | 7.8 (7.5,8.7) | 8.4 (7.7,9.1) | 0.15 |
|
22:4n-6 | 0.18
(0.14,0.22) | 0.15
(0.13,0.17) | 0.22
(0.21,0.26) | 0.03 |
|
22:5n-6 | 0.2
(0.14,0.26) | 0.16
(0.14,0.23) | 0.26
(0.22,0.31) | 0.06 |
| n-6:n-3 | 7.0 (5.7,8.6) | 5.8 (5.4,6.2) | 8.1 (7.2,9.3) | 0.11 |
Cut-off of total ω3 fatty acids and
22:4n6 to distinguish TA and NG groups
When the 75th percentile cutoff of the ω-3 fatty
acids and 22:4n-6 in the group from the first time point was
applied to the group from the second time point, the patients with
TA were 100% correctly classified (Tables IV and V). Consistent with the significant
difference (Table II), it
demonstrates that ω-3 PUFAs and 22:4n-6 are helpful to distinguish
TA and NG among three diseases although sample size is limited.
 | Table IV.The 75% threshold value for total ω-3
fatty acids was 6.550 (75th percentile), which was used to
distinguish TA from TC and NG. |
Table IV.
The 75% threshold value for total ω-3
fatty acids was 6.550 (75th percentile), which was used to
distinguish TA from TC and NG.
|
| Batch 1 | Batch 2 |
|---|
|
|
|
|
|---|
| Disease (%) | (Proportion
≥Q3) | (Proportion
≥Q3) |
|---|
| TC | 14/67=21 | 17/30=57 |
| TA | 6/9=67 | 2/2=100 |
| NG | 2/7=29 | 1/3=33 |
 | Table V.The 75% threshold value for 22:4n-6
was 0.209 (75th percentile), which was used to distinguish NG from
TC and TA. |
Table V.
The 75% threshold value for 22:4n-6
was 0.209 (75th percentile), which was used to distinguish NG from
TC and TA.
|
| Batch 1 | Batch 2 |
|---|
|
|
|
|
|---|
| Disease (%) | (Proportion
≥Q3) | (Proportion
≥Q3) |
|---|
| TC | 17/67=25 | 12/30=40 |
| TA | 0/9=0 | 0/2=0 |
| NG | 4/7=57 | 2/3=67 |
Discussion
The present study identified that the plasma levels
of 22:6n-3, total ω-3 fatty acids, and PUFAs were significantly
increased in the TA group compared with those of the TC and NG
groups and that 22:4n-6 level was significantly increased in NG
group compared with that of TC and TA groups.
Thyroid diseases are common endocrine diseases,
although some investigators hypothesize that thyroid diseases,
especially TC, are over-diagnosed (1,25,26). Conversely, other researchers postulate
that the incidence rate of TC is increasing worldwide (2,4); however,
this increasing trend may be associated with environmental changes
and the development of detection technology (3). Regardless, the clear etiological
mechanism of this substantial rise requires examination. It is
difficult to distinguish the different thyroid diseases of
follicular based on clinical symptoms and cytology (3,9–11). Therefore, it is important to develop
biomarkers and easier-to-use technologies to facilitate clinical
practice.
The estimated numbers of new cases of TC in 2015
were 40,200 in east, 14,300 in central, 10,700 in northeast and
<10,000 in other regions of China (4). A number of studies reported that the
fish and seafood-rich dietary intake was associated with the
increased risk of TC (27,28), in particular dried or salted fish that
specifically occur among Asian seasonings (29). Wenzhou is an eastern coastal city
where people consume an increased amount of seafood compared with
the national average (30), and
seafood is rich in ω-3 fatty acids. Therefore, evaluating the link
between thyroid disease and fatty acids may provide beneficial
insights; hence the patient sample was obtained from a
Wenzhou-based hospital.
Activation of de novo lipogenesis may result
in TC tumorigenesis through fatty acid synthase (FASN) catalyzing
the synthesis of 16-carbon saturated fatty acids and AKT pathway
signaling (31). However, consistent
with the results of previous studies (18,21,23), the
present study identified that the ω-3 and ω-6 fatty acids with
chains longer than 16 carbons were not increased in patients with
cancer. We hypothesize that this is due to long chain ω-3 and ω-6
fatty acids being primarily from diet, and produced through other
enzymatic reactions.
ω-3 and ω-6 fatty acids, including 18:3n-3, 20:5n-3,
22:5n-3, 22:6n-3 and 18:2n-6, 18:3n-6, 20:2n-6, 20:3n-6, 20:4n-6,
22:4n-6, 22:5n-6, are involved in two different metabolic pathways
in KEGG. It is reported that ω-3 fatty acids are protective in the
majority of diseases, while ω-6 fatty acids do not exhibit this
activity type (32). The present
study identified that ω-3 and ω-6 fatty acid profiles were
exhibited differently among three diseases: ω-3 fatty acids,
including 20:5n-3, 22:5n-3, and 22:6n-3, were highest in the TA
group; and ω-6 fatty acids, including 18:3n-6, 20:2n-6, 20:3n-6,
20:4n-6, 22:4n-6, and 22:5n-6, were highest in the NG group. We
hypothesize that ω-3 and ω-6 fatty acids may have different
metabolic pathways in TA and NG compared with TC, which may result
in the varying etiological characteristics of those diseases.
The incidence of nodules generally increases with
age (33). The present study
corroborated this, as NG patients of the study sample were older
than in the other two groups. TGA and TPOA are related to
autoimmune thyroid disease and thyroiditis (34,35). TSH
level, which is a risk factor for TC (3,36), was
highest in the TC group followed by the TA group in our study.
Total PUFA levels were significantly increased in
the TA group compared with the TC and NG groups in the present
study. Berg et al (18)
reported that the sum of arachidonic acid (20:4n-6) and
docosahexaenoic acid (DHA) concentrations and the sum of
arachidonic acid, EPA, and DHA were significant decreased in
patients with cancer compared with healthy controls. Guo et
al (23) reported C20:4, C22:4
and C22:5 were significantly decreased in six types of cancer
tissues (breast, lung, colorectal, esophageal and gastric cancer
and TC); however, C22:4 and C22:5 were increased in TC tissue
compared with normal tissue. Furthermore, urinary PUFAs are
increased in TC compared with healthy controls (21). To date, the results of previous
studies on total PUFA in cancer have been inconclusive, suggesting
that total PUFA may not serve as an appropriate indicator for
thyroid disease assessment.
The present study demonstrated that DHA and total
ω-3 fatty acids were significantly increased in TA compared with TC
and NG. If confirmed through further study, this exhibits potential
to aid in distinguishing TA from other thyroid diseases. Although
statistically significant differences were not identified for other
ω-3 fatty acids, they exhibited similar trends. Therefore a study
with a larger cohort would be required to examine this. Consistent
with our results, Xu et al (37) reported that DHA levels were increased
in TA compared with TC tissue. Yao et al (20) reported serum DHA levels were decreased
in PTC and NG compared with healthy controls; however, they did not
identify a difference between PTC and NG. Zhang et al
(19) reported that the serum DHA and
18:3n-3 were significantly increased in TC patients compared with
those with benign thyroid diseases, and the difference may be due
to the different types of fatty acids. Zhang et al (19) measured the free fatty acids (FFAs)
while Yao et al (20) measured
the total fatty acids, including FFA and esterified fatty acids.
Furthermore, Zhang et al (19)
used concentration (µM), whereas the present study merely obtained
percentages of fatty acids, which may be responsible for the
differences observed.
In the present study, 22:4n-6 was significantly
decreased in the TC and TA groups compared with NG group; a number
of other ω-6 fatty acids, including 18:3n-6, 20:2n-6, 20:3n-6,
20:4n-6, 22:4n-6, and 22:5n-6, exhibited similar, though
statistically insignificant trends. ω-6 fatty acids also can be
helpful to differentiate NG from other thyroid diseases. In a study
by Kim et al (21), the
urinary concentrations of ω-6 fatty acids, including 18:2n-6 and
20:4n-6, were decreased in TC compared with healthy controls.
However, Yao et al (20)
reported that serum 18:2n-6, 20:4n-6 and 22:4n-6 were increased in
PTC compared with NG. Furthermore, Zhang et al (19) reported the serum 20:4n-6 was
significantly increased in TC patients compared with those with
benign thyroid diseases. However, the differences may be related to
the types of diseases examined and the units used.
When the analysis was restricted to female subjects,
the DHA and total ω-3 fatty acids did not demonstrate a significant
difference. This may be due to the smaller sample size. The 20:4n-6
was significantly decreased in the TC and TA groups compared with
the NG group in female subjects, which may suggest that more
20:4n-6 would be transferred into the downstream product. This is
due to arachidonic acid being the substrate of prostaglandin E2,
which may inhibit the anti-tumor reaction of the immune system
(38). Therefore, 20:4n-6 may be
associated with a high incidence of TC in female subjects.
To the best of our knowledge, the present study is
amongst the first to explore the plasma fatty acid profiles among
three common thyroid diseases. TA was identified to be different
from TC and NG in ω-3 fatty acids, while NG was different from TC
and TA in ω-6 fatty acids. FNA is the most common tool for thyroid
disease diagnosis; however, its sampling accuracy is limited. While
the mutational analyses of BRAF, RAS, RET/PTC, and PAX8-PPARγ
rearrangement contribute to distinguishing follicular lesions
(39,40), the tissue damage resulting from
surgery is not negligible. Furthermore, this method also has
uncertainty due to the dependency of accurate sampling on the
equipment and the operator. However, if validated, fatty acids have
the potential to serve as diagnostic biomarkers, which may
facilitate the diagnosis of thyroid diseases.
The present study is not without limitations. It had
a small sample size, which limited the ability to detect moderate
differences. Second, fatty acids in the plasma only reflect
short-term dietary exposure, and the absence of analysis of PUFAs
in a healthy population is a limitation. However, the present study
focused on comparing three thyroid diseases, as opposed to a
case-control study with healthy controls, in order to provide
insight on the etiological evolution of the diseases. This could
aid in the understanding of whether NG or TA are precancerous forms
of TC from the aspect of fatty acid metabolism. The results
obtained may also provide data for the prevention/treatment of TC;
however, further studies with somatic tissues are required to
investigate the long-term effects of the exposure to ω-3 and ω-6
PUFAs.
In summary, the present study demonstrated the
differences in clinical parameters and plasma ω-3 and ω-6 PUFA
profiles among three different common thyroid diseases, namely NG,
TA and TC. The results of the present study suggest that ω-3 fatty
acids, especially 22:6n-3, are advantageous for distinguishing TA
from NG and TC; and ω-6 fatty acids, especially 22:4n-6, are
effective for distinguishing NG from TA and TC. However, further
study is required with a larger cohort and prospective design,
including used of somatic tissues.
Acknowledgements
Not applicable.
Funding
This research was supported by funds from the Key
Laboratory of Nutrition and Metabolism (awarded to OW and YG) and
the 100 Talented Plan of Chinese Academy of Sciences (awarded to
YG).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
YG and FC designed the current study. OW, LJ, and FY
obtained biological samples. XL, JZ, QD and CH performed the
experiments. XL and HL analyzed the data. All authors were involved
in the interpretation of the results. XL, FC and YG wrote the
manuscript. All authors read, gave comments, and approved the final
version of the manuscript. All authors had taken responsibility for
the integrity of the data and the accuracy of the data
analysis.
Ethics approval and consent to
participate
Institutional Review Board of the First Affiliated
Hospital of Wenzhou Medical University approved the study and
informed consent was obtained from all individual participants
included in the study.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
GC-FID
|
gas chromatography flame ionization
detector
|
|
IQR
|
interquartile range
|
|
SEER
|
Surveillance, Epidemiology, and End
Results
|
|
NCCR
|
National Central Cancer Registry
|
|
BMI
|
body mass index
|
|
FAME
|
fatty acid methyl esters
|
|
ANOVA
|
analysis of variance
|
References
|
1
|
Davies L, Ouellette M, Hunter M and Welch
HG: The increasing incidence of small thyroid cancers: Where are
the cases coming from? Laryngoscope. 120:2446–2451. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Torre LA, Bray F, Siegel RL, Ferlay J,
Lortet-Tieulent J and Jemal A: Global cancer statistics, 2012. CA
Cancer J Clin. 65:87–108. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Schneider DF and Chen H: New developments
in the diagnosis and treatment of thyroid cancer. CA Cancer J Clin.
63:374–394. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Chen W, Zheng R, Baade PD, Zhang S, Zeng
H, Bray F, Jemal A, Yu XQ and He J: Cancer statistics in China,
2015. CA Cancer J Clin. 66:115–132. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Burman KD and Wartofsky L: Clinical
Practice. Thyroid nodules. N Engl J Med. 373:2347–2356. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Hedinger C, Williams ED and Sobin LH: The
WHO histological classification of thyroid tumors: A commentary on
the second edition. Cancer. 63:908–911. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
La Vecchia C, Malvezzi M, Bosetti C,
Garavello W, Bertuccio P, Levi F and Negri E: Thyroid cancer
mortality and incidence: A global overview. Int J Cancer.
136:2187–2195. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Xing M: Molecular pathogenesis and
mechanisms of thyroid cancer. Nat Rev Cancer. 13:184–199. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
American Thyroid Association Guidelines
Taskforce on Thyroid Nodules and Differentiated Thyroid Cancer, ;
Cooper DS, Doherty GM, Haugen BR, Kloos RT, Lee SL, Mandel SJ,
Mazzaferri EL, McIver B, Pacini F, et al: Revised American Thyroid
Association management guidelines for patients with thyroid nodules
and differentiated thyroid cancer. Thyroid. 19:1167–1214. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Crippa S, Mazzucchelli L, Cibas ES and Ali
SZ: The Bethesda System for reporting thyroid fine-needle
aspiration specimens. Am J Clin Pathol. 134:343–345. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zablotska LB, Nadyrov EA, Polyanskaya ON,
McConnell RJ, O'Kane P, Lubin J, Hatch M, Little MP, Brenner AV,
Veyalkin IV, et al: Risk of thyroid follicular adenoma among
children and adolescents in Belarus exposed to iodine-131 after the
Chornobyl accident. Am J Epidemiol. 182:781–790. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Dutta-Roy AK: Cellular uptake of
long-chain fatty acids: Role of membrane-associated
fatty-acid-binding/transport proteins. Cell Mol Life Sci.
57:1360–1372. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Nickerson JG, Alkhateeb H, Benton CR,
Lally J, Nickerson J, Han XX, Wilson MH, Jain SS, Snook LA, Glatz
JF, et al: Greater transport efficiencies of the membrane fatty
acid transporters FAT/CD36 and FATP4 compared with FABPpm and FATP1
and differential effects on fatty acid esterification and oxidation
in rat skeletal muscle. J Biol Chem. 284:16522–16530. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Santos CR and Schulze A: Lipid metabolism
in cancer. FEBS J. 279:2610–2623. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
de Souza RJ, Mente A, Maroleanu A, Cozma
AI, Ha V, Kishibe T, Uleryk E, Budylowski P, Schünemann H, Beyene J
and Anand SS: Intake of saturated and trans unsaturated fatty acids
and risk of all-cause mortality, cardiovascular disease, and type 2
diabetes: Systematic review and meta-analysis of observational
studies. BMJ. 351:h39782015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Lagrou A, Dierick W, Christophe A and
Verdonk G: Lipid composition of normal and hypertrophic bovine
thyroids. Lipids. 9:870–875. 1974. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Butte NF, Puyau MR, Vohra FA, Adolph AL,
Mehta NR and Zakeri I: Body size, body composition, and metabolic
profile explain higher energy expenditure in overweight children. J
Nutr. 137:2660–2667. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Berg JP, Glattre E, Haldorsen T, Høstmark
AT, Bay IG, Johansen AF and Jellum E: Longchain serum fatty acids
and risk of thyroid cancer: A population-based case-control study
in Norway. Cancer Causes Control. 5:433–439. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zhang Y, Qiu L, He C, Wang Y, Liu Y, Zhang
D and Li Z: Serum unsaturated free fatty acids: A potential
biomarker panel for differentiating benign thyroid diseases from
thyroid cancer. J Cancer. 6:1276–1281. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Yao Z, Yin P, Su D, Peng Z, Zhou L, Ma L,
Guo W, Ma L, Xu G, Shi J and Jiao B: Serum metabolic profiling and
features of papillary thyroid carcinoma and nodular goiter. Mol
Biosyst. 7:2608–2614. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Kim KM, Jung BH, Lho DS, Chung WY, Paeng
KJ and Chung BC: Alteration of urinary profiles of endogenous
steroids and polyunsaturated fatty acids in thyroid cancer. Cancer
Lett. 202:173–179. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Leng J, Guan Q, Sun T, Wu Y, Cao Y and Guo
Y: Application of isotope-based carboxy group derivatization in
LC-MS/MS analysis of tissue free-fatty acids for thyroid carcinoma.
J Pharm Biomed Anal. 84:256–262. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Guo S, Wang Y, Zhou D and Li Z:
Significantly increased monounsaturated lipids relative to
polyunsaturated lipids in six types of cancer microenvironment are
observed by mass spectrometry imaging. Sci Rep. 4:59592014.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Chinese Society of Endocrinology, Chinese
Medical Association, . China Thyroid Disease Diagnosis and
Treatment Guidelines. Zhonghua Nei Ke Za Zhi. 47:867–868. 2008.(In
Chinese).
|
|
25
|
Davies L and Welch HG: Current thyroid
cancer trends in the United States. JAMA Otolaryngol Head Neck
Surg. 140:317–322. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Hoang JK, Nguyen XV and Davies L:
Overdiagnosis of thyroid cancer: Answers to five key questions.
Acad Radiol. 22:1024–1029. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Kolonel LN, Hankin JH, Wilkens LR,
Fukunaga FH and Hinds MW: An epidemiologic study of thyroid cancer
in Hawaii. Cancer Causes Control. 1:223–234. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Glattre E, Haldorsen T, Berg JP, Stensvold
I and Solvoll K: Norwegian case-control study testing the
hypothesis that seafood increases the risk of thyroid cancer.
Cancer Causes Control. 4:11–16. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Horn-Ross PL, Morris JS, Lee M, West DW,
Whittemore AS, McDougall IR, Nowels K, Stewart SL, Spate VL, Shiau
AC and Krone MR: Iodine and thyroid cancer risk among women in a
multiethnic population: the Bay Area Thyroid Cancer Study. Cancer
Epidemiol Biomarkers Prev. 10:979–985. 2001.PubMed/NCBI
|
|
30
|
Wang Yilong: Study of Wenzhou food
culture. Zhejiang Ocean University; 2014, (In Chinese).
|
|
31
|
Uddin S, Siraj AK, Al-Rasheed M, Ahmed M,
Bu R, Myers JN, Al-Nuaim A, Al-Sobhi S, Al-Dayel F, Bavi P, et al:
Fatty acid synthase and AKT pathway signaling in a subset of
papillary thyroid cancers. J Clin Endocrinol Metab. 93:4088–4097.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Calder PC: Polyunsaturated fatty acids,
inflammation, and immunity. Lipids. 36:1007–1024. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Hegedus L: Clinical practice. The thyroid
nodule. N Engl J Med. 351:1764–1771. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Bjoro T, Holmen J, Kruger O, Midthjell K,
Hunstad K, Schreiner T, Sandnes L and Brochmann H: Prevalence of
thyroid disease, thyroid dysfunction and thyroid peroxidase
antibodies in a large, unselected population. The Health Study of
Nord-Trondelag (HUNT). Eur J Endocrinol. 143:639–647. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Prummel MF and Wiersinga WM: Thyroid
peroxidase autoantibodies in euthyroid subjects. Best Pract Res
Clin Endocrinol Metab. 19:1–15. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Fiore E and Vitti P: Serum TSH and risk of
papillary thyroid cancer in nodular thyroid disease. J Clin
Endocrinol Metab. 97:1134–1145. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Xu Y, Zheng X, Qiu Y, Jia W, Wang J and
Yin S: Distinct metabolomic profiles of papillary thyroid carcinoma
and benign thyroid adenoma. J Proteome Res. 14:3315–3321. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Chen JH, Perry CJ, Tsui YC, Staron MM,
Parish IA, Dominguez CX, Rosenberg DW and Kaech SM: Prostaglandin
E2 and programmed cell death 1 signaling coordinately impair CTL
function and survival during chronic viral infection. Nat Med.
21:327–334. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Nikiforova MN, Lynch RA, Biddinger PW,
Alexander EK, Dorn GW III, Tallini G, Kroll TG and Nikiforov YE:
RAS point mutations and PAX8-PPAR gamma rearrangement in thyroid
tumors: Evidence for distinct molecular pathways in thyroid
follicular carcinoma. J Clin Endocrinol Metab. 88:2318–2326. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Fugazzola L, Puxeddu E, Avenia N, Romei C,
Cirello V, Cavaliere A, Faviana P, Mannavola D, Moretti S, Rossi S,
et al: Correlation between B-RAFV600E mutation and
clinico-pathologic parameters in papillary thyroid carcinoma: Data
from a multicentric Italian study and review of the literature.
Endocr Relat Cancer. 13:455–464. 2006. View Article : Google Scholar : PubMed/NCBI
|















